Expanded Application of the Passive Flux Meter: In-Situ Measurements of 1,4-Dioxane, Sulfate, Cr(VI) and RDX
Abstract
1. Introduction
2. Material and Methods
2.1. Passive Flux Meter Technology
2.2. Sorbent Materials
2.3. Extraction and Analytical Procedures
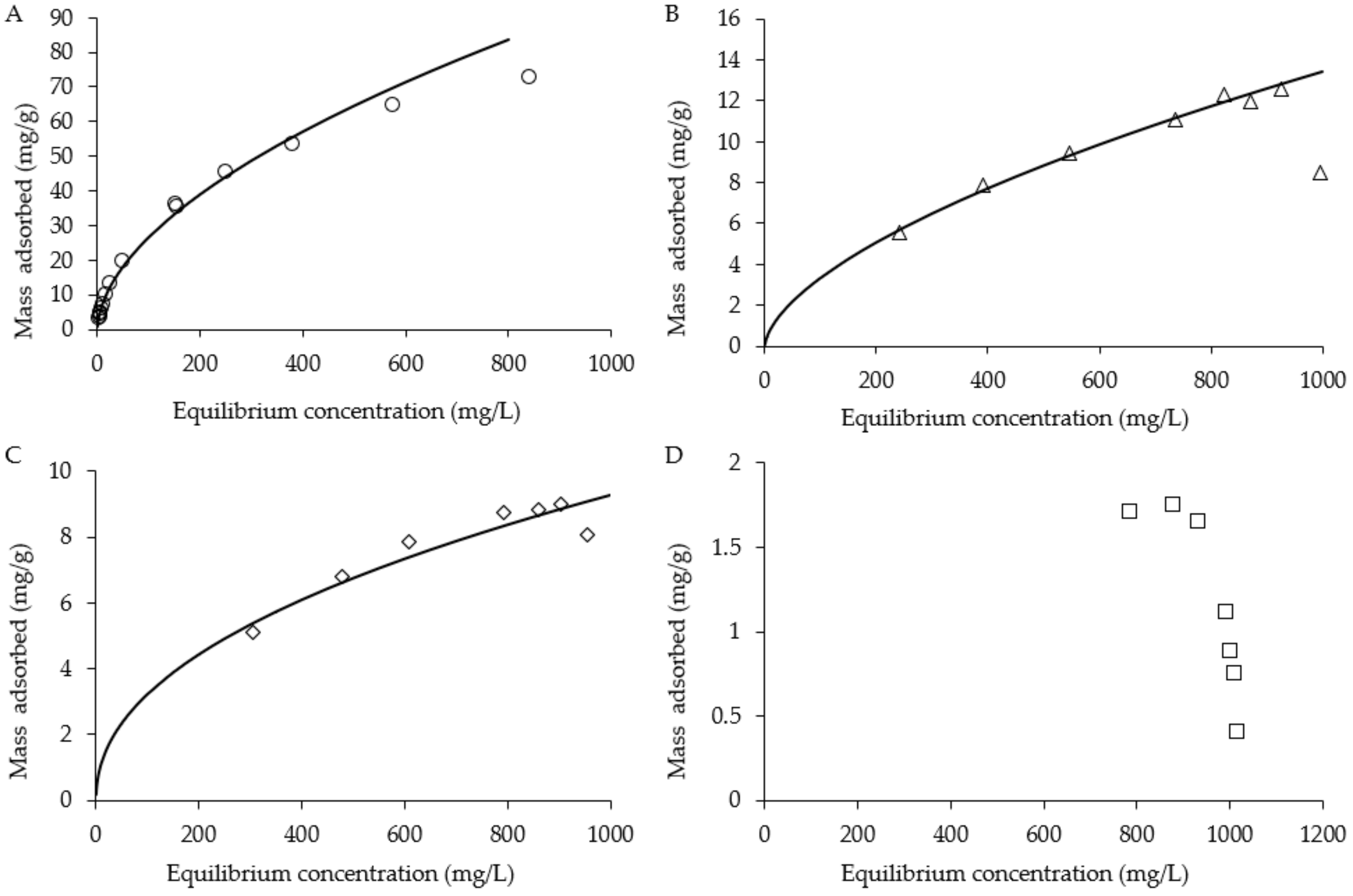
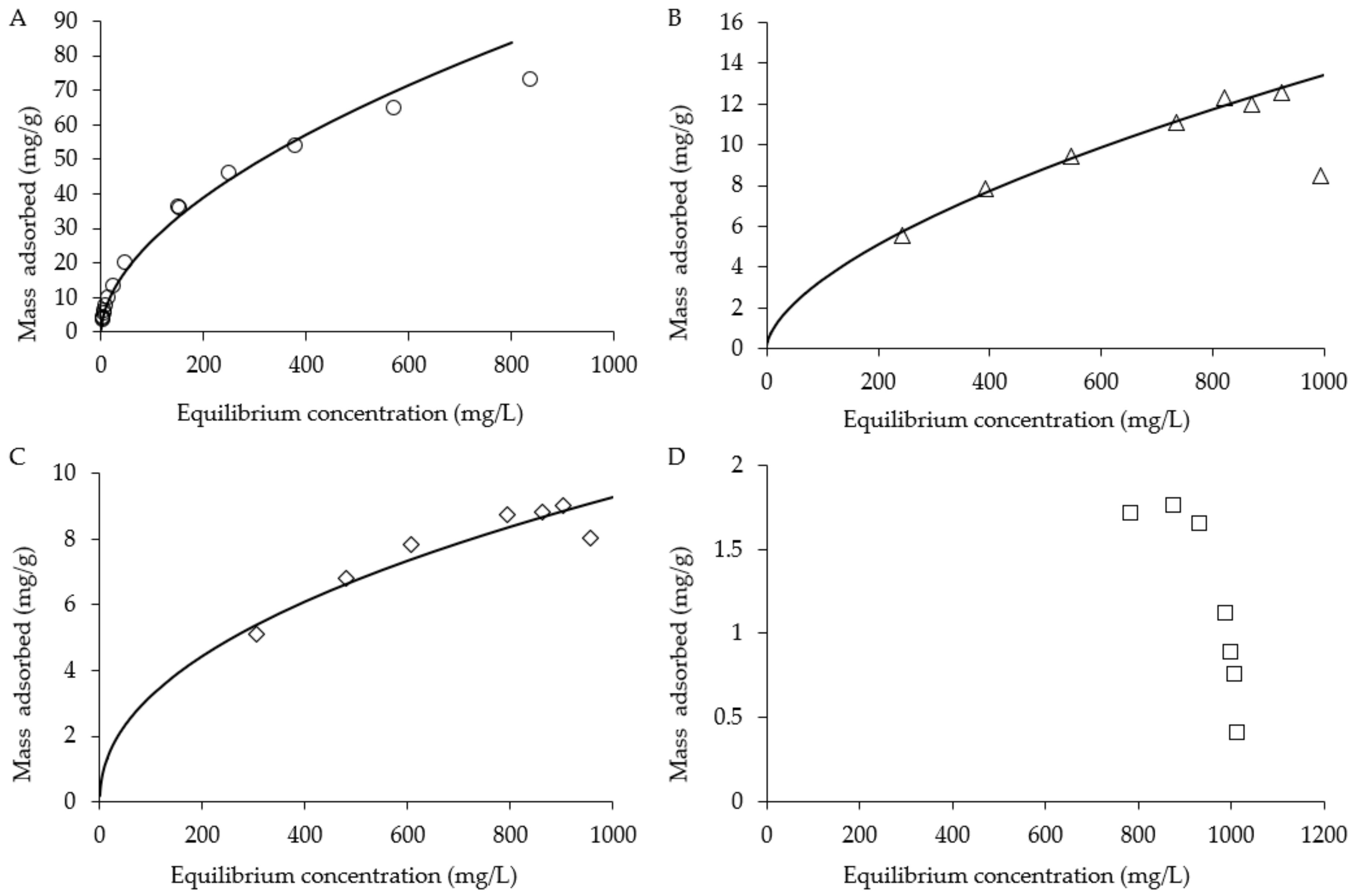
2.4. Batch Adsorption Isotherms and Recovery
2.5. Column Studies
2.6. Bench-Scale Aquifer Testing of 1,4-Dioxane PFM
2.7. Field Studies
2.7.1. RDX and Cr(VI) Measurements
2.7.2. 1,4-Dioxane Measurements
2.7.3. Sulfate Measurements
3. Results
3.1. RDX and Cr(VI)
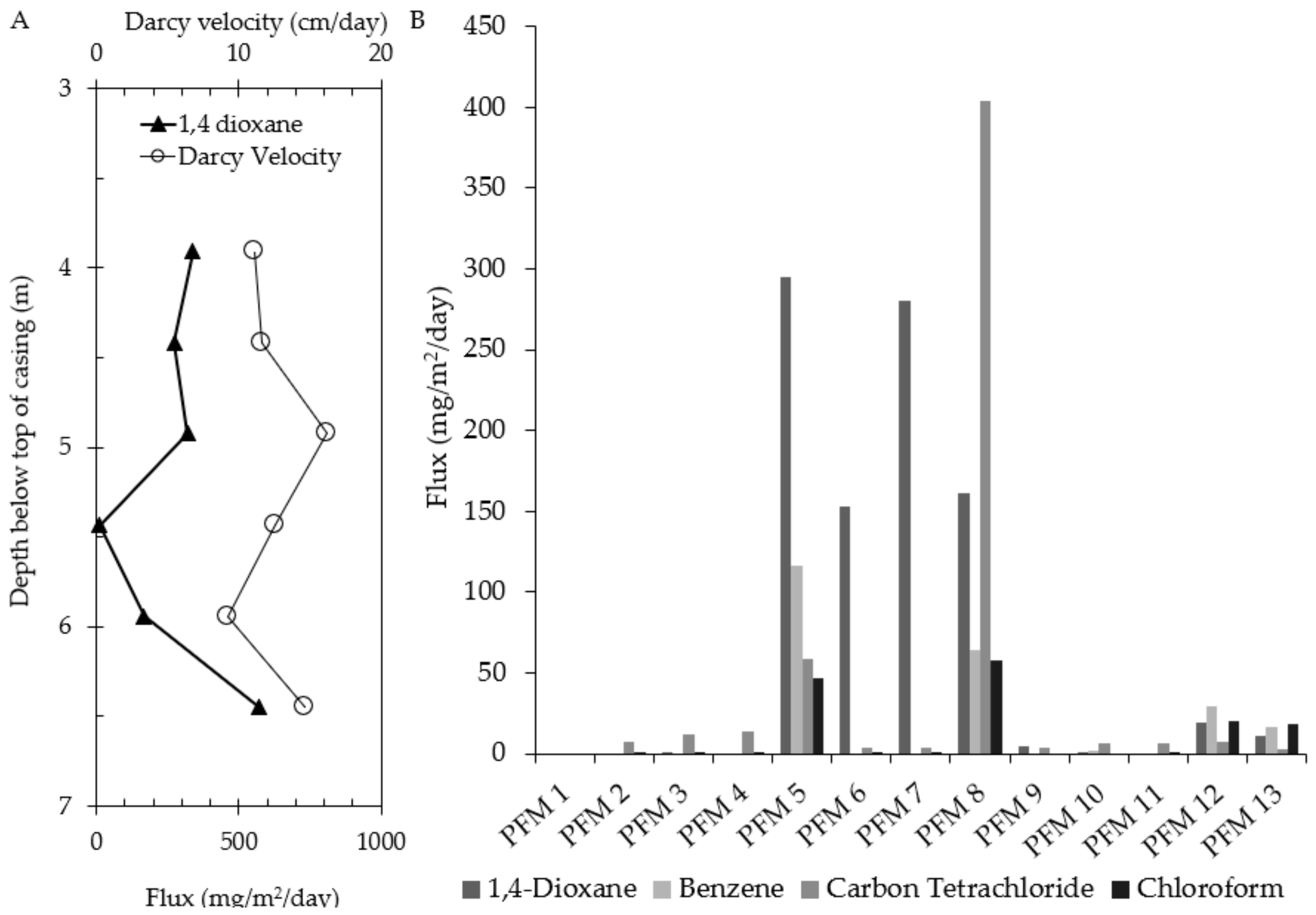
3.2. 1,4-Dioxane
3.3. In-situ Biogenic SO42− Reduction Rates
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rao, P.S.C.; Jawitz, J.W.; Enfield, C.G.; Falta, R.W.; Annable, M.D.; Wood, A.L. Technology Integration for Contaminated Site Remediation: Clean-Up Goals and Performance Criteria. In Groundwater Quality: Natural and Enhanced Restoration of Groundwater Pollution; International Association of Hydrological Sciences: Sheffield, UK, 2002; Volume 571–578, Available online: http://hydrologie.org/redbooks/a275/iahs_275_571.pdf (accessed on 16 September 2018).
- Verreydt, G.; Bronders, J.; Van Keer, I.; Diels, L.; Vanderauwera, P. Passive samplers for monitoring vocs in groundwater and the prospects related to mass flux measurements. Ground Water Monit. Remed. 2010, 30, 114–126. [Google Scholar] [CrossRef]
- Martin, H.; Piepenbrink, M.; Grathwohl, P. Ceramic dosimeters for time-integrated contaminant monitoring. J Process Anal. Chem. 2001, 6, 68–73. [Google Scholar]
- Vroblesky, D.A.; Campbell, T.R. Equilibration times, compound selectivity, and stability of diffusion samplers for collection of ground-water VOC concentrations. Adv. Environ. Res. 2001, 5, 1–12. [Google Scholar] [CrossRef]
- Harter, T.; Talozi, S. Evaluation of a simple, inexpensive dialysis sampler for small diameter monitoring wells. Ground Water Monit. Remed. 2004, 24, 97–105. [Google Scholar] [CrossRef]
- Jalalizadeh, M.; Ghosh, U. In situ passive sampling of sediment porewater enhanced by periodic vibration. Environ. Sci. Technol. 2016, 50, 8741–8749. [Google Scholar] [CrossRef] [PubMed]
- Webster, I.T.; Teasdale, P.R.; Grigg, N.J. Theoretical and experimental analysis of peeper equilibration dynamics. Environ. Sci. Technol. 1998, 32, 1727–1733. [Google Scholar] [CrossRef]
- Vrana, B.; Mills, G.A.; Dominiak, E.; Greenwood, R. Calibration of the chemcatcher passive sampler for the monitoring of priority organic pollutants in water. Environ. Pollut. 2006, 142, 333–343. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, H.; Rothenberg, G. New device and method for flux-proportional sampling of mobile solutes in soil and groundwater. Environ. Sci. Technol. 2005, 39, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Jalalizadeh, M.; Ghosh, U. Analysis of measurement errors in passive sampling of porewater PCB concentrations under static and periodically vibrated conditions. Environ. Sci. Technol. 2017, 51, 7018–7027. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, R. Critical review of low-density polyethylene’s partitioning and diffusion coefficients for trace organic contaminants and implications for its use as a passive sampler. Environ. Sci. Technol. 2015, 49, 3985. [Google Scholar] [CrossRef] [PubMed]
- Apell, J.N.; Gschwend, P.M. Validating the use of performance reference compounds in passive samplers to assess porewater concentrations in sediment beds. Environ. Sci. Technol. 2014, 48, 10301–10307. [Google Scholar] [CrossRef] [PubMed]
- Basu, N.B.; Suresh, P.; Rao, C.; Poyer, I.C.; Nandy, S.; Mallavarapu, M.; Naidu, R.; Davis, G.B.; Patterson, B.M.; Annable, M.D.; et al. Integration of traditional and innovative characterization techniques for flux-based assessment of dense non-aqueous phase liquid (DNAPL) sites. J. Contam. Hydrol. 2009, 105, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Brooks, M.C.; Wood, A.L.; Annable, M.D.; Hatfield, K.; Cho, J.; Holbert, C.; Rao, R.S.C.; Enfield, C.G.; Lynch, K.; Smith, R.E. Changes in contaminant mass discharge from dnapl source mass depletion: Evaluation at two field sites. J. Contam. Hydrol. 2008, 102, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, K.; Annable, M.; Cho, J.H.; Rao, P.S.C.; Klammler, H. A direct passive method for measuring water and contaminant fluxes in porous media. J. Contam. Hydrol. 2004, 75, 155–181. [Google Scholar] [CrossRef] [PubMed]
- Annable, M.D.; Hatfield, K.; Cho, J.; Klammler, H.; Parker, B.L.; Cherry, J.A.; Rao, P.S.C. Field-scale evaluation of the passive flux meter for simultaneous measurement of groundwater and contaminant fluxes. Environ. Sci. Technol. 2005, 39, 7194–7201. [Google Scholar] [CrossRef] [PubMed]
- Klammler, H.; Hatfield, K.; Annable, M.D. Concepts for measuring horizontal groundwater flow directions using the passive flux meter. Adv. Water Resour. 2007, 30, 984–997. [Google Scholar] [CrossRef]
- Klammler, H.; Hatfield, K.; da Luz, J.A.G.; Annable, M.D.; Newman, M.; Cho, J.; Peacock, A.; Stucker, V.; Ranville, J.; Cabaniss, S.A.; et al. Contaminant discharge and uncertainty estimates from passive flux meter measurements. Water Resour. Res. 2012, 48, 19. [Google Scholar] [CrossRef]
- Padowski, J.C.; Rothfus, E.A.; Jawitz, J.W.; Klammler, H.; Hatfield, K.; Annable, M.D. Effect of passive surface water flux meter design on water and solute mass flux estimates. J. Hydrol. Eng. 2009, 14, 1334–1342. [Google Scholar] [CrossRef]
- Layton, L.; Klammler, H.; Hatfield, K.; Cho, J.; Newman, M.A.; Annable, M.D. Development of a passive sensor for measuring vertical cumulative water and solute mass fluxes in lake sediments and streambeds. Adv. Water Resour. 2017, 105, 1–12. [Google Scholar] [CrossRef]
- Klammler, H.; Hatfield, K.; Newman, M.A.; Cho, J.; Annable, M.D.; Parker, B.L.; Cherry, J.A.; Perminova, I. A new device for characterizing fracture networks and measuring groundwater and contaminant fluxes in fractured rock aquifers. Water Resour. Res. 2016, 52, 5400–5420. [Google Scholar] [CrossRef]
- Kunz, J.V.; Annable, M.D.; Cho, J.; von Tumpling, W.; Hatfield, K.; Rao, S.; Borchardt, D.; Rode, M. Quantifying nutrient fluxes with a new hyporheic passive flux meter (HPFM). Biogeosciences 2017, 14, 631–649. [Google Scholar] [CrossRef]
- Lee, J.; Rao, P.S.C.; Poyer, I.C.; Toole, R.M.; Annable, M.D.; Hatfield, K. Oxyanion flux characterization using passive flux meters: Development and field testing of surfactant-modified granular activated carbon. J. Contam. Hydrol. 2007, 92, 208–229. [Google Scholar] [CrossRef] [PubMed]
- Campbell, T.J.; Hatfield, K.; Klammler, H.; Annable, M.D.; Rao, P.S.C. Magnitude and directional measures of water and Cr(VI) fluxes by passive flux meter. Environ. Sci. Technol. 2006, 40, 6392–6397. [Google Scholar] [CrossRef] [PubMed]
- Verreydt, G.; Annable, M.D.; Kaskassian, S.; Van Keer, I.; Bronders, J.; Diels, L.; Vanderauwera, P. Field demonstration and evaluation of the passive flux meter on a CAH groundwater plume. Environ. Sci. Pollut. Res. 2013, 20, 4621–4634. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.D.; Davis, G.B.; Bastow, T.P.; Woodbury, R.J.; Rao, P.S.C.; Annable, M.D.; Rhodes, S. Mass discharge assessment at a brominated DNAPL site: Effects of known DNAPL source mass removal. J. Contam. Hydrol. 2014, 164, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.J.; Hatfield, K.; Annable, M.D.; Gupta, P.; Chirenje, T. Estimation of arsenic contamination in groundwater by the passive flux meter. Environ. Forensics 2005, 6, 77–82. [Google Scholar] [CrossRef]
- Cho, J.Y.; Annable, M.D.; Jawitz, J.W.; Hatfield, K. Passive flux meter measurement of water and nutrient flux in saturated porous media: Bench-scale laboratory tests. J. Environ. Qual. 2007, 36, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Kunz, J.V.; Annable, M.D.; Rao, S.; Rode, M.; Borchardt, D. Hyporheic passive flux meters reveal inverse vertical zonation and high seasonality of nitrogen processing in an anthropogenically modified stream (Holtemme, Germany). Water Resour. Res. 2017, 53, 10155–10172. [Google Scholar] [CrossRef]
- Stucker, V.; Ranville, J.; Newman, M.; Peacock, A.; Cho, J.; Hatfield, K. Evaluation and application of anion exchange resins to measure groundwater uranium flux at a former uranium mill site. Water Res. 2011, 45, 4866–4876. [Google Scholar] [CrossRef] [PubMed]
- Zenker, M.J.; Borden, R.C.; Barlaz, M.A. Occurrence and treatment of 1,4-dioxane in aqueous environments. Environ. Eng. Sci. 2003, 20, 423–432. [Google Scholar] [CrossRef]
- Zhang, S.; Gedalanga, P.B.; Mahendra, S. Advances in bioremediation of 1,4-dioxane-contaminated waters. J. Environ. Manag. 2017, 204, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, P.J.J.; Illman, W.A. Bioremediation and Natural Attenuation; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Howard, P. Handbook of Environmental Fate and Exposure Data for Organic Chemicals; Lewis Publishers, Inc.: Chelsea, MI, USA, 1990. [Google Scholar]
- ITRC. Protocol for Use of Five Passive Sampler to Sample for a Variety of Contaminants in Groundwater; ITRC: Washington, DC, USA, 2007.
- Paquet, L.; Monteil-Rivera, F.; Hatzinger, P.B.; Fuller, M.E.; Hawari, J. Analysis of the key intermediates of RDX (hexahydro-1,3,5-trinitro-1,3,5-triazine) in groundwater: Occurrence, stability and preservation. J. Environ. Monit. 2011, 13, 2304–2311. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.C.; Myers, K.F.; Brannon, J.M.; Delfino, J.J. Effects of pH and temperature on the aqueous solubility and dissolution rate of 2,4,6-trinitrotoluene (TNT), hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX), and octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX). J. Chem. Eng. Data 2001, 46, 1549–1555. [Google Scholar] [CrossRef]
- Pascoe, G.A.; Kroeger, K.; Leisle, D.; Feldpausch, R.J. Munition constituents: Preliminary sediment screening criteria for the protection of marine benthic invertebrates. Chemosphere 2010, 81, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.H.; Smith, P.N.; Anderson, T.A. Evaluating the bioavailability of explosive metabolites, hexahydro-1-nitroso-3,5-dinitro-1,3,5-triazine (MNX) and hexahydro-1,3,5-trinitroso-1,3,5-triazine (TNX), in soils using passive sampling devices. J. Chromatogr. A 2006, 1101, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Rosen, G.; Wild, B.; George, R.D.; Belden, J.B.; Lotufo, G.R. Optimization and field demonstration of a passive sampling technology for monitoring conventional munition constituents in aquatic environments. Mar. Technol. Soc. J. 2016, 50, 23–32. [Google Scholar] [CrossRef]
- Morley, M.C.; Henke, J.L.; Speitel, G.E. Adsorption of rdx and hmx in rapid small-scale column tests: Implications for full-scale adsorbers. J. Environ. Eng.-ASCE 2005, 131, 29–37. [Google Scholar] [CrossRef]
- Heilmann, H.M.; Wiesmann, U.; Stenstrom, M.K. Kinetics of the alkaline hydrolysis of high explosives RDX and HMX in aqueous solution and adsorbed to activated carbon. Environ. Sci. Technol. 1996, 30, 1485–1492. [Google Scholar] [CrossRef]
- Millerick, K.; Drew, S.R.; Finneran, K.T. Electron shuttle-mediated biotransformation of hexahydro-1,3,5-trinitro-1,3,5-triazine adsorbed to granular activated carbon. Environ. Sci. Technol. 2013, 47, 8743–8750. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.A.; Mueller, J.G.; Seech, A.G.; Henderson, J.K.; Wilson, J.T. Interactions between biological and abiotic pathways in the reduction of chlorinated solvents. Remedation 2009, 20, 9–20. [Google Scholar] [CrossRef]
- Butler, E.C.; Hayes, K.F. Kinetics of the transformation of trichloroethylene and tetrachloroethylene by iron sulfide. Environ. Sci. Technol. 1999, 33, 2021–2027. [Google Scholar] [CrossRef]
- Butler, E.C.; Hayes, K.F. Factors influencing rates and products in the transformation of trichloroethylene by iron sulfide and iron metal. Environ. Sci. Technol. 2001, 35, 3884–3891. [Google Scholar] [CrossRef] [PubMed]
- Ferrey, M.L.; Wilkin, R.T.; Ford, R.G.; Wilson, J.T. Nonbiological removal of cis-dichloroethylene and 1,1-dichloroethylene in aquifer sediment containing magnetite. Environ. Sci. Technol. 2004, 38, 1746–1752. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.Y.; Hayes, K.F. Reductive dechlorination of tetrachloroethylene and trichloroethylene by mackinawite (FeS) in the presence of metals: Reaction rates. Environ. Sci. Technol. 2007, 41, 6390–6396. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.Y.; Anantharaman, K.; Han, Y.S.; Hayes, K.F. Abiotic reductive dechlorination of cis-dichloroethylene by Fe species formed during iron- or sulfate-reduction. Environ. Sci. Technol. 2011, 45, 5186–5194. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Batchelor, B. Abiotic reductive dechlorination of chlorinated ethylenes by iron-bearing soil minerals. 1. Pyrite and magnetite. Environ. Sci. Technol. 2002, 36, 5147–5154. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Batchelor, B. Abiotic, reductive dechlorination of chlorinated ethylenes by iron-bearing soil minerals. 2. Green rust. Environ. Sci. Technol. 2002, 36, 5348–5354. [Google Scholar] [CrossRef] [PubMed]
- Evans, P.J.; Nguyen, D.; Chappell, R.W.; Whiting, K.; Gillette, J.; Bodour, A.; Wilson, J.T. Factors controlling in situ biogeochemical transformation of trichloroethene: Column study. Ground Water Monit. Remed. 2014, 34, 65–78. [Google Scholar] [CrossRef]
- Whiting, K.; Evans, P.J.; Lebron, C.; Henry, B.; Wilson, J.T.; Becvar, E. Factors controlling in situ biogeochemical transformation of trichloroethene: Field survey. Ground Water Monit. Remed. 2014, 34, 79–94. [Google Scholar] [CrossRef]
- Kennedy, L.; Everett, J.W.; Gonzales, J. Aqueous and mineral intrinsic bioremediation assessment: Natural attenuation. J. Environ. Eng.-ASCE 2004, 130, 942–950. [Google Scholar] [CrossRef]
- Wilkin, R.T.; Bischoff, K.J. Coulometric determination of total sulfur and reduced inorganic sulfur fractions in environmental samples. Talanta 2006, 70, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Wiedemeier, T.H.; Swanson, M.A.; Moutoux, D.E.; Gordon, E.K.; Wilson, J.T.; Wilson, B.H.; Kampbell, D.H.; Haas, P.E.; Miller, R.N.; Hansen, J.E.; et al. Technical Protocol for Evaluating Natural Attenuation of Chlorinated Solvents in Ground Water; United States Environmental Protection Agency: Washington, DC, USA, 1998.
- Nobre, R.C.M.; Nobre, M.M.M.; Campos, T.M.P.; Ogles, D. In-situ biodegradation potential of 1,2-DCA and VC at sites with different hydrogeological settings. J. Hazard. Mater. 2017, 340, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Madsen, E.L. Epistemology of environmental microbiology. Environ. Sci. Technol. 1998, 32, 429–439. [Google Scholar] [CrossRef]
- Baruthio, F. Toxic effects of chromium and its compounds. Biol. Trace Elem. Res. 1992, 32, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Grevatt, P.C. Toxicological Review of Trivalent Chromium; Agency, USEP: Washington, DC, USA, 1998.
- Driscoll, S.K.; McArdle, M.E.; Plumlee, M.H.; Proctor, D. Evaluation of hexavalent chromium in sediment pore water of the Hackensack river, New Jersey, USA. Environ. Toxicol. Chem. 2010, 29, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Hopp, L.; Peiffer, S.; Durner, W. Spatial variability of arsenic and chromium in the soil water at a former wood preserving site. J. Contam. Hydrol. 2006, 85, 159–178. [Google Scholar] [CrossRef] [PubMed]
- James, B.R. The challenge of remediating chromium-contaminated soil. Environ. Sci. Technol. 1996, 30, A248–A251. [Google Scholar] [CrossRef] [PubMed]
- Barbee, G.C.; Brown, K.W. Comparison between suction and free-drainage soil solution samplers. Soil Sci. 1986, 141, 149–154. [Google Scholar] [CrossRef]
- Basu, N.B.; Rao, P.S.C.; Poyer, I.C.; Annable, M.D.; Hatfield, K. Flux-based assessment at a manufacturing site contaminated with trichloroethylene. J. Contam. Hydrol. 2006, 86, 105–127. [Google Scholar] [CrossRef] [PubMed]
- Johns, M.M.; Marshall, W.E.; Toles, C.A. Agricultural by-products as granular activated carbons for adsorbing dissolved metals and organics. J. Chem. Technol. Biotechnol. 1998, 71, 131–140. [Google Scholar] [CrossRef]
- Otto, M.; Nagaraja, S. Treatment technologies for 1,4-dioxane: Fundamentals and field applications. Remedation 2007, 17, 81–88. [Google Scholar] [CrossRef]
- Navalon, S.; Alvaro, M.; Garcia, H. Analysis of organic compounds in an urban wastewater treatment plant effluent. Environ. Technol. 2011, 32, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Otero, M.; Zabkova, M.; Rodrigues, A.E. Comparative study of the adsorption of phenol and salicylic acid from aqueous solution onto nonionic polymeric resins. Sep. Purif. Technol. 2005, 45, 86–95. [Google Scholar] [CrossRef]
- Maloney, S.W.; Adrian, N.R.; Hickey, R.F.; Heine, R.L. Anaerobic treatment of pinkwater in a fluidized bed reactor containing GAC. J. Hazard. Mater. 2002, 92, 77–88. [Google Scholar] [CrossRef]
- Parette, R.; Cannon, F.S.; Weeks, K. Removing low ppb level perchlorate, RDX, and HMX from groundwater with cetyltrimethylammonium chloride (CTAC) pre-loaded activated carbon. Water Res. 2005, 39, 4683–4692. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G.; Dixon, J.B. Oxidation and fate of chromium in soils. Soil Sci. Plant Nutr. 2002, 48, 483–490. [Google Scholar] [CrossRef]
- Daoud, W.; Ebadi, T.; Fahimifar, A. Optimization of hexavalent chromium removal from aqueous solution using acid-modified granular activated carbon as adsorbent through response surface methodology. Korean J. Chem. Eng. 2015, 32, 1119–1128. [Google Scholar] [CrossRef]
- Di Natale, F.; Lancia, A.; Molino, A.; Musmarra, D. Removal of chromium ions form aqueous solutions by adsorption on activated carbon and char. J. Hazard. Mater. 2007, 145, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Fetter, C.W. Contaminant Hydrology; Prentice Hall: Upper Saddle River, NJ, USA, 1999. [Google Scholar]
- Han, I.; Schlautman, M.A.; Batchelor, B. Removal of hexavalent chromium from groundwater by granular activated carbon. Water Environ. Res. 2000, 72, 29–39. [Google Scholar] [CrossRef]
- Piazzoli, A.; Antonelli, M. Feasibility assessment of chromium removal from groundwater for drinking purposes by sorption on granular activated carbon and strong base anion exchange. Water Air Soil Pollut. 2018, 229, 17. [Google Scholar] [CrossRef]
- Satapathy, D.; Natarajan, G.S.; Patil, S.J. Adsorption characteristics of chromium(VI) on granular activated carbon. J. Chin. Chem. Soc. 2005, 52, 35–44. [Google Scholar] [CrossRef]
- Singha, S.; Sarkar, U. Analysis of the dynamics of a packed column using semi-empirical models: Case studies with the removal of hexavalent chromium from effluent wastewater. Korean J. Chem. Eng. 2015, 32, 20–29. [Google Scholar] [CrossRef]
- Song, H.O.; Yao, Z.J.; Shuang, C.D.; Li, A.M. Accelerated removal of nitrate from aqueous solution by utilizing polyacrylic anion exchange resin with magnetic separation performance. J. Ind. Eng. Chem. 2014, 20, 2888–2894. [Google Scholar] [CrossRef]
- Song, H.O.; Yao, Z.J.; Wang, M.Q.; Wang, J.N.; Zhu, Z.L.; Li, A.M. Effect of dissolved organic matter on nitrate-nitrogen removal by anion exchange resin and kinetics studies. J. Environ. Sci. 2013, 25, 105–113. [Google Scholar] [CrossRef]
- Primo, O.; Rivero, M.J.; Urtiaga, A.M.; Ortiz, I. Nitrate removal from electro-oxidized landfill leachate by ion exchange. J. Hazard. Mater. 2009, 164, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, D.; Leao, V.A. Fundamental aspects related to batch and fixed-bed sulfate sorption by the macroporous type 1 strong base ion exchange resin Purolite A500. J. Environ. Manag. 2014, 145, 106–112. [Google Scholar] [CrossRef] [PubMed]






| Breakthrough | Methanol | 1,4-Dioxane | Methylene Chloride | cis-1,2-Dichloroethene | ||||
|---|---|---|---|---|---|---|---|---|
| Mass Retained by GAC | R Factor | Mass Retained by GAC | R Factor | Mass Retained by GAC | R Factor | Mass Retained by GAC | R Factor | |
| Initial (mg/g) | 0.04 | 1 | 6.4 | 62 | 16.6 | 147 | 71.3 | 713 |
| 50% (mg/g) | 0.12 | 3 | 13.3 | 192 | 28.2 | 455 | 98.1 | 1700 |
| 100% (mg/g) | 0.4 | 5 | 19.8 | 235 | 43.6 | 386 | 122.5 | 1167 |
| Well | PFM Flux-Averaged Concentration (μg/L) | Measured Aqueous Phase Concentration (μg/L) | Percent Difference (%) |
|---|---|---|---|
| PFM 1 | <5 | 2.3 | - |
| PFM 2 | <5 | 330 | - |
| PFM 3 | 18 | 8.6 | 68 |
| PFM 4 | <5 | 1.9 | - |
| PFM 5 | 5932 | 1600 | 115 |
| PFM 6 | 1663 | 1700 | 2 |
| PFM 7 | 2195 | 990 | 76 |
| PFM 8 | 1675 | 480 | 111 |
| PFM 9 | 55 | 46 | 18 |
| PFM 10 | 20 | <1 | - |
| PFM 11 | <5 | 2.3 | - |
| PFM 12 | 1384 | 280 | 133 |
| PFM 13 | 602 | 310 | 64 |
| Breakthrough | Sulfate | |
|---|---|---|
| Breakthrough (mg/g) | Retardation Factor | |
| Initial | 50.5 | 27 |
| 50% | 68 | 15 |
| 100% | 70.4 | 37 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haluska, A.A.; Thiemann, M.S.; Evans, P.J.; Cho, J.; Annable, M.D. Expanded Application of the Passive Flux Meter: In-Situ Measurements of 1,4-Dioxane, Sulfate, Cr(VI) and RDX. Water 2018, 10, 1335. https://doi.org/10.3390/w10101335
Haluska AA, Thiemann MS, Evans PJ, Cho J, Annable MD. Expanded Application of the Passive Flux Meter: In-Situ Measurements of 1,4-Dioxane, Sulfate, Cr(VI) and RDX. Water. 2018; 10(10):1335. https://doi.org/10.3390/w10101335
Chicago/Turabian StyleHaluska, Alexander A., Meghan S. Thiemann, Patrick J. Evans, Jaehyun Cho, and Michael D. Annable. 2018. "Expanded Application of the Passive Flux Meter: In-Situ Measurements of 1,4-Dioxane, Sulfate, Cr(VI) and RDX" Water 10, no. 10: 1335. https://doi.org/10.3390/w10101335
APA StyleHaluska, A. A., Thiemann, M. S., Evans, P. J., Cho, J., & Annable, M. D. (2018). Expanded Application of the Passive Flux Meter: In-Situ Measurements of 1,4-Dioxane, Sulfate, Cr(VI) and RDX. Water, 10(10), 1335. https://doi.org/10.3390/w10101335





