Stream Health Evaluation Using a Combined Approach of Multi-Metric Chemical Pollution and Biological Integrity Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description and Study Duration
2.2. Water Chemistry Analyses
2.3. Fish Sampling
2.4. Modified Multi-Metric Water Pollution Index (WPI)
2.5. Modified Multi-Metric Index of Biotic Integrity (IBI)
2.6. Statistical Analysis
3. Results and Discussion
3.1. Seasonal Variations in Water Chemistry
3.2. Inter-Annual Variations in Water Chemistry
3.3. Empirical Modelling on Chlorophyll and Nutrient Contributing Factors
3.4. Correlation of Water Chemistry Parameters
3.5. Chemical Health Evaluation Based on Modified Multi-Metric Water Pollution Index (WPI)
3.6. Biological Health Evaluation Based on Modified Multi-Metric Index of Biotic Integrity
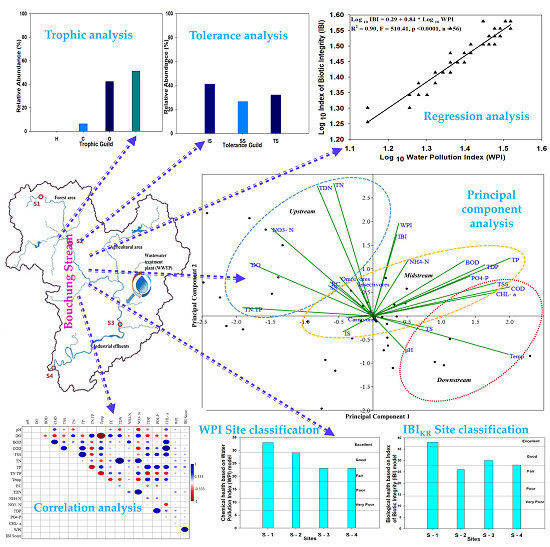
3.7. Responses of Trophic and Tolerant Guilds to Water Chemistry
3.8. Key Ecological Factor Identification with PCA
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Barbour, M.T.; Gerritsen, J.; Snyder, B.D.; Stribling, J.B. Rapid Bioassessment Protocols for Use in Streams and Wadeable Rivers: Periphyton, Benthic Macroinvertebrates and Fish, 2nd ed.; EPA 841-B-99-002; U.S. Environmental Protection Agency, Office of Water: Washington, DC, USA, 1999; p. 235.
- Long, J.M.; Walker, D.J. Small scale application and assessment of an Index of Biotic Integrity for a large boreal river. Hydrobiologia 2005, 544, 177–187. [Google Scholar] [CrossRef]
- Dahl, J.; Johnson, R.K.; Sandin, L. Detection of organic pollution of streams in southern Sweden using benthic macroinvertebrates. Hydrobiologia 2004, 516, 161–172. [Google Scholar] [CrossRef]
- Böhmer, J.; Rawer-Jost, C.; Zenker, A.; Meier, C.; Feld, C.K.; Biss, R.; Hering, D. Assessing streams in Germany with benthic invertebrates: Development of a multimetric invertebrate based assessment system. Limnologica 2004, 34, 416–432. [Google Scholar] [CrossRef]
- Noble, R.A.A.; Cowx, I.G.; Starkie, A. Development of fish-based methods for the assessment of ecological status in English and Welsh rivers. Fish. Manag. Ecol. 2007, 14, 495–508. [Google Scholar] [CrossRef]
- Choi, J.W.; Kumar, H.K.; Han, J.H.; An, K.G. The development of a regional multimetric fish model based on biological integrity in lotic ecosystems and some factors influencing the stream health. Water Air Soil Pollut. 2011, 217, 3–24. [Google Scholar] [CrossRef]
- Kim, J.Y.; An, K.G. Integrated Ecological River Health Assessments, Based on Water Chemistry, Physical Habitat Quality and Biological Integrity. Water 2015, 7, 6378–6403. [Google Scholar] [CrossRef]
- Dudgeon, D. Large-scale hydrological changes in tropical Asia: Prospects for riverine biodiversity the construction of large dams will have an impact on the biodiversity of tropical Asian rivers and their associated wetlands. BioScience 2000, 50, 793–806. [Google Scholar] [CrossRef]
- Ouyang, Y.; Higman, J.; Thompson, J.; O’Toole, T.; Campbell, D. Characterization and spatial distribution of heavy metals in sediment from Cedar and Ortega rivers sub-basin. J. Contam. Hydrol. 2002, 54, 19–35. [Google Scholar] [CrossRef]
- Cooper, M.J.; Uzarski, D.G.; Burton, T.M.; Rediske, R.R. Macroinvertebrate community composition, chemical/physical variables, land use and cover, and vegetation types within a Lake Michigan drowned river mouth wetland. Aquat. Ecosyst. Health Manag. Soc. 2006, 9, 463–479. [Google Scholar] [CrossRef]
- Burcher, C.L.; Valett, H.M.; Benfield, E.F. The land-cover cascade: Relationships coupling land and water. Ecology 2007, 88, 229–242. [Google Scholar] [CrossRef]
- Stoddard, J.L.; Larsen, D.P.; Hawkins, C.P.; Johnson, R.K.; Norris, R.H. Setting expectations for the ecological condition of streams: The concept of reference condition. Ecol. Appl. 2006, 16, 1267–1276. [Google Scholar] [CrossRef]
- Qadir, A.; Malik, R.N. Assessment of an index of biological integrity (IBI) to quantify the quality of two tributaries of river Chenab, Sialkot, Pakistan. Hydrobiologia 2009, 621, 127–153. [Google Scholar] [CrossRef]
- An, K.G.; Park, S.S.; Shin, J.Y. An evaluation of a river health using the index of biological integrity along with relations to chemical and habitat conditions. Environ. Int. 2002, 28, 411–420. [Google Scholar] [CrossRef]
- Lee, J.H.; An, K.G. Integrative restoration assessment of an urban stream using multiple modeling approaches with physical chemical, and biological integrity indicators. Ecol. Eng. 2014, 62, 153–167. [Google Scholar] [CrossRef]
- Karr, J.R. Assessment of biotic integrity using fish communities. Fisheries 1981, 6, 21–27. [Google Scholar] [CrossRef]
- Barbour, M.T.; Gerritsen, J.; Griffity, G.E.; Frydenborg, R.; McCarron, E.; White, J.S.; Bastian, M.L. A framework for biological criteria for Florida streams using benthic macroinvertebrates. J. N. Am. Benthol. Soc. 1996, 15, 185–211. [Google Scholar] [CrossRef]
- Yoder, C.O.; Rankin, E.T. The role of biological indicators in a state water quality management process. Environ. Monit. Assess. 1998, 51, 61–88. [Google Scholar] [CrossRef]
- Bach, E. A chemical index for surveillance of river water quality. Dtsch. Gewasserkd. Mitt. 1980, 24, 102–106. [Google Scholar]
- Trivedi, R.C.; De-Kruijf, H.A.M.; de-Zwart, D. The development and application of a yardstick for water quality evaluation. Sci. Total Environ. 1993, 134, 1191–1202. [Google Scholar] [CrossRef]
- Dodds, W.K.; Jones, J.R.; Welch, E.B. Suggested classification of stream trophic state: Distributions of temperate stream types by chlorophyll, total nitrogen and phosphorus. Water Res. 1998, 32, 1455–1462. [Google Scholar] [CrossRef]
- Lee, H.J.; An, K.G. The Development and Application of Multi-metric Water Quality Assessment Model for Reservoir Managements in Korea. Korean J. Limnol. 2009, 42, 242–252. [Google Scholar]
- Atique, U.; An, K.G. Water quality assessment based on chemical parameters and the chlorophyll dynamics in relation to nutrient regime in Chungju Reservoir. Pol. J. Environ. Stud. 2018, in press. [Google Scholar]
- Leopold, A. A Sand County Almanac, and Sketches Here and There; Oxford University Press, Inc.: New York, NY, USA, 1989. [Google Scholar]
- Angermeier, P.L.; Karr, J.R. Biological integrity versus biological diversity as policy directives. Bioscience 1993, 4, 690–697. [Google Scholar] [CrossRef]
- Karr, J.R. Ecological integrity and ecological health are not the same. In Engineering within Ecological Constraints; Schulze, P., Ed.; National Academy Press: Washington, DC, USA, 1996; pp. 100–113. [Google Scholar]
- Karr, J.R.; Chu, E.W. Restoring Life in Running Waters: Better Biological Monitoring; Island Press: Washington, DC, USA, 1999. [Google Scholar]
- Karr, J.R. Biological integrity: A long-neglected aspect of water resource management. Ecol. Appl. 1991, 1, 66–84. [Google Scholar] [CrossRef] [PubMed]
- Karr, J.R. Seven foundations of biological monitoring and assessment. Biol. Ambient. 2006, 20, 7–18. [Google Scholar]
- Westra, L. Ecological integrity. In Encyclopedia of Science, Technology, and Ethics; Mitcham, C., Ed.; Macmillan Reference: New York, NY, USA, 2005; Volume 2, pp. 574–578. [Google Scholar]
- Dale, V.H.; Beyeler, S.C. Challenges in the development and use of ecological indicators. Ecol. Indic. 2001, 1, 3–10. [Google Scholar] [CrossRef]
- Niemi, G.J.; McDonald, M.E. Application of ecological indicators. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 89–111. [Google Scholar] [CrossRef]
- Wilson, M.J.; Bayley, S.E. Use of single versus multiple biotic communities as indicators of biological integrity in northern prairie wetlands. Ecol. Indic. 2012, 20, 187–195. [Google Scholar] [CrossRef]
- Ruaro, R.; Gubiani, E.A. A scientometric assessment of 30 years of the index of biotic integrity in aquatic ecosystems: Applications and main flaws. Ecol. Indic. 2013, 29, 105–110. [Google Scholar] [CrossRef]
- Medeiros, H.R.; Bochio, G.M.; Ribeiro, M.C.; Torezan, J.M.; Anjos, L. Combining plant and bird data increases the accuracy of an index of biotic integrity to assess conservation levels of tropical forest fragments. J. Nat. Conserv. 2015, 25, 1–7. [Google Scholar] [CrossRef]
- Zampella, R.A.; Bunnell, J.F.; Laidig, K.J.; Procopio, N.A. Using multiple indicators to evaluate the ecological integrity of a coastal plain stream system. Ecol. Indic. 2006, 6, 644–663. [Google Scholar] [CrossRef]
- Joy, M.K.; Death, R.G. Application of the index of biotic integrity methodology to New Zealand freshwater fish communities. Environ. Manag. 2004, 34, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; An, K.G. Nutrient regime, N: P ratios and suspended solids as key factors influencing fish tolerance, trophic compositions, and stream ecosystem health. J. Ecol. Environ. 2015, 38, 505–515. [Google Scholar] [CrossRef]
- Ganasan, V.; Hughes, R.M. Application of an index of biological integrity (IBI) to fish assemblages of the rivers Khan and Kshipra (Madhya Pradesh), India. Freshw. Biol. 1998, 40, 367–383. [Google Scholar] [CrossRef]
- An, K.G.; Lee, J.Y.; Bae, D.Y.; Kim, J.H.; Hwang, S.J.; Won, D.H.; Lee, J.K.; Kim, C.S. Ecological assessments of aquatic environment using multi-metric model in major nationwide stream watersheds. J. Korean Soc. Water Qual. 2006, 22, 796–804. [Google Scholar]
- Choi, J.W.; Han, J.H.; Park, C.S.; Ko, D.G.; Kang, H.I.; Kim, J.Y.; Yun, Y.J.; Kwon, H.H.; An, K.G. Nutrients and sestonic chlorophyll dynamics in Asian lotic ecosystems and ecological stream health in relation to land-use patterns and water chemistry. Ecol. Eng. 2015, 79, 15–31. [Google Scholar] [CrossRef]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: New York, NY, USA, 2005. [Google Scholar]
- Crumpton, W.G.; Isenhart, T.M.; Mitchell, P.D. Nitrate and organic N analyses with second-derivative spectroscopy. Limnol. Oceanogr. 1992, 37, 907–913. [Google Scholar] [CrossRef]
- Eaton, A.D.; Franson, M.A.H. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Prepas, E.E.; Rigler, F.A. Improvements in qualifying the phosphorus concentration in lake water. Can. J. Fish. Aquat. Sci. 1982, 39, 822–829. [Google Scholar] [CrossRef]
- The Ministry of Environment (MOE). Standard Methods for the Examination of Water Quality Contamination, 7th ed.; Gwacheon Korea, 2000; p. 435. (In Korean)
- An, K.G.; Yeom, D.H.; Lee, S.K. Rapid Bioassessments of Kap Stream Using the Index of Biological Integrity. Korean J. Environ. Biol. 2001, 19, 261–269. [Google Scholar]
- The Ministry of Environment/National Institute of Environmental Research (MOE/NIER). The Survey and Evaluation of Aquatic Ecosystem Health in Korea; The Ministry of Environment/National Institute of Environmental Research (NIER): Incheon, Korea, 2008.
- United States Environmental Protection Agency (U.S. EPA). Fish Field and Laboratory Methods for Evaluating the Biological Integrity of Surface Waters; EPA 600-R-92-111; Environmental Monitoring Systems Laboratory-Cincinnati Office of Modeling, Monitoring Systems, and Quality Assurance Office of Research Development; U.S. EPA: Cincinnati, OH, USA, 1993; p. 348.
- Kim, I.S.; Park, J.Y. Freshwater Fish of Korea; Kyohak Publishing: Seoul, Korea, 2002; p. 465. [Google Scholar]
- Nelson, J.S. Fishes of the World, 4th ed.; John Wiley & Sons: New York, NY, USA, 2006; p. 624. [Google Scholar]
- Sanders, R.E.; Milter, R.J.; Yondr, C.O.; Rankin, E.T. The use of external deformities, erosion, lesions, and tumors (DELT anormalies) in fish assemblages for characterizing aquatic resources: A case study of seven Ohio streams. In Assessing the Sustainability and Biological Integrity of Water Resources Using Fish Communities; Simon, T.P., Ed.; CRC Press: Boca Raton, FL, USA, 1999; pp. 225–245. [Google Scholar]
- An, K.G.; Kim, D.S.; Kong, D.S.; Kim, S.D. Integrative assessments of a temperate stream based on a multimetric determination of biological integrity, physical habitat evaluations, and toxicity tests. Bull. Environ. Contam. Toxicol. 2004, 73, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Rankin, E.T.; Yoder, C.O. Adjustments to the index of biotic integrity: A summary of Ohio experiences and some suggested modifications. In Assessing the Sustainability and Biological Integrity of Water Resources Using Fish Communities; Simon, T.P., Ed.; CRC Press: Boca Raton, FL, USA, 1999; p. 672. [Google Scholar]
- Sigma Plot, version 10; Systat Software, Inc.: San Jose, CA, USA; Available online: www.systatsoftware.com (accessed on 13 April 2018).
- Hammer, Ø. The Past of the Future; PAST, Version 3.18 (Software); Natural History Museum, University of Oslo: Oslo, Norway, 2018. [Google Scholar]
- Krishnaram, H.; Mohan, M.; Ramchandra; Vishalkashi, Y. Limnological studies on Kolaramma lake Kolar, Karnataka. Environ. Ecol. 2007, 52, 364–367. [Google Scholar]
- Vijayakumar, S.K.; Rajesh, K.M.; Mendon, R.; Hariharan, V. Seasonal distribution and behavior of nutrients with reference to tidal rhythm in the Mulki estuary, Southwest coast of India. J. Mar. Biol. Assoc. India 2000, 42, 21–31. [Google Scholar]
- Manivasakam, N. Physico-Chemical Examination of Water, Sewage and Industrial Effluents; Pragati Prakashan: New Delhi, India, 2003. [Google Scholar]
- Scott, T.M.; Salina, P.; Portier, K.M.; Rose, J.B.; Tamplin, M.L.; Farra, S.R.; Koo, A.; Lukasik, J. Geographical variation in ribotype profiles of Escherichia coli isolates from human, swan, poultry, beef and dairy cattle in Florida. Appl. Environ. Microbiol. 2003, 69, 1089–1092. [Google Scholar] [CrossRef] [PubMed]
- Pipes, W.O. Bacteriological Indicators of Pollution; CRC Press: Boca Raton, FL, USA, 1981; p. 242. [Google Scholar]
- Sood, A.; Singh, K.D.; Pandey, P.; Sharma, S. Assessment of bacterial indicators and physicochemical parameters to investigate pollution status of Gangetic river system of Uttarakhand (India). Ecol. Indic. 2008, 8, 709–717. [Google Scholar] [CrossRef]
- Han, J.H.; Kim, B.; Kim, C.; An, K.G. Ecosystem health evaluation of agricultural reservoirs using multi-metric lentic ecosystem health assessment (LEHA) model. Paddy Water Environ. 2014, 12 (Suppl. 1), 7–18. [Google Scholar] [CrossRef]
- Carlson, R.E. Expanding the trophic state concept to identify non-nutrient limited lakes and reservoirs. In Proceedings of a National Conference on Enhancing the States Lake Management Programs; North American Lake Management Society: Chicago, IL, USA, 1991; pp. 59–71. [Google Scholar]
- Lind, O.T.; Terrell, T.T.; Kimmel, B.L. Problems in reservoir trophic-state classification and implications for reservoir management. In Comparative Reservoir Limnology and Water Quality Management; Straskraba, M., Tundisi, J.G., Duncan, A., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1993; pp. 57–67. [Google Scholar]
- Hill, W.R.; Ryon, M.G.; Schilling, E.M. Light limitation in a stream ecosystem: Responses by primary producers and consumers. Ecology 1995, 76, 1297–1309. [Google Scholar] [CrossRef]
- Song, E.S.; Jeon, S.M.; Lee, E.J.; Park, D.J.; Shin, Y.S. Long-term trend analysis of chlorophyll a and water quality in the Yeongsan River. Korean J. Ecol. Environ. 2012, 45, 302–313. [Google Scholar]
- Downing, J.A.; McCauley, E. The nitrogen: Phosphorus relationship in lakes. Limnol. Oceanogr. 1992, 37, 936–945. [Google Scholar] [CrossRef]
- Smith, V.H. Low nitrogen to phosphorus ratios favor dominance by blue-green algae in Lake Phytoplankton. Science 1983, 221, 669–671. [Google Scholar] [CrossRef] [PubMed]
- An, K.G.; Jones, J.R. Temporal and spatial patterns in ionic salinity and suspended solids in a reservoir influenced by the Asian monsoon. Hydrobiologia 2000, 436, 179–189. [Google Scholar] [CrossRef]
- Fujimoto, N.; Sudo, R. Nutrient-limited growth of Microcystis aerugimosa and phormidium tenue and competition under various N:P supply ratios and temperatures. Limnol. Oceonogr. 1997, 42, 250–256. [Google Scholar] [CrossRef]
- Seppala, J.; Tamminen, T.; Kaitala, S. Experimental evaluation of nutrient limitation of phytoplankton communities in the Gulf of Riga. J. Mar. Syst. 1999, 23, 107–126. [Google Scholar] [CrossRef]
- Han, J.H.; An, K.G. Chemical Water Quality and Fish Community Characteristics in the Mid- to Downstream Reach of Geum River. Korean J. Environ. Biol. 2013, 31, 180–188. [Google Scholar] [CrossRef]
- An, K.G.; Kim, J.H. A Diagnosis of Ecological health Using a Physical Habitat Assessment and Multimetric Fish Model in Daejeon Stream. Korean J. Limnol. 2005, 38, 361–371. [Google Scholar]
- Bae, E.Y.; An, K.G. Stream Ecosystem Assessments, based on a Biological Multimetric Parameter Model and Water Chemistry Analysis. Korean J. Limnol. 2006, 39, 198–208. [Google Scholar]
- McCune, B.; Mefford, M.J. PC-ORD: Multivariate Analysis of Ecological Data; MjM Software Design: Gleneden Beach, OR, USA, 1999. [Google Scholar]














| Category | Model Metrics (M) | Scoring Criteria | Mean ± Standard Deviation (Score) | |||||
|---|---|---|---|---|---|---|---|---|
| 5 | 3 | 1 | S1 | S2 | S3 | S4 | ||
| Nutrient Regime | M1: Total Nitrogen (mg/L) | <1.5 | 1.5–3.0 | >3 | 1.98 ± 0.069 (3) | 1.98 ± 0.63 (3) | 2.26 ± 0.614 (3) | 1.56 ± 0.60 (3) |
| M2: Total Phosphorus (µg/L) | <30 | 30–100 | >100 | 23.57 ± 23.02 (5) | 32.23 ± 23.72 (3) | 43.83 ± 44.02 (3) | 39.96 ± 79.64 (3) | |
| M3: TN:TP ratio | >50 | 20–50 | <20 | 163.36 ± 179.34 (5) | 100.71 ± 98.99 (5) | 86.53 ± 68.08 (5) | 101.08 ± 101.44 (5) | |
| Organic Matter | M4: Biological Oxygen Demand (mg/L) | <1 | 1–2.5 | >2.5 | 0.563 ± 0.241 (5) | 0.99 ± 0.66 (5) | 1.15 ± 0.48 (3) | 0.85 ± 0.53 (5) |
| Ionic Contents and Solids | M5: Total Suspended Solid (mg/L) | <4 | 4–10 | >10 | 1.67 ± 1.66 (5) | 3.44 ± 4.45 (5) | 8.40 ± 11.50 (3) | 12.86 ± 52.50 (1) |
| M6: Electrical Conductivity (µS/cm) | <180 | 180–300 | >300 | 163.85 ± 43.03 (5) | 198.46 ± 52.67 (3) | 231.19 ± 46.19 (3) | 204.92 ± 44.44 (3) | |
| Primary Production Indicator | M7: Sestonic Chlorophyll (µg/L) | <3 | 3–10 | >10 | 0.671 ± 1.08 (5) | 1.31 ± 1.24 (5) | 6.81 ± 5.58 (3) | 5.37 ± 6.72 (3) |
| Final WPI Scores | 33 | 29 | 23 | 23 | ||||
| Water Quality Criteria | Excellent | Good | Fair | Fair | ||||
| Category | Model Metrics (M) | Scoring Criteria (5–1) | Sampling Sites (Obtained Model Values) | |||||
|---|---|---|---|---|---|---|---|---|
| 5 | 3 | 1 | S1 | S2 | S3 | S4 | ||
| Species Richness and Composition | M1: Total Number of Native Fish Species | Expectations of M1 vary with stream size and region. | 29 (5) | 39 (5) | 46 (5) | 46 (5) | ||
| M2: Number of Riffle Benthic Species | Expectations of M2 vary with stream size and region. | 06 (5) | 08 (5) | 10 (5) | 09 (5) | |||
| M3: Number of Sensitive Species | Expectations of M3 vary with stream size and region. | 08 (3) | 07 (3) | 13 (5) | 13 (5) | |||
| M4: Proportion of Individuals as Tolerant Species | <5 | 5-20 | >20 | 05 (5) | 58 (1) | 22 (1) | 45 (1) | |
| Trophic Composition | M5: Proportion of Individual as Omnivore Species | <20 | 20-45 | >45 | 05 (5) | 58 (1) | 46 (1) | 58 (1) |
| M6: Proportion of Individuals as Native Insectivore Species | >45 | 45-20 | <20 | 85 (5) | 32 (3) | 51 (5) | 37 (3) | |
| Fish Abundance and Condition | M7: Total Number of Native Individuals | Expectations of M7 vary with stream size and region. | 1725 (5) | 748 (5) | 1316 (5) | 597 (5) | ||
| M8: Percent Individuals with Anomalies | 0 | 0-1 | >1 | 0.0 (5) | 0.43 (3) | 0.04 (3) | 0.06 (3) | |
| Final IBI Scores | 38 | 26 | 30 | 28 | ||||
| Biological Health Status of Stream | Excellent | Fair | Good | Good | ||||
| Sites | Attrib. | pH | DO | BOD | COD | TN | TP | TN:TP | Temp. | EC | TNCB | TDN | NH4-N | NO3-N | TDP | PO4-P | Chl-a |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | Min. | 6.10 | 7.20 | 0.20 | 0.50 | 0.64 | 2.00 | 9.50 | 0.80 | 90 | 0.00 | 0.62 | 0.00 | 0.48 | 0.00 | 0.00 | 0.00 |
| Max. | 8.90 | 19.30 | 1.30 | 3.80 | 4.62 | 140 | 1185.33 | 25 | 308 | 92,000 | 4.53 | 0.57 | 4.35 | 0.14 | 0.07 | 7.90 | |
| Mean | 7.59 | 11.10 | 0.56 | 1.61 | 1.99 | 23.57 | 163.36 | 12.99 | 163.86 | 6091.61 | 1.90 | 0.04 | 1.76 | 0.01 | 0.01 | 0.67 | |
| SD | 0.58 | 2.20 | 0.24 | 0.61 | 0.64 | 23.02 | 179.35 | 7.56 | 43.03 | 19,617.33 | 0.63 | 0.08 | 0.62 | 0.02 | 0.01 | 1.09 | |
| CV | 7.58 | 19.85 | 42.95 | 38.13 | 32.22 | 97.68 | 109.79 | 58.17 | 26.26 | 322.04 | 33.22 | 175.83 | 35.46 | 122.39 | 167.86 | 162.08 | |
| S2 | Min. | 6.70 | 6.40 | 0.20 | 0.60 | 0.57 | 2.00 | 14.46 | 1.40 | 6 | 0.00 | 0.55 | 0.00 | 0.37 | 0.00 | 0.00 | 0.00 |
| Max. | 8.60 | 19.20 | 4.30 | 5.40 | 3.86 | 140 | 644 | 26.50 | 520 | 240,000 | 3.79 | 1.69 | 3.41 | 0.12 | 0.11 | 6.90 | |
| Mean | 7.57 | 10.89 | 0.99 | 2.32 | 1.98 | 32.24 | 100.71 | 14.45 | 198.46 | 6138.71 | 1.89 | 0.12 | 1.60 | 0.02 | 0.01 | 1.31 | |
| SD | 0.41 | 2.39 | 0.67 | 1.06 | 0.63 | 23.73 | 99 | 7.94 | 52.68 | 28,947.48 | 0.62 | 0.25 | 0.58 | 0.02 | 0.02 | 1.24 | |
| CV | 5.39 | 21.93 | 66.90 | 45.73 | 31.78 | 73.60 | 98.30 | 54.93 | 26.54 | 471.56 | 33.08 | 205.96 | 35.97 | 87.10 | 169.35 | 94.69 | |
| S3 | Min. | 6.90 | 8.70 | 0.50 | 1.20 | 1.00 | 9.00 | 10.99 | −0.50 | 122 | 6 | 0.81 | 0.01 | 0.02 | 0.00 | 0.00 | 0.40 |
| Max. | 8.20 | 15.70 | 2.90 | 8.40 | 5.19 | 266 | 408.56 | 26.90 | 337 | 140,000 | 3.54 | 0.32 | 2.85 | 0.17 | 0.12 | 30.20 | |
| Mean | 7.69 | 11.68 | 1.15 | 3.78 | 2.26 | 43.83 | 86.53 | 13.86 | 231.19 | 3262.68 | 2.08 | 0.05 | 1.55 | 0.02 | 0.01 | 6.82 | |
| SD | 0.23 | 2.06 | 0.48 | 1.30 | 0.61 | 44.02 | 68.09 | 8.46 | 46.20 | 15,677.68 | 0.58 | 0.05 | 0.61 | 0.03 | 0.02 | 5.59 | |
| CV | 2.97 | 17.67 | 41.68 | 34.45 | 27.13 | 100.44 | 78.69 | 61.02 | 19.98 | 480.52 | 28.03 | 93.82 | 39.74 | 112.84 | 172.74 | 81.94 | |
| S4 | Min. | 7.10 | 6.20 | 0.30 | 0.90 | 0.35 | 3.00 | 4.44 | 0.00 | 103 | 2 | 0.34 | 0.00 | 0.11 | 0.00 | 0.00 | 0.10 |
| Max. | 9.00 | 16.50 | 2.60 | 18.70 | 2.94 | 661 | 569.25 | 30.70 | 300 | 40,000 | 2.43 | 0.43 | 2.25 | 0.13 | 0.12 | 37.50 | |
| Mean | 8.07 | 10.65 | 0.85 | 3.50 | 1.56 | 39.96 | 101.09 | 14.64 | 204.93 | 1540.23 | 1.42 | 0.04 | 1.13 | 0.02 | 0.01 | 5.37 | |
| SD | 0.37 | 2.55 | 0.53 | 1.98 | 0.60 | 79.64 | 101.45 | 9.41 | 44.44 | 4844.51 | 0.54 | 0.08 | 0.54 | 0.02 | 0.02 | 6.73 | |
| CV | 4.63 | 23.97 | 62.51 | 56.57 | 38.41 | 199.28 | 100.36 | 64.32 | 21.69 | 314.53 | 38.03 | 175.59 | 47.46 | 125.30 | 194.79 | 125.25 |
| Species | Type of Fish Guild | Sampling Sites | TNI | RA (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Tol. G. | Tro. G. | Hab. G. | S1 | S2 | S3 | S4 | |||
| Zacco platypus | TS | O | -- | 67 | 1137 | 471 | 673 | 2348 | 27.35 |
| Zacco koreanus | SS | I | 788 | 14 | 169 | 27 | 998 | 11.62 | |
| Rhinogobius brunneus | IS | I | RB | 23 | 53 | 403 | 189 | 668 | 7.78 |
| Zacco temminckii | SS | I | -- | 445 | 0 | 71 | 19 | 535 | 6.23 |
| Pseudogobio esocinus | IS | I | -- | 9 | 358 | 79 | 84 | 530 | 6.17 |
| Hamibarbus longirostris | IS | I | -- | 315 | 112 | 43 | 8 | 478 | 5.57 |
| Acheilognathus lanceolatus | IS | O | -- | 5 | 59 | 238 | 42 | 344 | 4.01 |
| Acheilognathus koreensis | IS | O | -- | 3 | 8 | 265 | 46 | 322 | 3.75 |
| Pungtungia herzi | IS | I | -- | 21 | 78 | 114 | 38 | 251 | 2.92 |
| Microphysogobio yaluensis | IS | O | RB | 4 | 56 | 163 | 22 | 245 | 2.85 |
| Odontobutis platycephala | SS | C | -- | 134 | 10 | 15 | 42 | 201 | 2.34 |
| Coreoleuciscus splendidus | SS | I | RB | 0 | 0 | 130 | 27 | 157 | 1.83 |
| Iksookimia koreensis | IS | I | RB | 17 | 13 | 85 | 36 | 151 | 1.76 |
| Odontobutis interrupta | IS | C | -- | 58 | 45 | 23 | 3 | 129 | 1.50 |
| Opsarichthys uncirostris amurensis | TS | C | -- | 0 | 99 | 11 | 6 | 116 | 1.35 |
| Acheilognathus yamatsuatea | IS | O | -- | 0 | 5 | 9 | 77 | 91 | 1.06 |
| Gobiobotia brevibarba | SS | I | RB | 0 | 6 | 73 | 0 | 79 | 0.92 |
| Micropterus salmoides | TS | C | -- | 2 | 52 | 18 | 4 | 76 | 0.89 |
| Cobitis lutheri | IS | I | -- | 0 | 76 | 0 | 0 | 76 | 0.89 |
| Sarcocheilichthys variegatus wakiyae | SS | I | -- | 0 | 1 | 10 | 63 | 74 | 0.86 |
| Squalidus chankaensis tsuchigae | IS | O | -- | 0 | 10 | 10 | 50 | 70 | 0.82 |
| Rhynchocypris oxycephalus | SS | I | -- | 64 | 0 | 1 | 0 | 65 | 0.76 |
| Tridentiger brevispinis | IS | I | RB | 0 | 1 | 5 | 36 | 42 | 0.49 |
| Pseudopungtungia nigra | SS | I | -- | 0 | 0 | 0 | 41 | 41 | 0.48 |
| Gasterosteus aculeatus | IS | I | -- | 0 | 0 | 40 | 0 | 40 | 0.47 |
| Pseudobagrus koreanus | SS | I | RB | 0 | 6 | 27 | 5 | 38 | 0.44 |
| Leiocassis nitidus | TS | I | -- | 1 | 2 | 29 | 5 | 37 | 0.43 |
| Microphysogobio koreensis | SS | O | RB | 3 | 15 | 10 | 4 | 32 | 0.37 |
| Squalidus gracilis majimae | SS | I | -- | 17 | 0 | 12 | 1 | 30 | 0.35 |
| Misgurnus anguillicaudatus | TS | O | -- | 2 | 16 | 8 | 1 | 27 | 0.31 |
| Pseudorasbora parva | TS | O | -- | 0 | 7 | 0 | 15 | 22 | 0.26 |
| Acheilognathus rhombeus | IS | O | -- | 4 | 1 | 15 | 1 | 21 | 0.24 |
| Pseudobagrus fulvidraco | TS | I | -- | 5 | 4 | 11 | 0 | 20 | 0.23 |
| Misgurnus mizolepis | TS | O | -- | 2 | 9 | 7 | 1 | 19 | 0.22 |
| Carassius auratus | TS | O | -- | 0 | 15 | 4 | 0 | 19 | 0.22 |
| Lepomis macrochirus | TS | I | -- | 12 | 1 | 0 | 3 | 16 | 0.19 |
| Sarcocheilichthys nigripinnis morii | IS | I | -- | 0 | 12 | 0 | 4 | 16 | 0.19 |
| Oreochromis niloticus | TS | O | -- | 0 | 4 | 0 | 11 | 15 | 0.17 |
| Hamibarbus labeo | TS | I | -- | 0 | 5 | 0 | 9 | 14 | 0.16 |
| Squalidus japonicus coreanus | TS | O | -- | 5 | 0 | 1 | 7 | 13 | 0.15 |
| Koreocobitis naktongensis | SS | O | RB | 7 | 2 | 2 | 2 | 13 | 0.15 |
| Micropercops swinhonis | IS | O | -- | 0 | 12 | 0 | 0 | 12 | 0.14 |
| Gnathopogon strigatus | IS | I | -- | 4 | 6 | 1 | 0 | 11 | 0.13 |
| Takifugu niphobles | IS | I | -- | 10 | 0 | 0 | 0 | 10 | 0.12 |
| Total Number of Species | 29 | 39 | 46 | 46 | 64 | ||||
| Total Number of Individuals | 2034 | 2315 | 2597 | 1640 | 8586 | ||||
| Factors | PC 1 | PC 2 | PC 3 | PC 4 | PC 5 | PC 6 |
|---|---|---|---|---|---|---|
| pH | 0.07 | −0.11 | 0.39 | −0.16 | 0.25 | 0.12 |
| DO | −0.29 | 0.18 | 0.19 | −0.14 | −0.06 | 0.01 |
| BOD | 0.22 | 0.19 | −0.09 | −0.27 | 0.25 | 0.01 |
| COD | 0.33 | 0.10 | 0.11 | −0.21 | 0.14 | 0.08 |
| TSS | 0.29 | 0.12 | 0.10 | 0.02 | −0.01 | 0.16 |
| TN | −0.09 | 0.46 | −0.11 | −0.13 | −0.13 | 0.15 |
| TP | 0.46 | 0.20 | 0.02 | 0.05 | −0.10 | 0.08 |
| TN:TP | −0.31 | 0.03 | 0.03 | −0.13 | 0.03 | 0.13 |
| Temp. | 0.32 | −0.13 | −0.19 | 0.12 | −0.02 | −0.01 |
| EC | −0.10 | 0.12 | 0.21 | −0.40 | 0.08 | −0.13 |
| TDN | −0.13 | 0.45 | −0.13 | −0.09 | −0.12 | 0.12 |
| NH4-N | 0.08 | −0.57 | −0.27 | −0.04 | 0.10 | −0.06 |
| NO3-N | −0.24 | 0.51 | −0.13 | 0.06 | −0.21 | 0.14 |
| TDP | −0.45 | 0.18 | −0.16 | 0.19 | −0.17 | −0.03 |
| PO4-P | 0.53 | 0.14 | −0.08 | 0.23 | −0.16 | −0.03 |
| CHL-a | 0.29 | 0.08 | 0.13 | −0.25 | 0.13 | −0.50 |
| WPI | 0.06 | 0.32 | −0.50 | 0.20 | 0.09 | −0.34 |
| IBI | 0.06 | 0.28 | 0.58 | 0.16 | 0.02 | −0.37 |
| IS | −0.07 | −0.05 | −0.19 | 0.24 | 0.58 | 0.20 |
| TS | 0.12 | −0.04 | 0.11 | −0.15 | −0.50 | 0.43 |
| SS | −0.11 | 0.10 | 0.11 | 0.32 | 0.28 | 0.44 |
| Omnivores | −0.08 | 0.13 | 0.27 | 0.43 | 0.36 | 0.57 |
| Carnivores | −0.06 | 0.00 | −0.02 | 0.14 | 0.03 | −0.58 |
| Insectivores | −0.04 | 0.12 | −0.48 | −0.11 | −0.50 | −0.05 |
| Eigenvalue | 3.26 | 1.84 | 1.40 | 1.30 | 1.07 | 0.87 |
| % Variance | 24.10 | 13.58 | 10.34 | 9.57 | 7.91 | 6.46 |
| CPV | 24.10 | 37.68 | 48.02 | 57.59 | 65.50 | 71.96 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atique, U.; An, K.-G. Stream Health Evaluation Using a Combined Approach of Multi-Metric Chemical Pollution and Biological Integrity Models. Water 2018, 10, 661. https://doi.org/10.3390/w10050661
Atique U, An K-G. Stream Health Evaluation Using a Combined Approach of Multi-Metric Chemical Pollution and Biological Integrity Models. Water. 2018; 10(5):661. https://doi.org/10.3390/w10050661
Chicago/Turabian StyleAtique, Usman, and Kwang-Guk An. 2018. "Stream Health Evaluation Using a Combined Approach of Multi-Metric Chemical Pollution and Biological Integrity Models" Water 10, no. 5: 661. https://doi.org/10.3390/w10050661
APA StyleAtique, U., & An, K.-G. (2018). Stream Health Evaluation Using a Combined Approach of Multi-Metric Chemical Pollution and Biological Integrity Models. Water, 10(5), 661. https://doi.org/10.3390/w10050661