Collapse Mechanisms of Nascent and Aged Sea Spray Aerosol Proxy Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Surface Pressure–Area Isotherms
2.3. Brewster Angle Microscopy (BAM)
3. Results and Discussion
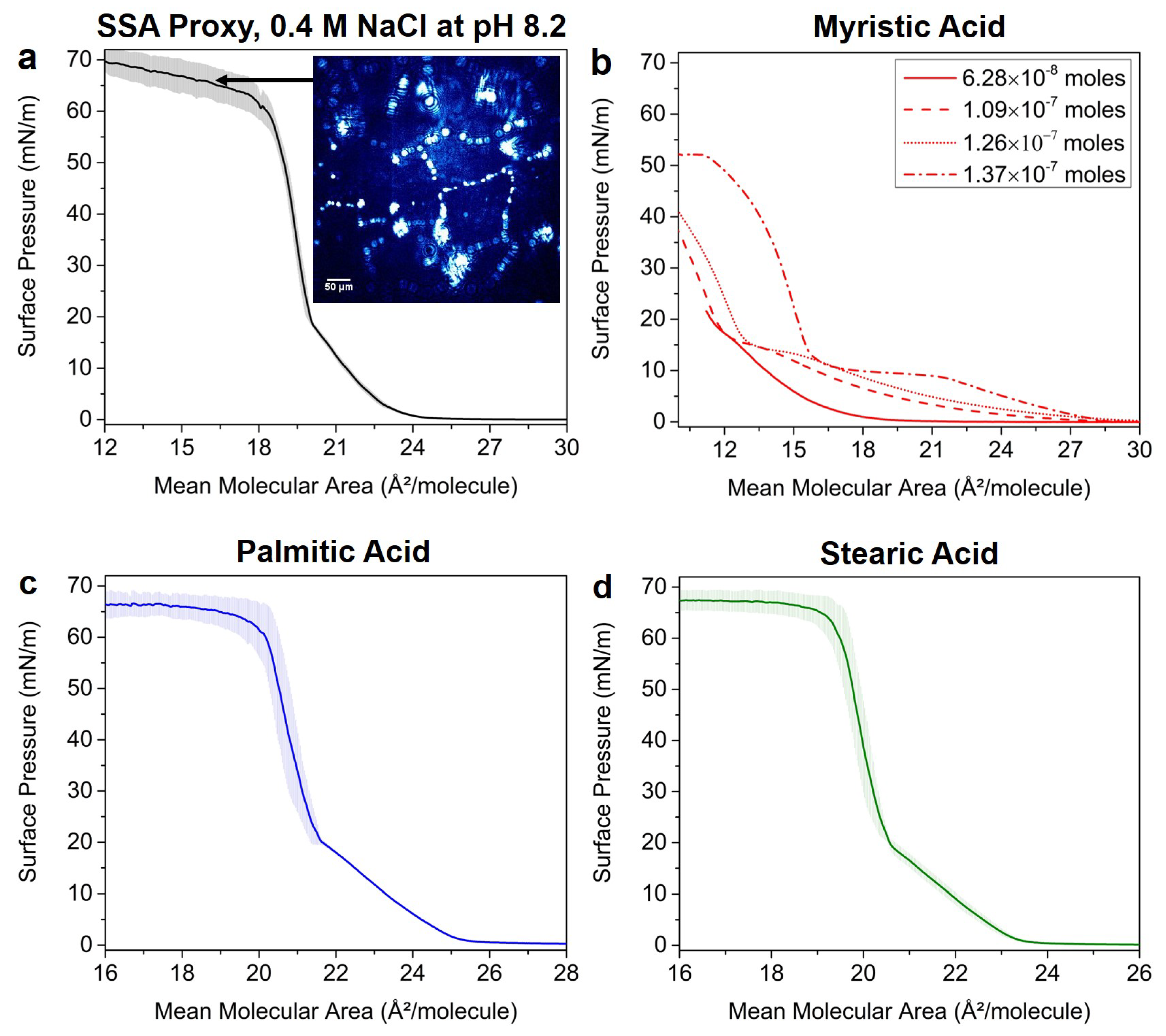
3.1. Nascent SSA Proxy Film Phase Behavior
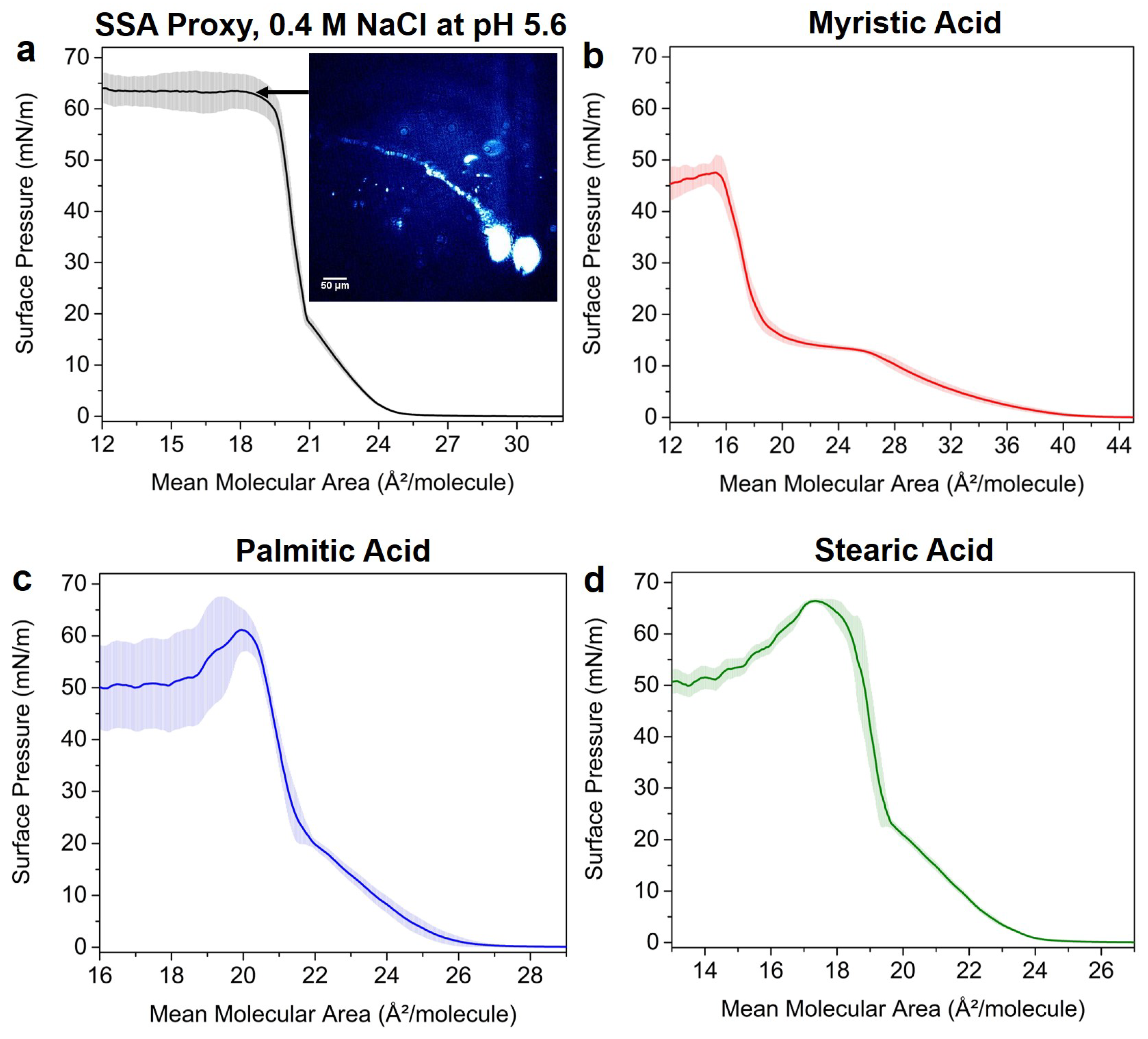
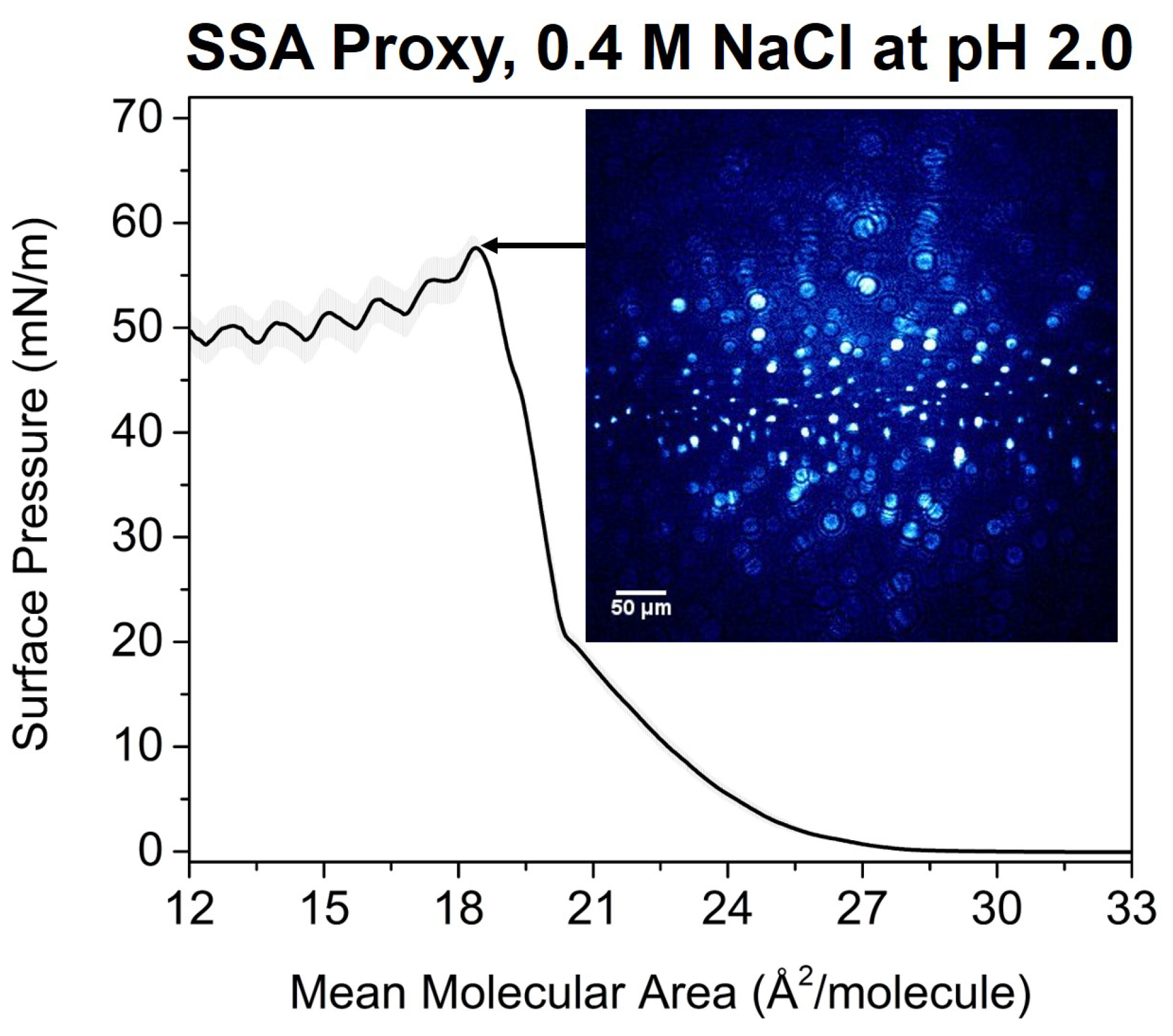
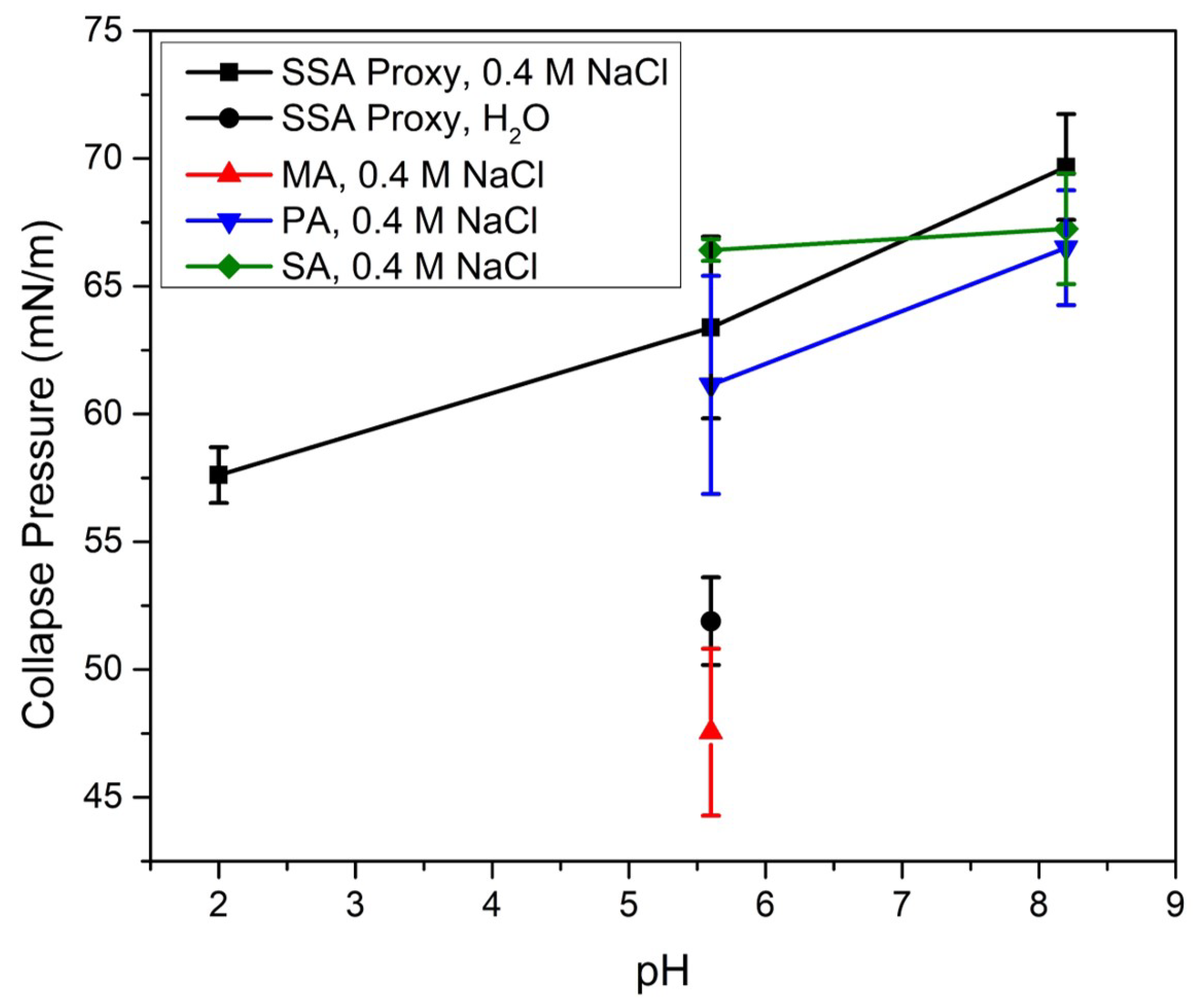
3.2. Effects of SSA Proxy Aqueous Composition on the Film Phase Behavior
3.3. Aged SSA Proxy Film Phase Behavior
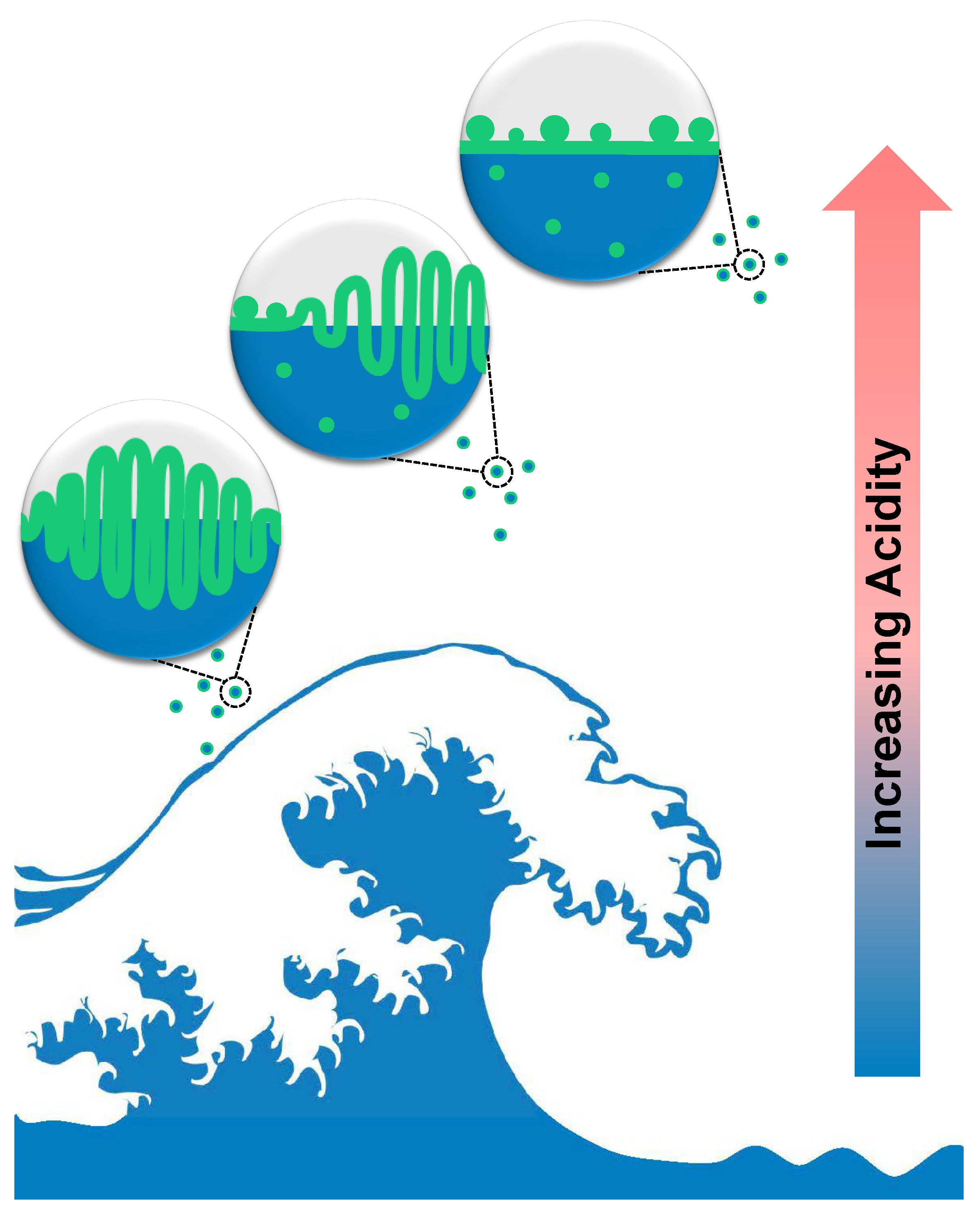
3.4. SSA Film Collapse Mechanisms and Their Atmospheric Implications
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Leeuw, G.; Andreas, E.L.; Anguelova, M.D.; Fairall, C.W.; Lewis, E.R.; O’Dowd, C.; Schulz, M.; Schwartz, S.E. Production Flux of Sea Spray Aerosol. Rev. Geophys. 2011, 49, RG2001. [Google Scholar] [CrossRef]
- Carslaw, K.S.; Lee, L.A.; Reddington, C.L.; Pringle, K.J.; Rap, A.; Forster, P.M.; Mann, G.W.; Spracklen, D.V.; Woodhouse, M.T.; Regayre, L.A.; et al. Large Contribution of Natural Aerosols to Uncertainty in Indirect Forcing. Nature 2013, 503, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Haywood, J.; Boucher, O. Estimates of the Direct and Indirect Radiative Forcing Due to Tropospheric Aerosols: A Review. Rev. Geophys. 2000, 38, 513–543. [Google Scholar] [CrossRef]
- Alpert, P.A.; Aller, J.Y.; Knopf, D.A. Ice Nucleation from Aqueous NaCl Droplets with and without Marine Diatoms. Atmos. Chem. Phys. 2011, 11, 5539–5555. [Google Scholar] [CrossRef]
- Knopf, D.A.; Alpert, P.A.; Wang, B.; Aller, J.Y. Stimulation of Ice Nucleation by Marine Diatoms. Nat. Geosci. 2011, 4, 88–90. [Google Scholar] [CrossRef]
- Pummer, B.G.; Budke, C.; Augustin-Bauditz, S.; Niedermeier, D.; Felgitsch, L.; Kampf, C.J.; Huber, R.G.; Liedl, K.R.; Loerting, T.; Moschen, T.; et al. Ice Nucleation by Water-Soluble Macromolecules. Atmos. Chem. Phys. 2015, 15, 4077–4091. [Google Scholar] [CrossRef]
- Wilson, T.W.; Ladino, L.A.; Alpert, P.A.; Breckels, M.N.; Brooks, I.M.; Browse, J.; Burrows, S.M.; Carslaw, K.S.; Huffman, J.A.; Judd, C.; Kilthau, W.P.; et al. A Marine Biogenic Source of Atmospheric Ice-Nucleating Particles. Nature 2015, 525, 234–238. [Google Scholar] [CrossRef] [PubMed]
- DeMott, P.J.; Hill, T.C.J.; McCluskey, C.S.; Prather, K.A.; Collins, D.B.; Sullivan, R.C.; Ruppel, M.J.; Mason, R.H.; Irish, V.E.; Lee, T.; et al. Sea Spray Aerosol as a Unique Source of Ice Nucleating Particles. Proc. Natl. Acad. Sci. USA 2016, 113, 5797–5803. [Google Scholar] [CrossRef] [PubMed]
- Ladino, L.A.; Yakobi-Hancock, J.D.; Kilthau, W.P.; Mason, R.H.; Si, M.; Li, J.; Miller, L.A.; Schiller, C.L.; Huffman, J.A.; Aller, J.Y.; et al. Addressing the Ice Nucleating Abilities of Marine Aerosol: A Combination of Deposition Mode Laboratory and Field Measurements. Atmos. Environ. 2016, 132, 1–10. [Google Scholar] [CrossRef]
- McCluskey, C.S.; Hill, T.C.J.; Malfatti, F.; Sultana, C.M.; Lee, C.; Santander, M.V.; Beall, C.M.; Moore, K.A.; Cornwell, G.C.; Collins, D.B.; et al. A Dynamic Link between Ice Nucleating Particles Released in Nascent Sea Spray Aerosol and Oceanic Biological Activity during Two Mesocosm Experiments. J. Atmos. Sci. 2016, 74, 151–166. [Google Scholar] [CrossRef]
- Chance, R.J.; Hamilton, J.F.; Carpenter, L.J.; Hackenberg, S.C.; Andrews, S.J.; Wilson, T.W. Water-Soluble Organic Composition of the Arctic Sea Surface Microlayer and Association with Ice Nucleation Ability. Environ. Sci. Technol. 2018, 52, 1817–1826. [Google Scholar] [CrossRef] [PubMed]
- McCluskey, C.S.; Hill, T.C.J.; Sultana, C.M.; Laskina, O.; Trueblood, J.; Santander, M.V.; Beall, C.M.; Michaud, J.M.; Kreidenweis, S.M.; Prather, K.A.; et al. A Mesocosm Double Feature: Insights into the Chemical Make-up of Marine Ice Nucleating Particles. J. Atmos. Sci. 2018. [Google Scholar] [CrossRef]
- Moore, M.J.; Furutani, H.; Roberts, G.C.; Moffet, R.C.; Gilles, M.K.; Palenik, B.; Prather, K.A. Effect of Organic Compounds on Cloud Condensation Nuclei (CCN) Activity of Sea Spray Aerosol Produced by Bubble Bursting. Atmos. Environ. 2011, 45, 7462–7469. [Google Scholar] [CrossRef]
- Gantt, B.; Xu, J.; Meskhidze, N.; Zhang, Y.; Nenes, A.; Ghan, S.J.; Liu, X.; Easter, R.; Zaveri, R. Global Distribution and Climate Forcing of Marine Organic Aerosol—Part 2: Effects on Cloud Properties and Radiative Forcing. Atmos. Chem. Phys. 2012, 12, 6555–6563. [Google Scholar] [CrossRef]
- Modini, R.L.; Frossard, A.A.; Ahlm, L.; Russell, L.M.; Corrigan, C.E.; Roberts, G.C.; Hawkins, L.N.; Schroder, J.C.; Bertram, A.K.; Zhao, R.; et al. Primary Marine Aerosol-Cloud Interactions off the Coast of California. J. Geophys. Res. Atmos. 2015, 120, 4282–4303. [Google Scholar] [CrossRef]
- Collins, D.B.; Bertram, T.H.; Sultana, C.M.; Lee, C.; Axson, J.L.; Prather, K.A. Phytoplankton Blooms Weakly Influence the Cloud Forming Ability of Sea Spray Aerosol. Geophys. Res. Lett. 2016, 43, 2016GL069922. [Google Scholar] [CrossRef]
- Quinn, P.K.; Coffman, D.J.; Johnson, J.E.; Upchurch, L.M.; Bates, T.S. Small Fraction of Marine Cloud Condensation Nuclei Made up of Sea Spray Aerosol. Nat. Geosci. 2017, 10, 674–679. [Google Scholar] [CrossRef]
- Brooks, S.D.; Thornton, D.C. Marine Aerosols and Clouds. Annu. Rev. Mar. Sci. 2018, 10, 289–313. [Google Scholar] [CrossRef] [PubMed]
- Forestieri, S.D.; Staudt, S.M.; Kuborn, T.M.; Faber, K.; Ruehl, C.R.; Bertram, T.H.; Cappa, C.D. Establishing the Impact of Model Surfactants on Cloud Condensation Nuclei Activity of Sea Spray Aerosol Mimics. Atmos. Chem. Phys. 2018, 18, 10985–11005. [Google Scholar] [CrossRef]
- Gaston, C.; Cahill, J.; Collins, D.; Suski, K.; Ge, J.; Barkley, A.; Prather, K.; Gaston, C.J.; Cahill, J.F.; Collins, D.B.; et al. The Cloud Nucleating Properties and Mixing State of Marine Aerosols Sampled along the Southern California Coast. Atmosphere 2018, 9, 52. [Google Scholar] [CrossRef]
- Ault, A.P.; Guasco, T.L.; Baltrusaitis, J.; Ryder, O.S.; Trueblood, J.V.; Collins, D.B.; Ruppel, M.J.; Cuadra-Rodriguez, L.A.; Prather, K.A.; Grassian, V.H. Heterogeneous Reactivity of Nitric Acid with Nascent Sea Spray Aerosol: Large Differences Observed between and within Individual Particles. J. Phys. Chem. Lett. 2014, 5, 2493–2500. [Google Scholar] [CrossRef] [PubMed]
- Estillore, A.D.; Trueblood, J.V.; Grassian, V.H. Atmospheric Chemistry of Bioaerosols: Heterogeneous and Multiphase Reactions with Atmospheric Oxidants and Other Trace Gases. Chem. Sci. 2016, 7, 6604–6616. [Google Scholar] [CrossRef] [PubMed]
- Trueblood, J.V.; Estillore, A.D.; Lee, C.; Dowling, J.A.; Prather, K.A.; Grassian, V.H. Heterogeneous Chemistry of Lipopolysaccharides with Gas-Phase Nitric Acid: Reactive Sites and Reaction Pathways. J. Phys. Chem. A 2016, 120, 6444–6450. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, M.; Luo, M.; Li, Y.; Xiang, B.; Xiong, W.; Grassian, V.H. Let There Be Light: Stability of Palmitic Acid Monolayers at the Air/Salt Water Interface in the Presence and Absence of Simulated Solar Light and a Photosensitizer. Chem. Sci. 2018, 9, 5716–5723. [Google Scholar] [CrossRef] [PubMed]
- Veghte, D.P.; Altaf, M.B.; Freedman, M.A. Size Dependence of the Structure of Organic Aerosol. J. Am. Chem. Soc. 2013, 135, 16046–16049. [Google Scholar] [CrossRef] [PubMed]
- Altaf, M.B.; Zuend, A.; Freedman, M.A. Role of Nucleation Mechanism on the Size Dependent Morphology of Organic Aerosol. Chem. Commun. 2016, 52, 9220–9223. [Google Scholar] [CrossRef] [PubMed]
- Losey, D.J.; Parker, R.G.; Freedman, M.A. pH Dependence of Liquid–Liquid Phase Separation in Organic Aerosol. J. Phys. Chem. Lett. 2016, 7, 3861–3865. [Google Scholar] [CrossRef] [PubMed]
- Freedman, M.A. Phase Separation in Organic Aerosol. Chem. Soc. Rev. 2017, 46, 7694–7705. [Google Scholar] [CrossRef] [PubMed]
- Altaf, M.B.; Dutcher, D.D.; Raymond, T.M.; Freedman, M.A. Effect of Particle Morphology on Cloud Condensation Nuclei Activity. ACS Earth Space Chem. 2018, 2, 634–639. [Google Scholar] [CrossRef]
- Gavish, M.; Popovitz-Biro, R.; Lahav, M.; Leiserowitz, L. Ice Nucleation by Alcohols Arranged in Monolayers at the Surface of Water Drops. Science 1990, 250, 973–975. [Google Scholar] [CrossRef] [PubMed]
- Ochshorn, E.; Cantrell, W. Towards Understanding Ice Nucleation by Long Chain Alcohols. J. Chem. Phys. 2006, 124, 054714. [Google Scholar] [CrossRef] [PubMed]
- Knopf, D.A.; Forrester, S.M. Freezing of Water and Aqueous NaCl Droplets Coated by Organic Monolayers as a Function of Surfactant Properties and Water Activity. J. Phys. Chem. A 2011, 115, 5579–5591. [Google Scholar] [CrossRef] [PubMed]
- Lupi, L.; Peters, B.; Molinero, V. Pre-Ordering of Interfacial Water in the Pathway of Heterogeneous Ice Nucleation Does Not Lead to a Two-Step Crystallization Mechanism. J. Chem. Phys. 2016, 145, 211910. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Odendahl, N.; Hudait, A.; Mason, R.; Bertram, A.K.; Paesani, F.; DeMott, P.J.; Molinero, V. Ice Nucleation Efficiency of Hydroxylated Organic Surfaces Is Controlled by Their Structural Fluctuations and Mismatch to Ice. J. Am. Chem. Soc. 2017, 139, 3052–3064. [Google Scholar] [CrossRef] [PubMed]
- Takahama, S.; Liu, S.; Russell, L.M. Coatings and Clusters of Carboxylic Acids in Carbon-Containing Atmospheric Particles from Spectromicroscopy and Their Implications for Cloud-Nucleating and Optical Properties. J. Geophys. Res. 2010, 115. [Google Scholar] [CrossRef]
- Estillore, A.D.; Morris, H.S.; Or, V.W.; Lee, H.D.; Alves, M.R.; Marciano, M.A.; Laskina, O.; Qin, Z.; Tivanski, A.V.; Grassian, V.H. Linking Hygroscopicity and the Surface Microstructure of Model Inorganic Salts, Simple and Complex Carbohydrates, and Authentic Sea Spray Aerosol Particles. Phys. Chem. Chem. Phys. 2017, 19, 21101–21111. [Google Scholar] [CrossRef] [PubMed]
- Cochran, R.E.; Jayarathne, T.; Stone, E.A.; Grassian, V.H. Selectivity Across the Interface: A Test of Surface Activity in the Composition of Organic-Enriched Aerosols from Bubble Bursting. J. Phys. Chem. Lett. 2016, 7, 1692–1696. [Google Scholar] [CrossRef] [PubMed]
- Burrows, S.M.; Ogunro, O.; Frossard, A.A.; Russell, L.M.; Rasch, P.J.; Elliott, S.M. A Physically Based Framework for Modeling the Organic Fractionation of Sea Spray Aerosol from Bubble Film Langmuir Equilibria. Atmos. Chem. Phys. 2014, 14, 13601–13629. [Google Scholar] [CrossRef]
- Elliott, S.; Burrows, S.M.; Deal, C.; Liu, X.; Long, M.; Ogunro, O.; Russell, L.M.; Wingenter, O. Prospects for Simulating Macromolecular Surfactant Chemistry at the Ocean–Atmosphere Boundary. Environ. Res. Lett. 2014, 9, 064012. [Google Scholar] [CrossRef]
- Elliott, S.; Burrows, S.; Cameron-Smith, P.; Hoffman, F.; Hunke, E.; Jeffery, N.; Liu, Y.; Maltrud, M.; Menzo, Z.; Ogunro, O.; et al. Does Marine Surface Tension Have Global Biogeography? Addition for the OCEANFILMS Package. Atmosphere 2018, 9, 216. [Google Scholar] [CrossRef]
- Yoon, Y.J.; Ceburnis, D.; Cavalli, F.; Jourdan, O.; Putaud, J.P.; Facchini, M.C.; Decesari, S.; Fuzzi, S.; Sellegri, K.; Jennings, S.G.; et al. Seasonal Characteristics of the Physicochemical Properties of North Atlantic Marine Atmospheric Aerosols. J. Geophys. Res. 2007, 112, D04206. [Google Scholar] [CrossRef]
- O’Dowd, C.D.; de Leeuw, G. Marine Aerosol Production: A Review of the Current Knowledge. Philos. Trans. R. Soc. Lond. Math. Phys. Eng. Sci. 2007, 365, 1753–1774. [Google Scholar] [CrossRef] [PubMed]
- Gantt, B.; Meskhidze, N.; Facchini, M.C.; Rinaldi, M.; Ceburnis, D.; O’Dowd, C.D. Wind Speed Dependent Size-Resolved Parameterization for the Organic Mass Fraction of Sea Spray Aerosol. Atmos. Chem. Phys. 2011, 11, 8777–8790. [Google Scholar] [CrossRef]
- Quinn, P.K.; Collins, D.B.; Grassian, V.H.; Prather, K.A.; Bates, T.S. Chemistry and Related Properties of Freshly Emitted Sea Spray Aerosol. Chem. Rev. 2015, 115, 4383–4399. [Google Scholar] [CrossRef] [PubMed]
- O’Dowd, C.D.; Facchini, M.C.; Cavalli, F.; Ceburnis, D.; Mircea, M.; Decesari, S.; Fuzzi, S.; Yoon, Y.J.; Putaud, J.P. Biogenically Driven Organic Contribution to Marine Aerosol. Nature 2004, 431, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Facchini, M.C.; Rinaldi, M.; Decesari, S.; Carbone, C.; Finessi, E.; Mircea, M.; Fuzzi, S.; Ceburnis, D.; Flanagan, R.; Nilsson, E.D.; et al. Primary Submicron Marine Aerosol Dominated by Insoluble Organic Colloids and Aggregates. Geophys. Res. Lett. 2008, 35, L17814. [Google Scholar] [CrossRef]
- Sciare, J.; Favez, O.; Sarda-Estève, R.; Oikonomou, K.; Cachier, H.; Kazan, V. Long-Term Observations of Carbonaceous Aerosols in the Austral Ocean Atmosphere: Evidence of a Biogenic Marine Organic Source. J. Geophys. Res. 2009, 114. [Google Scholar] [CrossRef]
- Gantt, B.; Meskhidze, N. The Physical and Chemical Characteristics of Marine Primary Organic Aerosol: A Review. Atmos. Chem. Phys. 2013, 13, 3979–3996. [Google Scholar] [CrossRef]
- Prather, K.A.; Bertram, T.H.; Grassian, V.H.; Deane, G.B.; Stokes, M.D.; DeMott, P.J.; Aluwihare, L.I.; Palenik, B.P.; Azam, F.; Seinfeld, J.H.; et al. Bringing the Ocean into the Laboratory to Probe the Chemical Complexity of Sea Spray Aerosol. Proc. Natl. Acad. Sci. USA 2013, 110, 7550–7555. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Sultana, C.M.; Collins, D.B.; Santander, M.V.; Axson, J.L.; Malfatti, F.; Cornwell, G.C.; Grandquist, J.R.; Deane, G.B.; Stokes, M.D.; et al. Advancing Model Systems for Fundamental Laboratory Studies of Sea Spray Aerosol Using the Microbial Loop. J. Phys. Chem. A 2015, 119, 8860–8870. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sultana, C.M.; Trueblood, J.; Hill, T.C.J.; Malfatti, F.; Lee, C.; Laskina, O.; Moore, K.A.; Beall, C.M.; McCluskey, C.S.; et al. Microbial Control of Sea Spray Aerosol Composition: A Tale of Two Blooms. ACS Cent. Sci. 2015, 1, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Cochran, R.E.; Laskina, O.; Trueblood, J.V.; Estillore, A.D.; Morris, H.S.; Jayarathne, T.; Sultana, C.M.; Lee, C.; Lin, P.; Laskin, J.; et al. Molecular Diversity of Sea Spray Aerosol Particles: Impact of Ocean Biology on Particle Composition and Hygroscopicity. Chem 2017, 2, 655–667. [Google Scholar] [CrossRef]
- Pham, D.Q.; O’Brien, R.; Fraund, M.; Bonanno, D.; Laskina, O.; Beall, C.; Moore, K.A.; Forestieri, S.; Wang, X.; Lee, C.; et al. Biological Impacts on Carbon Speciation and Morphology of Sea Spray Aerosol. ACS Earth Space Chem. 2017, 1, 551–561. [Google Scholar] [CrossRef]
- Cochran, R.E.; Laskina, O.; Jayarathne, T.; Laskin, A.; Laskin, J.; Lin, P.; Sultana, C.; Lee, C.; Moore, K.A.; Cappa, C.D.; Bertram, T.H.; et al. Analysis of Organic Anionic Surfactants in Fine and Coarse Fractions of Freshly Emitted Sea Spray Aerosol. Environ. Sci. Technol. 2016, 50, 2477–2486. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.P.; Collins, D.B.; Michaud, J.M.; Axson, J.L.; Sultana, C.M.; Moser, T.; Dommer, A.C.; Conner, J.; Grassian, V.H.; Stokes, M.D.; et al. Sea Spray Aerosol Structure and Composition Using Cryogenic Transmission Electron Microscopy. ACS Cent. Sci. 2016, 2, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Aller, J.Y.; Radway, J.C.; Kilthau, W.P.; Bothe, D.W.; Wilson, T.W.; Vaillancourt, R.D.; Quinn, P.K.; Coffman, D.J.; Murray, B.J.; Knopf, D.A. Size-Resolved Characterization of the Polysaccharidic and Proteinaceous Components of Sea Spray Aerosol. Atmos. Environ. 2017, 154, 331–347. [Google Scholar] [CrossRef]
- Bertram, T.H.; Cochran, R.E.; Grassian, V.H.; Stone, E.A. Sea Spray Aerosol Chemical Composition: Elemental and Molecular Mimics for Laboratory Studies of Heterogeneous and Multiphase Reactions. Chem. Soc. Rev. 2018, 47, 2374–2400. [Google Scholar] [CrossRef] [PubMed]
- Jayarathne, T.; Sultana, C.M.; Lee, C.; Malfatti, F.; Cox, J.L.; Pendergraft, M.A.; Moore, K.A.; Azam, F.; Tivanski, A.V.; Cappa, C.D.; et al. Enrichment of Saccharides and Divalent Cations in Sea Spray Aerosol During Two Phytoplankton Blooms. Environ. Sci. Technol. 2016, 50, 11511–11520. [Google Scholar] [CrossRef] [PubMed]
- Collins, D.B.; Zhao, D.F.; Ruppel, M.J.; Laskina, O.; Grandquist, J.R.; Modini, R.L.; Stokes, M.D.; Russell, L.M.; Bertram, T.H.; Grassian, V.H.; et al. Direct Aerosol Chemical Composition Measurements to Evaluate the Physicochemical Differences between Controlled Sea Spray Aerosol Generation Schemes. Atmos. Meas. Tech. 2014, 7, 3667–3683. [Google Scholar] [CrossRef]
- Frossard Amanda, A.; Russell Lynn, M.; Burrows Susannah, M.; Elliott Scott, M.; Bates Timothy, S.; Quinn Patricia, K. Sources and Composition of Submicron Organic Mass in Marine Aerosol Particles. J. Geophys. Res. Atmos. 2014, 119, 12977–13003. [Google Scholar] [CrossRef]
- Wang, X.; Deane, G.B.; Moore, K.A.; Ryder, O.S.; Stokes, M.D.; Beall, C.M.; Collins, D.B.; Santander, M.V.; Burrows, S.M.; Sultana, C.M.; et al. The Role of Jet and Film Drops in Controlling the Mixing State of Submicron Sea Spray Aerosol Particles. Proc. Natl. Acad. Sci. USA 2017, 114, 6978–6983. [Google Scholar] [CrossRef] [PubMed]
- Tervahattu, H.; Hartonen, K.; Kerminen, V.M.; Kupiainen, K.; Aarnio, P.; Koskentalo, T.; Tuck, A.F.; Vaida, V. New Evidence of an Organic Layer on Marine Aerosols. J. Geophys. Res. 2002, 107, AAC 1-1–AAC 1-8. [Google Scholar] [CrossRef]
- Tervahattu, H.; Juhanoja, J.; Kupiainen, K. Identification of an Organic Coating on Marine Aerosol Particles by TOF-SIMS. J. Geophys. Res. 2002, 107, ACH 18–1. [Google Scholar] [CrossRef]
- Donaldson, D.J.; Vaida, V. The Influence of Organic Films at the Air-Aqueous Boundary on Atmospheric Processes. Chem. Rev. 2006, 106, 1445–1461. [Google Scholar] [CrossRef] [PubMed]
- Griffith, E.C.; Adams, E.M.; Allen, H.C.; Vaida, V. Hydrophobic Collapse of a Stearic Acid Film by Adsorbed l-Phenylalanine at the Air–Water Interface. J. Phys. Chem. B 2012, 116, 7849–7857. [Google Scholar] [CrossRef] [PubMed]
- Gaines, G.L. Insoluble Monolayers at Liquid-Gas Interfaces; Interscience Publishers: New York, NY, USA, 1966. [Google Scholar]
- Joos, P. Effect of the pH on the Collapse Pressure of Fatty Acid Monolayers Evaluation of the Surface Dissociation Constant. Bull. SociÉtÉs Chim. Belg. 1971, 80, 277–281. [Google Scholar] [CrossRef]
- Pezron, E.; Claesson, P.M.; Berg, J.M.; Vollhardt, D. Stability of Arachidic Acid Monolayers on Aqueous Salt Solutions. J. Colloid Interface Sci. 1990, 138, 245–254. [Google Scholar] [CrossRef]
- Angelova, A.; Reiche, J.; Ionov, R.; Janietz, D.; Brehmer, L. Control of the Structure of Langmuir-Blodgett Films of a Discotic Liquid Crystalline Compound via the Subphase Composition and the Adjacent Molecular Environment. Thin Solid Film. 1994, 242, 289–294. [Google Scholar] [CrossRef]
- Angelova, A.; Vollhardt, D.; Ionov, R. 2D-3D Transformations of Amphiphilic Monolayers Influenced by Intermolecular Interactions: A Brewster Angle Microscopy Study. J. Phys. Chem. 1996, 100, 10710–10720. [Google Scholar] [CrossRef]
- Minassian-Saraga, L.T. Recent Work on Spread Monolayers, Adsorption and Desorption. J. Colloid Sci. 1956, 11, 398–418. [Google Scholar] [CrossRef]
- Patil, G.S.; Matthews, R.H.; Cornwell, D.G. Kinetics of the Processes of Desorption from Fatty Acid Monolayers. J. Lipid Res. 1973, 14, 26–31. [Google Scholar] [PubMed]
- Gershfeld, N.L. Physical Chemistry of Lipid Films at Fluid Interfaces. Annu. Rev. Phys. Chem. 1976, 27, 349–368. [Google Scholar] [CrossRef]
- Gilman, J.; Eliason, T.; Fast, A.; Vaida, V. Selectivity and Stability of Organic Films at the Air–Aqueous Interface. J. Colloid Interface Sci. 2004, 280, 234–243. [Google Scholar] [CrossRef] [PubMed]
- McLean, D.S.; Vercoe, D.; Stack, K.R.; Richardson, D. The Colloidal pKa of Lipophilic Extractives Commonly Found in Pinus Radiata. Appita J. J. Tech. Assoc. Aust. N. Z. Pulp Pap. Ind. 2005, 58, 362. [Google Scholar]
- Wellen, B.A.; Lach, E.A.; Allen, H.C. Surface pKa of Octanoic, Nonanoic, and Decanoic Fatty Acids at the Air–Water Interface: Applications to Atmospheric Aerosol Chemistry. Phys. Chem. Chem. Phys. 2017, 39, 26551–26558. [Google Scholar] [CrossRef] [PubMed]
- Dynarowicz-ątka, P.; Kita, K. Molecular Interaction in Mixed Monolayers at the Air/Water Interface. Adv. Colloid Interface Sci. 1999, 79, 1–17. [Google Scholar] [CrossRef]
- Rodrıǵuez Patino, J.M.; Rodrıǵuez Niño, M. Interfacial Characteristics of Food Emulsifiers (Proteins and Lipids) at the Air-Water Interface. Colloids Surf. B Biointerfaces 1999, 15, 235–252. [Google Scholar] [CrossRef]
- Brzozowska, A.M.; Mugele, F.; Duits, M.H.G. Stability and Interactions in Mixed Monolayers of Fatty Acid Derivatives on Artificial Sea Water. Colloids Surf. A Physicochem. Eng. Asp. 2013, 433, 200–211. [Google Scholar] [CrossRef]
- Khattari, Z.; Sayyed, M.I.; Qashou, S.I.; Fasfous, I.; Al-Abdullah, T.; Maghrabi, M. Interfacial Behavior of Myristic Acid in Mixtures with DMPC and Cholesterol. Chem. Phys. 2017, 490, 106–114. [Google Scholar] [CrossRef]
- McFate, C.; Ward, D.; Olmsted, J. Organized Collapse of Fatty Acid Monolayers. Langmuir 1993, 9, 1036–1039. [Google Scholar] [CrossRef]
- Ybert, C.; Lu, W.; Möller, G.; Knobler, C.M. Collapse of a Monolayer by Three Mechanisms. J. Phys. Chem. B 2002, 106, 2004–2008. [Google Scholar] [CrossRef]
- Kundu, S.; Datta, A.; Hazra, S. Effect of Metal Ions on Monolayer Collapses. Langmuir 2005, 21, 5894–5900. [Google Scholar] [CrossRef] [PubMed]
- Baoukina, S.; Monticelli, L.; Risselada, H.J.; Marrink, S.J.; Tieleman, D.P. The Molecular Mechanism of Lipid Monolayer Collapse. Proc. Natl. Acad. Sci. USA 2008, 105, 10803–10808. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.C. Collapse Mechanisms of Langmuir Monolayers. Annu. Rev. Phys. Chem. 2008, 59, 771–791. [Google Scholar] [CrossRef] [PubMed]
- Millero, F.J. Chemical Oceanography, 4th ed.; Taylor & Francis: Boca Raton, FL, USA, 2013. [Google Scholar]
- Fridlind, A.M.; Jacobson, M.Z. A Study of Gas-Aerosol Equilibrium and Aerosol pH in the Remote Marine Boundary Layer during the First Aerosol Characterization Experiment (ACE 1). J. Geophys. Res. Atmos. 2000, 105, 17325–17340. [Google Scholar] [CrossRef]
- Huang, Z.; Hua, W.; Verreault, D.; Allen, H.C. Influence of Salt Purity on Na+ and Palmitic Acid Interactions. J. Phys. Chem. A 2013, 117, 13412–13418. [Google Scholar] [CrossRef] [PubMed]
- Adams, E.M.; Allen, H.C. Palmitic Acid on Salt Subphases and in Mixed Monolayers of Cerebrosides: Application to Atmospheric Aerosol Chemistry. Atmosphere 2013, 4, 315–336. [Google Scholar] [CrossRef]
- Neal, J.F.; Zhao, W.; Grooms, A.J.; Flood, A.H.; Allen, H.C. Arginine–Phosphate Recognition Enhanced in Phospholipid Monolayers at Aqueous Interfaces. J. Phys. Chem. C 2018. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Millero, F.J.; Feistel, R.; Wright, D.G.; McDougall, T.J. The Composition of Standard Seawater and the Definition of the Reference-Composition Salinity Scale. Deep Sea Res. Part Oceanogr. Res. Pap. 2008, 55, 50–72. [Google Scholar] [CrossRef]
- Durbin, M.K.; Shih, M.C.; Malik, A.; Zschack, P.; Dutta, P. Isotherm and X-ray Diffraction Studies of Mixed Monolayers. Colloids Surf. A Physicochem. Eng. Asp. 1995, 102, 173–179. [Google Scholar] [CrossRef]
- Nutting, G.C.; Harkins, W.D. Pressure-Area Relations of Fatty Acid and Alcohol Monolayers. J. Am. Chem. Soc. 1939, 61, 1180–1187. [Google Scholar] [CrossRef]
- Fischer, A.; Sackmann, E. Electron Microscopy and Electron Diffraction Study of Coexisting Phases of Pure and Mixed Monolayers Transferred onto Solid Substrates. J. Colloid Interface Sci. 1986, 112, 1–14. [Google Scholar] [CrossRef]
- Adams, E.M.; Wellen, B.A.; Thiraux, R.; Reddy, S.K.; Vidalis, A.S.; Paesani, F.; Allen, H.C. Sodium–Carboxylate Contact Ion Pair Formation Induces Stabilization of Palmitic Acid Monolayers at High pH. Phys. Chem. Chem. Phys. 2017, 19, 10481–10490. [Google Scholar] [CrossRef] [PubMed]
- Guyot-Sionnest, P.; Hunt, J.H.; Shen, Y.R. Sum-Frequency Vibrational Spectroscopy of a Langmuir Film: Study of Molecular Orientation of a Two-Dimensional System. Phys. Rev. Lett. 1987, 59, 1597–1600. [Google Scholar] [CrossRef] [PubMed]
- Gericke, A.; Huehnerfuss, H. In Situ Investigation of Saturated Long-Chain Fatty Acids at the Air/Water Interface by External Infrared Reflection-Absorption Spectrometry. J. Phys. Chem. 1993, 97, 12899–12908. [Google Scholar] [CrossRef]
- Smith, R.D.; Berg, J.C. The Collapse of Surfactant Monolayers at the Air—Water Interface. J. Colloid Interface Sci. 1980, 74, 273–286. [Google Scholar] [CrossRef]
- Baoukina, S.; Rozmanov, D.; Mendez-Villuendas, E.; Tieleman, D.P. The Mechanism of Collapse of Heterogeneous Lipid Monolayers. Biophys. J. 2014, 107, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- Gopal, A.; Lee, K.Y.C. Morphology and Collapse Transitions in Binary Phospholipid Monolayers. J. Phys. Chem. B 2001, 105, 10348–10354. [Google Scholar] [CrossRef]
- Ding, J.; Takamoto, D.Y.; von Nahmen, A.; Lipp, M.M.; Lee, K.Y.C.; Waring, A.J.; Zasadzinski, J.A. Effects of Lung Surfactant Proteins, SP-B and SP-C, and Palmitic Acid on Monolayer Stability. Biophys. J. 2001, 80, 2262–2272. [Google Scholar] [CrossRef]
- Takamoto, D.Y.; Lipp, M.M.; von Nahmen, A.; Lee, K.Y.C.; Waring, A.J.; Zasadzinski, J.A. Interaction of Lung Surfactant Proteins with Anionic Phospholipids. Biophys. J. 2001, 81, 153–169. [Google Scholar] [CrossRef]
- Ridsdale, R.; Palaniyar, N.; Possmayer, F.; Harauz, G. Formation of Folds and Vesicles by Dipalmitoylphosphatidylcholine Monolayers Spread in Excess. J. Membr. Biol. 2001, 180, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Pallas, N.R.; Pethica, B.A. Liquid-Expanded to Liquid-Condensed Transition in Lipid Monolayers at the Air/Water Interface. Langmuir 1985, 1, 509–513. [Google Scholar] [CrossRef]
- Ruckenstein, E. On the Nature of The Liquid Expanded/Liquid Condensed Phase Transition in Monolayers of Polar Molecules. J. Colloid Interface Sci. 1997, 196, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.Y.; Allen, H.C. Ionic Binding of Na+ versus K+ to the Carboxylic Acid Headgroup of Palmitic Acid Monolayers Studied by Vibrational Sum Frequency Generation Spectroscopy. J. Phys. Chem. A 2009, 113, 7383–7393. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carter-Fenk, K.A.; Allen, H.C. Collapse Mechanisms of Nascent and Aged Sea Spray Aerosol Proxy Films. Atmosphere 2018, 9, 503. https://doi.org/10.3390/atmos9120503
Carter-Fenk KA, Allen HC. Collapse Mechanisms of Nascent and Aged Sea Spray Aerosol Proxy Films. Atmosphere. 2018; 9(12):503. https://doi.org/10.3390/atmos9120503
Chicago/Turabian StyleCarter-Fenk, Kimberly A., and Heather C. Allen. 2018. "Collapse Mechanisms of Nascent and Aged Sea Spray Aerosol Proxy Films" Atmosphere 9, no. 12: 503. https://doi.org/10.3390/atmos9120503
APA StyleCarter-Fenk, K. A., & Allen, H. C. (2018). Collapse Mechanisms of Nascent and Aged Sea Spray Aerosol Proxy Films. Atmosphere, 9(12), 503. https://doi.org/10.3390/atmos9120503




