Abstract
Constructed wetlands are commonly used for sewage treatment. However, as the natural processes operate, these artificial ecosystems can also be used to enhance the equalization of water features to those of the receiving environments, thus reducing the impacts of the treated water on the natural systems. Here, we studied, by a year-round survey, the simultaneous and separated operation of two subsurface wetlands that were used as a tertiary treatment to enhance the naturalization of wastewaters that had already been treated in a waste water treatment plant (WWTP). These wetlands were operating serially, with the first wetland being covered by the riparian plant Helosciadum nodiflorum, which has not been described so far as being used in treatment wetland, whereas the second was covered by Typha latifolia. The changes in the concentrations and transformation among the different types of pollutants and other physical and chemical parameters, as well as in the bacterial abundance and activity, were studied under different operational conditions of serial co-operation or of separately-operating wetlands. Both wetlands were differentially efficient in the reduction and transformation of the remaining pollutants, with very active nitrification and denitrification processes, which reduced the ammonium concentrations by more than 65%, although they changed according to the operational status of each wetland. They also reduced the already low organic matter contents by around 30% and promoted slight shifts in the dominant types of dissolved organic matter to less labile compounds. To a certain extent, the Typha-covered wetland also contributed to phosphorus removal, by up to 35%. Noticeably, both of the wetlands contributed greatly to the reduction of bacterial abundance, which was even 50% lower after the wetland transit, although the resulting community increased its activity, thus keeping the capacity for pollutant removal and transformation. Overall, the wetlands’ operation increased the similarity between the poured waters and those of the receiving stream, thus diminishing its environmental impact.
1. Introduction
Non-naturalized waters that originated from wastewater discharges, despite being treated in waste water treatment plants (WWTP) achieving the legislated quality standards, show quite different features to those of the natural environments where they are poured. This can cause a drop in the concentration of dissolved oxygen in the receiving environment, as well as promote eutrophication, the loss of biodiversity, and the release pathogenic microorganisms [1,2]. All of this causes a degradation of the natural environment, diminishing its ecological integrity and its capacity to provide ecosystem services [3]. In Europe, the Directive 2013/39/EU has established the need to implement cost-effective purification treatments, which requires the development of innovative water treatment technologies, using more sustainable processes, such as artificial wetlands [4,5]. Furthermore, limits in the concentrations of the pollutants that have been fixed by European Directive 91/271/EEC for WWTP effluents may be excessively high for some deficit basins, such as those in the Mediterranean, where the discharge may constitute an important proportion of the total volume of the receiving water bodies. This problem, which has already been addressed by the European Commission, encumbers the achievement of the good ecological status of European water bodies that is required by the EU Water Framework Directive [6].
Constructed wetlands (CWs) are engineered systems that are designed to treat different types of polluted water using natural biogeochemical processes. These systems can effectively treat raw, primary, secondary, or tertiary treated sewage, as well as different types of industrial wastewater, and they are considered environmentally friendly and sustainable options for wastewater treatment [7], simulating the structure of natural wetlands. CWs are classified according to the wetland hydrology as free water surface and subsurface systems. Subsurface flow CWs are generally classified depending on the flow direction, as those with horizontal or vertical flow. The latter are usually fed intermittently and are generally capable of achieving higher nitrification rates because of the greater oxygenation of the wetland bed. The serial use of a combination of several types of wetlands is also common [8]. On the other hand, CW may contain both emergent and/or submerged vegetation, which determines the wetland metabolism and performance [9]. For instance, vegetation prevents the resuspension of solids and increases the oxygenation of the soil [9]. Nutrient removal by vegetation may occur either by direct plant uptake, particularly nitrogen, or by facilitating the uptake by microorganisms [10,11] and chemical precipitation [12]. Additionally, plants increase the available carbon in the sediment, which in turn can favour the nutrient-removal processes, such as denitrification [11].
The selection of the vegetation cover for the CW depends of several factors, such as its geographical distribution, growth capacity, climatic adaptation, the type of contamination to be treated, the tolerance to CW conditions, and the costs of maintenance. Despite this high number of factors, there are few species of aquatic plants that are overrepresented in CWs. In relation to this, Vymazal [13] describes the regular use of proliferative plants, such as those of the genera Typha, Scirpus, Phragmites, Juncus, and Eleocharis, which are usually planted as monocultures. Monocultures, especially of exotic invasive species, might represent a risk for the conservation of natural biodiversity of aquatic ecosystems downstream, but also misprizes the potential functional complementarity of combining the different plant species [14]. Moreover, some authors pointed out the suitability of using wild plant species for the treatment of effluents in constructed wetlands [15], an issue that had not been sufficiently addressed so far. For instance, there has been no mention in the scientific literature for the use of wild species, such as Helosciadum nodiflorum, which thrives in a wide variety of Mediterranean aquatic ecosystems, but more particularly, spreads within the nutrient-rich shores of Mediterranean streams.
In this study we evaluated the performance of a CW system, where two plant species (Helosciadum nodiflorum and Typha latifolia) were used separately in two different ponds that were working sequentially. This CW represents a tertiary treatment that receives a continuous flow from the outlet of a wastewater treatment plant (WWTP). Here, we assessed the pollution removal efficiency of the system and its capability to naturalize water, either by the simultaneous serial operation of both of the wetlands or by the single use of one of them. Our results will help to discern to what extent it is possible to gain advantages of the pollutants’ removal by using different plants species in combination, which shows a fast growth and have minimal maintenance costs. This would represent an inexpensive way to diminish the disturbance on the waterbody receiving the effluent, as the waters in the outlet of the CW system may then show more similar features to that of the receiving Mediterranean stream.
2. Materials and Methods
2.1. Study Site and Operational Phases
The studied constructed wetland (CW) is located down to the wastewater treatment plant (WWTP) of Higueruelas, a small town with 503 inhabitants. This town is located in a mountain area (767 m a.s.l.) at nearly 50 Km from the Mediterranean coast in the Valencia Region (Spain). This WWTP includes a pre-treatment and a biological (extended aeration activated sludge) treatment, and treated 275 m3 day−1 on average during the studied period, which corresponds to 715 population equivalents. This town also has an industry of electronic components and holds an increased population during the summer months. The WWTP-treated water (I) enter directly into Wetland 1, whereas the outlet from Wetland 1 (O1) directly enters Wetland 2, which in turn discharges (O2) to Rambla Castellana, a small Mediterranean stream (Figure 1). Both of the constructed wetlands have a subsurface flow design.
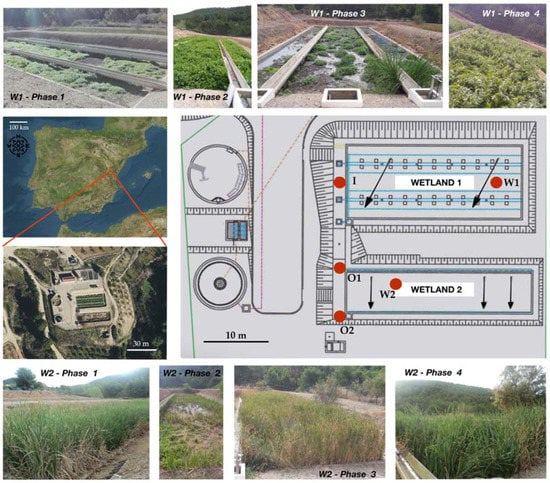
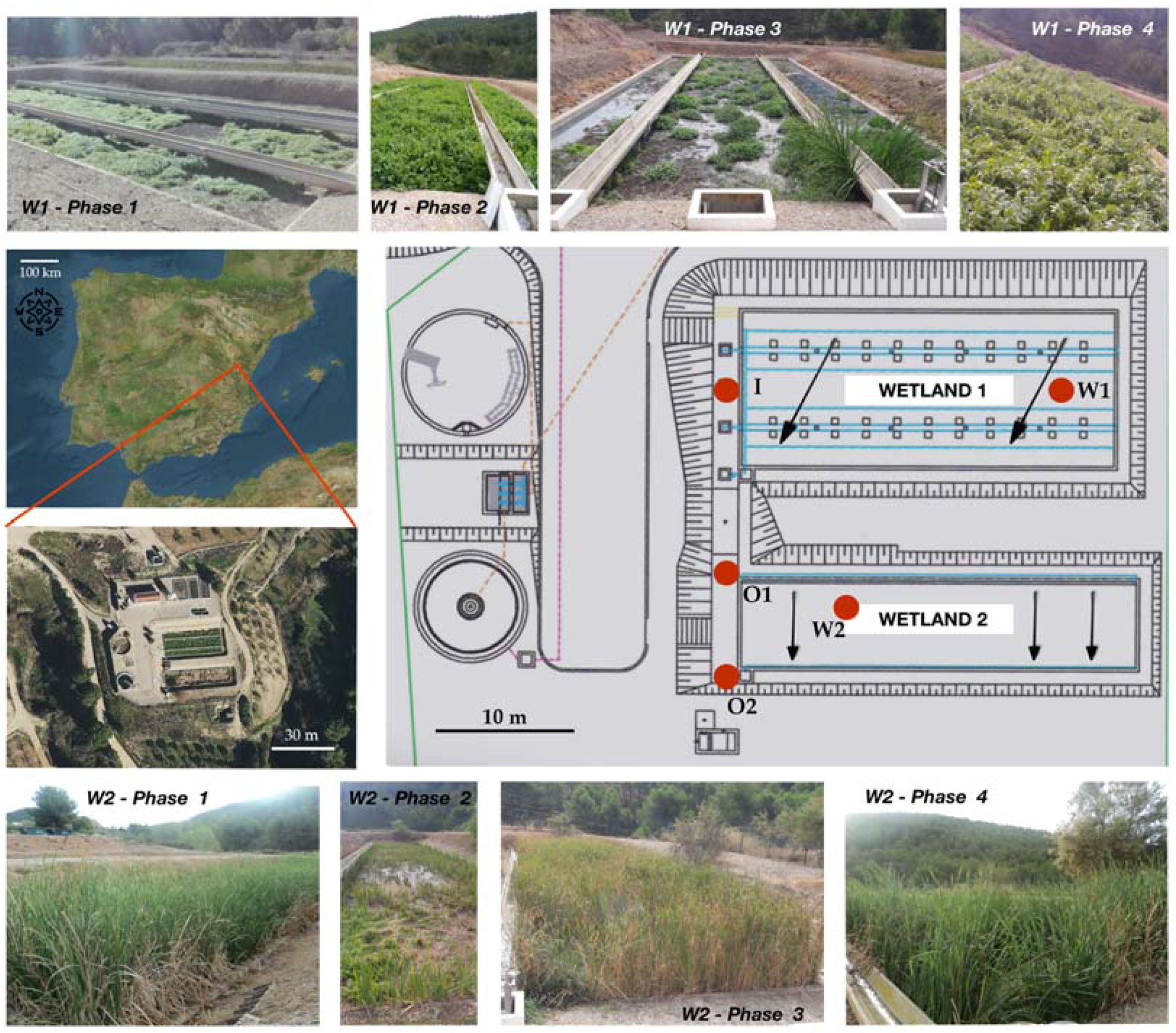
Figure 1.
Map with the location of the studied system, design of the waste water treatment plant (WWTP) and the associated constructed wetlands, and photographs of the vegetation coverage during different phases of the study of Wetland 1, covered by Helosciadum nodiflorum, and Wetland 2, covered by Typha latifolia. Red dots indicate the respective sampling points for the inlet to Wetland 1 (I), Wetland 1 (W1), the outlet from Wetland 1 entering Wetland 2 (O1), Wetland 2 (W2), and the outlet from the constructed wetland system (O2).
Each of the wetlands is a raft of parallelepiped shape, with the bottom covered by a layer of vegetal substrate overlying another layer of gravel of varied granulometry. The Wetland 1 area is around 333 m2, whereas that of Wetland 2 is 201 m2. The main constructing and operational features of the studied wetlands are shown in Table 1. Previously, both of the wetlands were covered by Typha latifolia, but this was displaced in the vertical subsurface flow Wetland 1 by riparian plants typical from nearby streams. This way, during the studied period, Wetland 1 was commonly covered by a continuous bed of Helosciadum nodiflorum (formerly Apium nodiflorum), a riparian plant which had not commonly been used in CW so far for the wastewater purification. Contrastingly, Wetland 2, which presented a horizontal subsurface flow, was still covered by Typha latifolia during the studied period, this plant commonly being used in wastewater treatment (Figure 1).

Table 1.
Main constructing and operational features of the studied wetlands.
The studied period was divided into four different phases, where the operational setting was different, as follows:
- Both of the wetlands (Wetland 1 and 2) were operating simultaneously, for two months, in late autumn (up to six sampling events).
- Wetland 1 was operating normally with its Helosciadum nodiflorum cover, whereas Wetland 2 was closed and the Typha cover was pruned. The length of phase 2 was around three months, from the beginning of winter to early spring (up to 10 sampling events).
- Wetland 1 was closed and Helosciadum was pruned, whereas Typha grew in Wetland 2 which was nearly operating normally at the end of this period. Phase 3 took place for around two months, from early to late spring (up to six sampling events).
- Both of the wetlands (Wetland 1 and 2) were operating sequentially. Although Helosciadum was growing slowly in Wetland 1, Wetland 2 was covered by healthy Typha and was operating normally. Phase 4 lasted for four months, during summer and early fall (up to 19 sampling events).
Thus, both of the wetlands were operative during Phases 1 and 4, whereas only Wetland 1 operated during phase 2, and only Wetland 2 operated during phase 3. The meteorological and plant conditions during the different phases are given in Supplementary Table S1.
2.2. Control Parameters. Sampling, and Analytical and Statistical Procedures
Five different sampling points were established for all of the studied variables, as follows: (i) I—Inlet to Wetland 1 from the WWTP; (ii) W1—inside Wetland 1; (iii) O1—Outlet from Wetland 1 to Wetland 2; (iv) W2—inside Wetland 2 and; (v) O2—Outlet from Wetland 2 to Rambla Castellana. Some of the variables were determined in situ or in the lab immediately after sampling, whereas the samples for the determination of some other variables were processed prior being stored, when possible, then were analysed later.
Conductivity and oxygen were measured weekly in situ, with a Hach Intellical CDC401 conductivity probe and a Hach Lange oximeter that was equipped with a LDO101 probe, respectively. For the determination of the concentrations of total nitrogen (TN) and total phosphorus (TP), the biological oxygen demand (BOD5), and the chemical oxygen demand (COD), the sampling points were screened weekly. For the measurement of the concentrations of the dissolved forms of nutrients (nitrate, ammonium, and phosphorus), as well as of chlorophyll-a concentration, dissolved organic matter (DOC), chromophoric dissolved organic matter (CDOM), and fluorescent organic matter (FDOM), the sampling points were screened bi-weekly. In both of the wetlands, the subsurface water was sampled from PVC tubes (piezometers). All of the containers that were used for sampling were rinsed several times with sample water before being filled. For the analyses of dissolved inorganic nutrients, DOC, CDOM, and FDOM, water samples were filtered through Whatman GF/F glass fibre filters.
The suspended solids were measured by filtering a volume of water through Whatman glass microfiber filters, grade 934-AH, and by heating them in a furnace at 105 °C for 2 h so as to obtain the dry weight. Alkalinity was measured after titration with sulphuric acid, to the equivalence end point pH. The analyses of nitrate, ammonium, and soluble reactive phosphorus were made using standard colorimetric methods [16]. Total N and total P were determined from unfiltered samples, after persulphatic-acid hydrolysis at 150 °C for 2 h. This procedure hydrolysed any N- and P-forms to nitrate and orthophosphate, respectively. These concentrations were measured following standard methods using spectrophotometric determination, as described above [16]. The concentration of DOC was analysed on a Shimadzu (Tokyo, Japan) TOC-VCSH analyser using potassium hydrogen phthalate as a standard. The biochemical (BOD5) and chemical (COD) oxygen demands were also measured following standard methods [16].
Spectrophotometric analyses of the dissolved organic matter (DOM) were conducted with samples that were acclimated at room temperature. The absorption spectra were obtained between 200 and 750 nm on a Beckman DU-700 spectrophotometer, using a 1 cm path length cuvette. The background was removed by subtracting the spectrum of ultrapure distilled water. The Napierian absorption coefficient at all of the obtained wavelengths was obtained by applying the following equation: a(λ) = 2.303 A/L; where, a(λ) is the absorption coefficient (m−1) at each wavelength, A is the absorbance at the wavelength λ, and L is the cuvette length in meters. With the obtained data, the spectral slopes were also determined for the intervals of 275–295 nm (S275–295) and 350–400 nm (S350–400), by fitting the absorption spectra to a linear regression on log-transformed spectra, following Helms et al. [17]. These slope coefficients represent a measure of how the absorption decreases with respect to wavelength. The slope ratio (Sr) was calculated as the ratio of S275–295 to S350–400. Slopes S275–295 and S350–400 are used as surrogates of DOM for low and high molecular weight, respectively [17].
The DOM fluorescence parameters were acquired from the excitation–emission matrices (EEMs), which were performed with an F-7000 Hitachi fluorescence spectrophotometer, following Coble [18]. The EEMs consisted of a series of emission scans (240–600 nm) that were collected over excitation wavelengths that ranged from 240 to 450 nm, by 5 nm increments. The bandwidth was set to 5 nm for both the excitation and emission. The water Raman scattering effects were removed by subtracting Milli-Q water fluorescence. The spectra corrections were performed following the specifications of the spectrophotometer manufacturer. The fluorescent components were identified and labelled, as previously described [18]. For the humic-like substances, a proxy of aged DOM, the maximum fluorescence signal at Ex/Em wavelengths of 250–260 nm/380–480 nm was defined as the peak FDOM-A, and that at 330–350 nm/420–480 nm was defined as the peak FDOM-C. For the protein-like substances, which are fresh DOM, the maximum fluorescence signals at Ex/Em wavelengths of 270–280 nm/320–350 nm were defined as the peak FDOM-T.
The bacterial numbers were acquired by flow cytometry using a Beckman Coulter flow cytometer (Cytomics FC 500 MPL), which was equipped with five fluorescent channels and two lasers emitting at 488 nm (argon laser) and 635 nm (red emitting diode). The procedures for sample preparation and the setting of the cytometer parameters are detailed elsewhere [19]. The cells were stained with SYBR Green-I (Molecular Probes), a regular stain that is used to detect double-stranded DNA, and the samples were run in the cytometer after 30 min of incubation in the dark. The bacterial cells were visualized during the acquisition by plotting the side scatter light (SSC), which is a proxy of the cell size, versus the green fluorescence (FL1) channel. In order to refine the analysis, the ratios of the green (FL1) versus red (FL4) fluorescence channels were also plotted, so as to discriminate the bacteria from cytometric noise. Fluorescent beads of 1 μm in diameter were used as size markers. This analysis allowed the separation of two main subpopulations of bacteria that were distinguished, depending of their green fluorescence intensity, being proportional to their DNA content. We referred to these as high DNA (HDNA) and low DNA (LDNA) bacteria. Given that the green fluorescence is related to the apparent nucleic acid content per cell, we assumed here that the HDNA subpopulation was metabolically more active and constituted the dynamic fraction of the whole bacterial assemblage [20]. The cell numbers were then obtained by considering gravimetrically the processed volume.
The yield in the removal of the pollutants or the changes in other water variables when passing through each wetland was calculated as a percentage, which compared the value of each variable in the wetland inlet to that in the wetland outlet. The overall yield of the whole wetland treatment was also calculated as a percentage, which compared the values of the studied variables in the inlet (I) of Wetland 1 to those of the outlet of Wetland 2 (O2). Mann–Whitney U-tests, with significance levels of 0.05, were used to compare the yields that were obtained among both of the wetlands, when they were simultaneously operating (Phases 1 and 4).
3. Results
The effect of both of the wetlands on the waters’ physical, chemical, and biological features differed throughout the different phases of the study, and was influenced by the operational mode. Some of the waters’ physical and chemical variables did not vary significantly when passing the wetlands, such as the water pH and electrical conductivity, whereas others showed significant variations (Supplementary Figure S1). The dissolved oxygen concentrations in the outlet of the WWTP (I, inlet to Wetland 1) were usually comprised between 1.7 and 2.2 mg L−1, although they occasionally dropped to values around 1 mg L−1, or increased to maximum values in Phase 4 that reached up to 3.7 mg L−1. The dissolved oxygen concentrations in the outlet of Wetland 1 (O1) commonly dropped slightly, to values around 1.6 mg L−1, and this decrease made a significant difference (p < 0.001) in the behaviour of the dissolved oxygen in Wetland 2 compared to Wetland 1, as in Wetland 2 they slightly increased to averages of around 2 mg L−1, especially in phase 4. Concerning the pH, it was slightly alkaline, although no statistically significant changes were found in the pattern that was shown by both of the wetlands (p = 0.843). The alkalinity was mostly maintained in Wetland 1, with respect to its inlet (I), and decreased slightly (2–17%) when passing through Wetland 2. Among the possible comparisons of the co-operational phases (1 & 4), this drop was significantly higher in Wetland 2 compared to Wetland 1 only in phase 4 (p = 0.007), when Wetland 2 was operating under optimal conditions. The water electrical conductivity was highly variable and fluctuated in the outlet (I) of the WWTP that entered Wetland 1, from 430 to around 1000 μS cm−1 and, as a general pattern, it dropped slightly (up to 6% maximum) in the transit through both of the wetlands, but without significant differences of the decreasing pattern when both of the wetlands were compared (p = 0.435). The removal effect on suspended solids, however, fluctuated very much in Wetland 1, since they increased in phase 1, whereas they decreased between 31% (phase 4) and 42% (phase 2). Contrastingly, the suspended solids were continuously removed in Wetland 2, with removal percentages of around of 9% (phases 3 and 4) or even higher (20% in phase 1), but the contrasting variation pattern among both of the wetlands was statistically significant (p = 0.015) only in Phase 1, when Wetland 2 reduced the suspended solids by 20%, whereas they increased by around 50% in Wetland 1. Nevertheless, the CW system generally contributed to the suspended solids removal. In summary, among these parameters, the combined effect of both of the wetlands contributed to a slightly decreased salt content, as it was revealed by the consistent decrease of conductivity, to a small drop in alkalinity and, especially, to the removal of the suspended solids, whereas both the dissolved oxygen and pH were mostly similar after the transit through the whole wetland system.
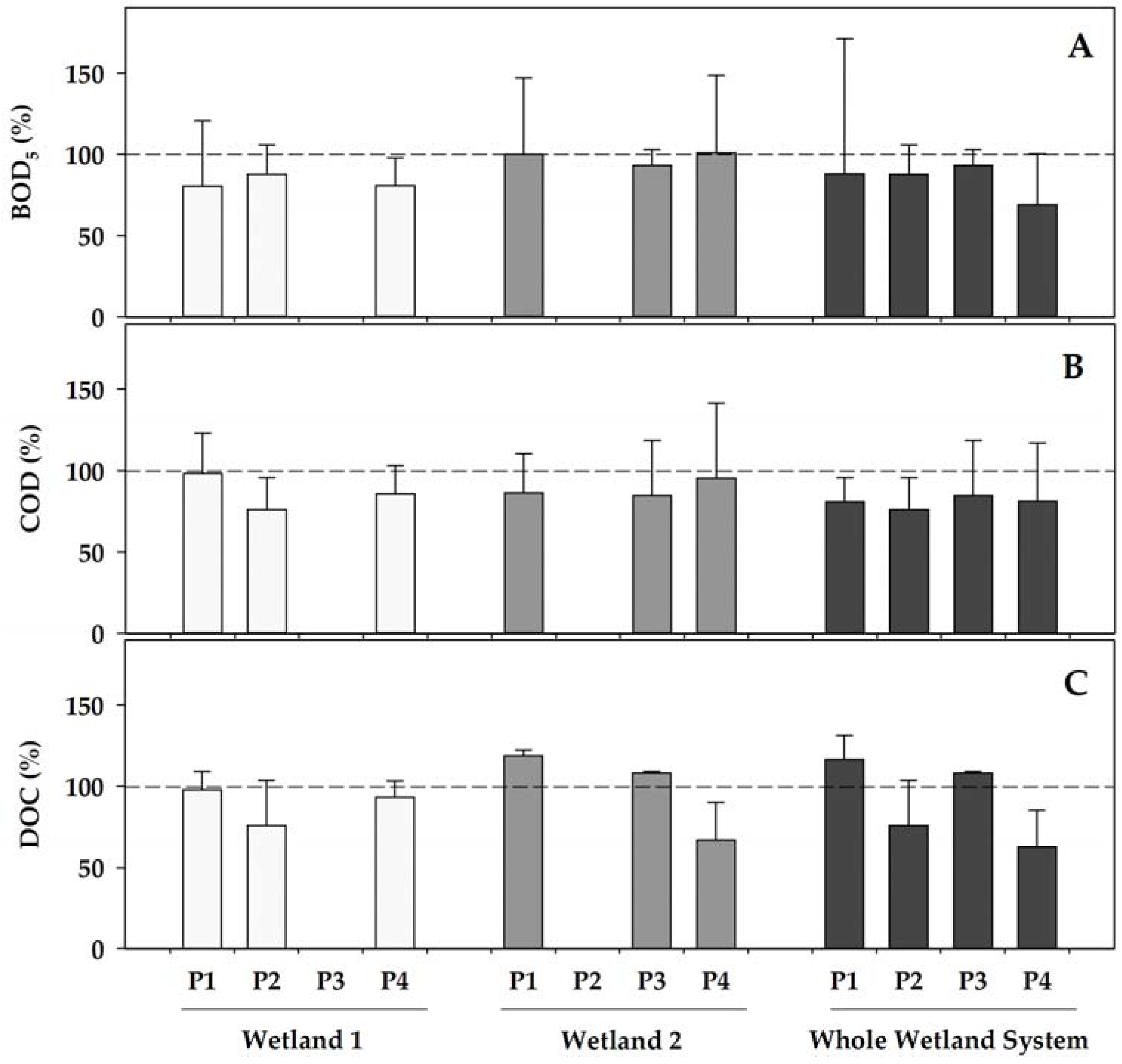
As a general pattern, both of the wetlands contributed to a further increase in the removal of the organic matter that was already initiated by the WWTP (Figure 2). When operating (phases 1, 2, and 4) the concentrations of the indicators of organic matter were reduced in Wetland 1 by 13–20% (for BOD5), by 2 to 23% (for COD), and by 2 to 24% (for DOC), with the highest decreases generally occurring in phase 2, when Wetland 1 was fully operational with a healthy coverage of Helosciadum nodiflorum, and the temperatures became the highest during the two fully operational initial phases (phases 1 and 2) of Wetland 1. Contrastingly, Wetland 2, which was covered by Typha latifolia, showed a more erratic pattern when operating, with small reductions (maximum 7% in Phase 3) in the BOD5, decreases in the COD in all of the operating phases, from 5 to 15%, whereas the DOC increased by 8–16% in phases 1 and 3, but decreased by 33% in phase 4. Among these, only the highest DOC reduction in Wetland 2 compared to Wetland 1 in phase 4 was statistically significant (p = 0.026) when comparing both of the co-operational phases (phase 1 and 4). In any case, the water organic content was reduced through its sequential transit over the combined constructed wetland showing total reductions ranging 7–30% for BOD5, 15–24% for COD, and up to 37% for DOC.
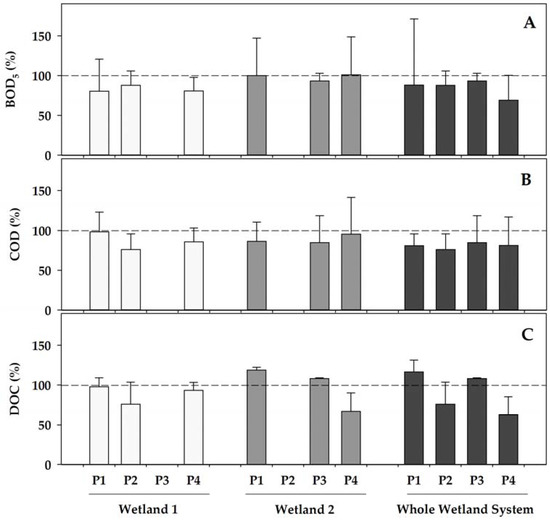
Figure 2.
Percentage variations in different water features after passing Wetland 1 (left), Wetland 2 (centre), and the overall constructed wetland (right), compared to each of their inlets at each of the operational phases (P). The yield in the removal of pollutants was calculated as a percentage, comparing the value of each variable in the wetland inlet to that in the wetland outlet. (A) BOD5—biochemical oxygen demand; (B) COD—chemical oxygen demand; and (C) DOC—dissolved organic carbon. Data represent the mean (bar) and the standard deviation (whisker). Dashed line represents 100%, this is, equal concentration in the inlet as in the outlet. Wetland 1 was inoperative during phase 3, whereas Wetland 2 was inoperative during phase 2.
Among the fractions of the chromophoric dissolved organic matter (CDOM), the most noticeable reductions, although modest, occurred for the CDOM with a high molecular weight (S350–400), particularly in phase 4, when, as mentioned previously, Wetland 2 operated more efficiently in removing the dissolved organic carbon (Figure 2C). During this period, the average decrease in the value of S350–400 that was attributable to this wetland was slightly above 10% (Supplementary Figure S2). However, there was no statistically significant differential reductions for any of the wetlands in any of the phases (p = 0.317). On the other hand, no significant differences (p = 0.744) in the transformation patterns were observed between Wetland 1 and Wetland 2 when comparing the changes for the fraction of low molecular weight (S275–295) during the co-operating phases. Consequently, the changes of the Sr ratio mainly mimicked the variations of the high molecular weight CDOM.
With respect to the fluorescent fraction of the dissolved organic matter (FDOM), although representing a limited fraction of the total DOM, higher dynamisms appeared. The FDOM of types A and C, which are attributable to the presence of humic and fulvic acids, showed a clear increase during the earlier period (Phase 1), which eventually might have come from the leaching of the wetlands ground (Supplementary Figure S2). On the contrary, from phase 2, the organic matter of these types that was entering the wetland system, particularly that of type C, began to be effectively removed, with maximum decreases close to 40% in Wetland 1 during phase 2, and around 25% for Wetland 2 in phase 4. This increased efficiency coincided with a progressive increase of the percentage of the bacteria with high DNA content (%HDNA). Nevertheless, the pattern of reduction for the humic types of FDOM was not significantly different for both of the wetlands, neither for type A (p = 0.423) nor for type C (p = 0.852). In parallel, the maximum removal of the FDOM of type T, which predominantly derives from tryptophan and tyrosine, thus indicating the presence of proteins, also reached its maximum in Wetland 1 during phase 2, with nearly 41% removal. Wetland 2, when operating, also removed efficiently this fraction of FDOM-T, especially in the warmer phase 4, when the removal reached up to 31% and was significantly higher (p = 0.013) for Wetland 2 than that of Wetland 1.
Overall, the higher organic matter removal capacity of Wetland 1, according to all of the parameters that were related to the organic matter, was then achieved in phase 2, when this wetland was fully operative with a healthy cover of Helosciadum nodiflorum. Contrastingly, most of these parameters showed a higher removal efficiency in phase 4 for Wetland 2, when the health status of its Typha latifolia cover was optimum, with the plants actively growing under warmer temperatures.
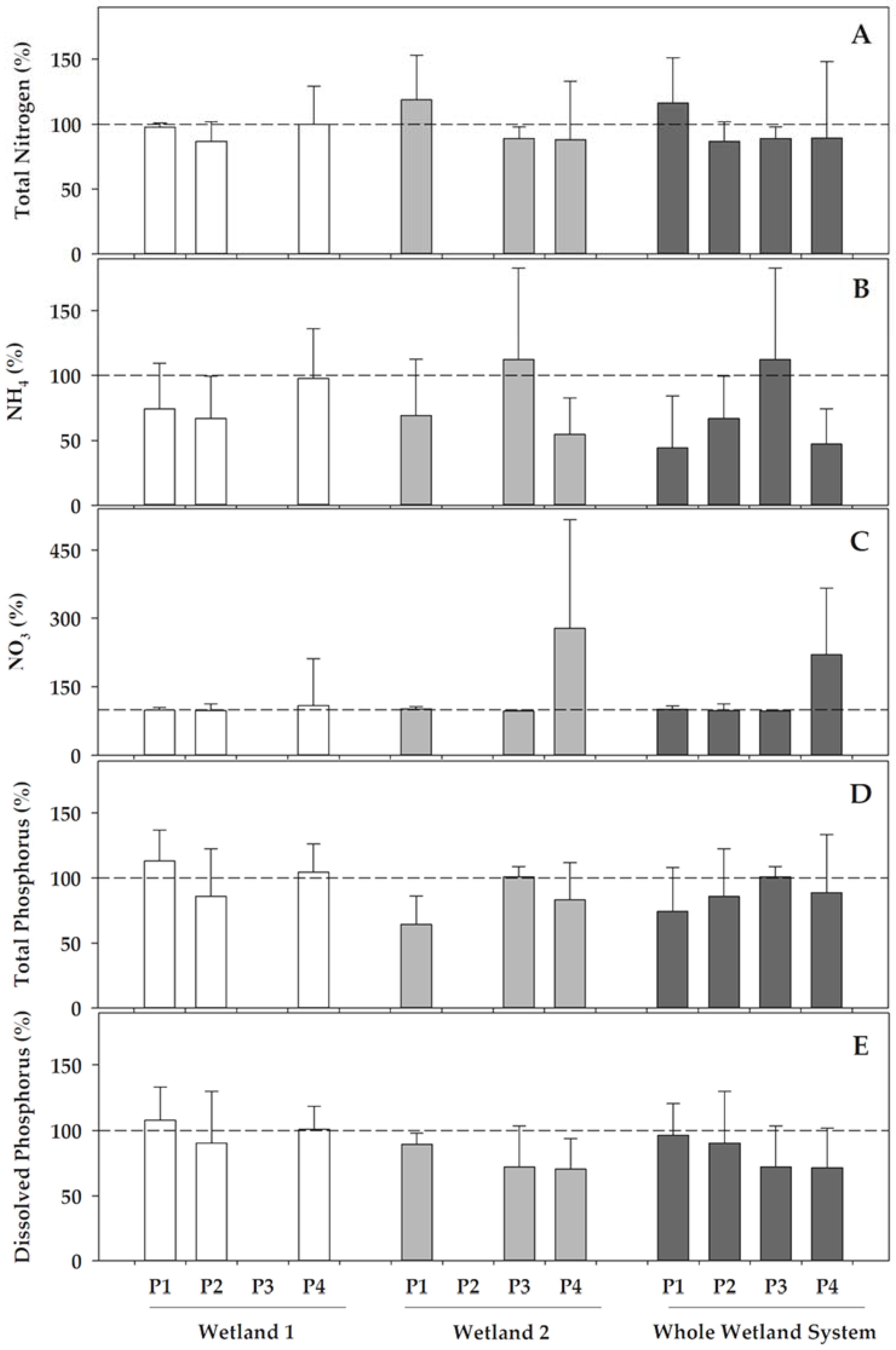
With regards to the nitrogen and phosphorus compounds, the studied constructed wetlands not only retained a part of these nutrients, but also influenced redox transformations (Figure 3). Concerning the nitrogen compounds, the most remarkable effect for both of the wetlands was that they generally favoured the ammonium nitrification, and this resulted in remarkable decreases of ammonium concentrations compared to the inlet (I) from the WWTP, which ranged 3–33% in Wetland 1 and 30–45% in Wetland 2, except, for the latter, in phase 3, where the ammonium did not decrease in the recently re-operating Wetland 2. The nitrification processes resulted in increases in the nitrate concentrations in water when passing through wetlands, although with a different behaviour in both of the wetlands. In Wetland 1, the denitrification should have occurred in phases 1 and 2, as the increase in nitrate concentration did not yet cover the decrease in ammonium, which was also reflected in the decrease of the total nitrogen, which ranged from 3 to 13% in these phases. Contrarily, the total nitrogen almost maintained its concentration in phase 4 in Wetland 1, and most of the ammonium was transformed into nitrate. Wetland 2 also presented nitrification, with ammonium decreases ranging from 30% and 45% in phases 1 and 4, respectively, although it did not decrease in phase 3, when Typha latifolia was recently re-growing after the biomass withdrawal. In Wetland 2, part of the ammonium was nitrified in phase 1, but there was also an additional increase in the concentration of the total nitrogen compared to the water entering this wetland. Similarly, ammonium decreased in phase 4 and nitrate increased concomitantly, but the decreases in total nitrogen also revealed that part of the nitrogen could have been denitrified. When compared between both of the wetlands, denitrification was only significantly different (p = 0.026) when it was linked to the higher reduction of the total nitrogen in Wetland 1 during phase 1. Contrastingly, nitrification, as deduced from the transformations of ammonium into nitrate was, in general, much more active in Wetland 2 during phase 4 than in Wetland 1 (p = 0.007 for ammonium reduction and p = 0.019 for nitrate increase).
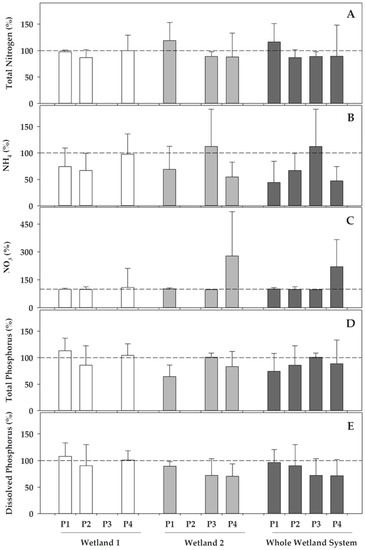
Figure 3.
Percentage variations in different water features after passing Wetland 1 (left), Wetland 2 (centre), and the overall constructed wetland (right), compared to each of their inlets at each of the operational phases (P). The yield in the removal of pollutants was calculated as a percentage, comparing the value of each variable in the wetland inlet to that in the wetland outlet. (A) Total nitrogen, (B) ammonium, (C) nitrate, (D) total phosphorus, and (E) soluble (dissolved) reactive phosphorus. Data represent the mean (bar) and the standard deviation (whisker). Dashed line represents 100%, this is, equal concentration in the inlet as in the outlet. Wetland 1 was inoperative during phase 3, whereas Wetland 2 was inoperative during phase 2.
Although the nitrogen was transformed and removed in Wetland 1, the phosphorus concentrations, both as orthophosphate and total phosphorus, were not reduced significantly in this wetland, but were instead varying with an unclear pattern, either increasing or decreasing compared to its the inlet (I) from the WWTP (Figure 3). In contrast, Wetland 2 remarkably reduced both total P (17–35%, except in phase 3, when it maintained on average the concentrations entering this wetland), and orthophosphate (12 to 30%), when comparing its outlet (O2) with its inlet (O1). Overall, Wetland 2 was much more efficient in phosphorus removal than Wetland 1, both for the total phosphorus (p = 0.001) and soluble orthophosphate, and accounted for most of the P-removal of the CW system.
Except for phase 1, both of the wetlands generally promoted a decrease in bacterial abundance, which was reduced by 15–50% in Wetland 1, and by 13–23% in Wetland 2. Concerning the bacterial activity, which was obtained from the relative abundance of the more active (HDNA) to less active (LDNA) bacteria, the relative abundance of the HDNA versus LDNA cells increased in all of the phases by 10 to 37% in Wetland 1 and by 7 to 65% in Wetland 2. Both trends were solid, and were not significantly different when comparing both of the wetlands (p = 0.394 for bacterial abundance, and p = 0.769 for the relative contribution of HDNA bacteria).
4. Discussion
The release of the used waters into the environment requires effective treatments that could reduce the amount of pollutants in such a way that they cause no degradation to the receiving water bodies [21]. However, even when these pollutants could have been reduced on a conventional WWTP, the physical, chemical, and biological features of these waters might differ very much to those from the receiving aquatic ecosystems [22,23], which strengthens the need to consider the role of constructed wetlands, not only as systems helping to reduce the amount of pollutants, but also, when these had already been reduced to acceptable levels, as driving these water features to others that are more similar to those of the receiving natural environment. Our work was conducted in a small constructed wetland that is annexed to a wastewater treatment plant (WWTP), the latter being efficient for pollutant removal. , Nevertheless, the conventional WWTP pours water of very different characteristics to those that circulate through the environment, in this case, a Mediterranean stream where the natural flows are very low (a few hundred litres per second, except during occasional storms) and, consequently, the effect of the treated waters on the environmental features could be strong. Our study provides evidences on how the ‘naturalization’ processes of the already treated waters benefits the receiving environment by decreasing the physical, chemical, and biological differences of the poured waters, compared to the small flowing waters of the receiving stream. These processes, for example, favour shifts to more oxidised compounds of certain elements (e.g., nitrogen), further degrading the dissolved organic matter towards more recalcitrant and inactive forms, and lowering bacterial loads. This type of studies, which is relatively uncommon on semiarid environments such as those of the Mediterranean basin, help by providing guidelines to avoid further deterioration of aquatic ecosystems, which is enforced by environmental laws such as, for Europe, the Water Framework Directive.
The studied system consists of two serial subsurface flow wetlands, one vertical and one horizontal, both of which contained different aquatic plant species (Helosciadium nodiflorum in Wetland 1 and Typha latifolia in Wetland 2). Apart from nicely illustrating the naturalization process of the already treated wastewater, the main novelty of our study was the use of H. nodiflorum, a native macrophyte that regularly appears in the streams of Europe. This plant shows a large standing biomass in rivers, even when temperatures and nutrients content are low [24], which makes it suitable for the water naturalization processes, even during the colder months under a Mediterranean climate. This naturalization consisted mainly in both the removal and the transformation of the remaining pollutants. As examples of the most remarkable effects on water renaturalization, the combined action of both of the wetlands allowed for the reduction of BOD5 from averages close to 9.5 mg O2 L−1 in the effluent of the WWTP (I) to averages of 4.8 mg O2 L−1 in the outlet of the CW system (O2), which are closer to the typical averages of the receiving streams (2.2 mg O2 L−1) in the area, according to public data available from the Natura 2000 monitoring network (http://www.agroambient.gva.es/documents/91061501/109939278/Evaluaci%C3%B3n+de+los+Datos+de+Calidad+de+Aguas+para+el+Seguimiento+de+Masas+de+Agua+Fluvial+en+los+Espacios+Red+Natura+2000/f9101f01-d819-4468-a6b7-e4fe823b6f08). Similarly, other pollutants, such as the suspended solids, dropped from higher concentrations in the WWTP effluent (8.4 mg L−1) to values that were even lower in the CW outlet (3.5 mg L−1) than those that are typically averaged in the receiving streams (5.8 mg L−1). These and other positive effects of the CWs treatments minimised the impacts of the poured treated wastewaters on the receiving stream.
Any interpretation of the results that were obtained here need to bear in mind the different phases of the study. Only two of the four studied phases (1 and 4) were co-operative for both of the wetlands. In phase 1, in the fall, the temperatures were still relatively mild during the first weeks, so the temperature dependent processes were still quite operative. However, the healthy status of the H. nodiflorum in Wetland 1 contrasted with the beginning of the autumnal senescence of T. latifolia in Wetland 2, which implied a higher operational status of Wetland 1. This wetland, which was covered by H. nodiflorum, was still fully operative (Figure 2 and Figure 3), even under the colder conditions at the end of phase 1 and the beginning of phase 2 (Supplementary Table S1). This supports one of the main discoveries of our study, which is the possibility of using the native plant H. nodiflorum for wastewater treatment during winter periods, when the other plants that are typically used in constructed wetlands cannot be operative because they remain in a vegetative status. The T. latifolia cover of Wetland 2 was pruned in phase 2, and this wetland was closed, so only Wetland 1 was operating during this phase. On the contrary, Wetland 2 was re-opened in phase 3 and T. latifolia started to grow, although slowly, whereas the H. nodiflorum cover in Wetland 1 was pruned and this wetland was closed. Later, in phase 4, both of the wetlands were co-operating, but the H. nodiflorum cover had just started to recover in Wetland 1, whereas Wetland 2 was already recolonized by a healthy and dense T. latifolia cover. This implies that, comparatively, the operational status of the latter was healthier than that of H. nodiflorum in Wetland 1, which coincided with the highest (summer) temperatures (Supplementary Table S1). Additionally, the higher hydraulic load that was experienced by the smaller Wetland 2 (Table 1) implies that the reductions in the pollution loads that were achieved in this wetland were relatively higher at equal values, when compared to those of Wetland 1 in terms of areal efficiency, although the T. latifolia cover of Wetland 2 was quite inefficient during the colder periods. Overall, our results also demonstrate the complementarity of both plants that were used in our wetland system, with Wetland 1 covered by H. nodiflorum being more capable of treating the WWTP during the colder months, contrasting with Wetland 2 covered by T. latifolia being more efficient during the warmer period. This makes an important advance in overcoming one of the main problems of the constructed wetlands that are used in wastewater treatment, namely its much lower efficiency in colder months because of the low operational status of the typically used plants.
Despite the strong reduction, the waters that were treated in the WWTP still contained higher levels of certain pollutants than those of the clean water that was previously taken from the environment that was to be used by population. This includes, for example, higher levels (and different relative amounts) of inorganic nitrogen and phosphorus forms, which are primary nutrients promoting eutrophication. For sure, the nutrient loads that entered the constructed wetland system from the WWTP of Higueruelas were not very high compared to those where the wastewater has not been so intensively treated [25]. However, these waters still showed the characteristic composition of effluents of urban wastewaters, with, for example, a relatively high concentration of ammonium and soluble phosphorus. Thus, the main function of this wetland system would be to naturalize the effluent, discharging in a small Mediterranean stream located in a mountain area, which is a sub-tributary of the Túria River, the main fluvial axis of the region. To this respect, the huge removal of ammonium and suspended solids in the constructed wetland system, if compared with the WWTP effluent, although being variable over time depending on the growth of the plants, equalled or was even higher than that shown by other studies that were conducted in south Europe aiming to recover the natural features of streams, which also involved the use of constructed wetlands as tertiary treatments. For example, Huertas et al. [26] described the use of recycled water for stream flow augmentation, which, as in our case, has the potential to improve the stream habitat in water courses suffering strong water abstractions, particularly in the Mediterranean region (East of Iberian Peninsula), where renewable water use is an important concern.
The design of the wetlands (either vertical or horizontal), together with the choice of the aquatic plant in each wetland, may be the main factors in determining the yields of nutrients and organic matter reduction, in such a way that a combination of horizontal and vertical flow wetlands could remove up to 90% of the organic load, and of the total N and P of the untreated sewage [11,27]. In addition, the horizontal flow wetlands have an advantage in long-term P-removal, because P is bound to organic substances [28]. Concerning phosphorus, we also found a similar behaviour, with most of the phosphorus removal occurring in the horizontal flow Wetland 2, and with no consistent P-removal in the vertically flowing Wetland 1, where the newly used of H. nodiflorum did not significantly contribute to a significant increase in phosphorus removal. The higher phosphorus reductions occurred in Wetland 2, during the periods of the healthier growing of Typha latifolia. This is also consistent with other studies, which also showed the role on P-removal involving this species [29,30]. However, P-retention by plants could not be the most effective option, since aquatic plant biomass may become P-saturated after a relatively short time [28], or it might even become an important P-source of after plant senescence [31]. By contrast, the P-retention capacity could be determined by the adsorption of phosphorus to soil particles, or by chemical precipitation, with either inorganic (i.e., Ca, Al, and Fe) and organic compounds, as previously mentioned. The calcareous regional lithology, providing water in the area with huge amounts of dissolved Ca, suggest that P bound to Ca was likely a prevailing form for P-retention in the soils of the studied CW.
Nitrification occurred in both of the wetlands, but with a higher transformation of ammonium into nitrate in Wetland 1 when both of the wetlands were operating simultaneously under similar operative conditions (Phase 1). This also agrees with the higher nitrification rates that were generally reported for vertical flow subsurface wetlands, compared to those with horizontal flow [32], which, in our case, is also demonstrated when the riparian H. nodiflorum is newly used. Additionally, both of the wetlands were capable of efficiently removing nitrogen through denitrification, but Wetland 1, especially in phase 1, presented higher removal rates. In this wetland, the anaerobic consumption of the remaining organic matter using nitrate (both that coming from the WWTP and the newly created by nitrification in the upper wetland layers) as an electron acceptor [33], was favoured by the lower oxygen content in phase 1. In this co-operating period, only Wetland 1 was capable of nitrogen removal through denitrification. However, during the other co-operative period (Phase 4), Wetland 2, which was then more healthfully operating than Wetland 1, was more efficient than Wetland 1 in removing nitrogen, although nitrification was the dominating nitrogen pathway in this horizontally flowing subsurface wetland covered by T. latifolia. Nevertheless, the removal of ammonium by nitrification, with further partial denitrification to remove nitrogen from the poured waters, occurred in both of the wetlands when each was fully functional. This also enhances the importance of the novelty of our study in showing the utility of H. nodiflorum as a plant that can be usable in treatment wetlands.
As a general pattern, the studied CW system contributed to further enhancing the removal and transformation of organic matter that was already performed by the WWTP, with the removal in the wetlands system reaching up to 40% (depending on the studied variable) of the organic loads that were already reduced to low levels by the WWTP. The changes that were observed in the optical properties of organic matter might shed light on these transformations. With regards to the colourless dissolved organic matter (CDOM), most of the variability was found for the fraction of high molecular weight, which was parametrized by the slope between wavelengths of 350 and 400 nm (S350–400), when compared to that of the low molecular weight (S275–295), which indeed remained almost unchanged. The increases of S275–295 values are typically related with photodegradation processes, which cause bond cleavage and/or the formation of photoproducts with a low molecular weight [34]. Accordingly, the limited exposure of wastewater to light in the studied wetland system would explain the minimal variations that were observed for this DOM fraction. A high exposure of DOM to sun light would have caused a shift from high to low molecular compounds that distinctly would yield an increase of Sr values, which did not occur in our case. The decreases that were observed during some of the periods in the slopes at the higher wavelength range (S350–400) seem to be, however, more related to the biodegradation processes, as also indicated by the net loss of DOC, COD, and BOD5 that were observed during these periods. Instead, the observed net increment in the outlet of the S350–400 values in phase 2 might have been caused by the increase of the lignin derived from vegetation, which was likely originating from the H. nodiflorum that was present in Wetland 1, which experienced a slightly less healthy status during the winter period.
The reductions of the organic matter loads were also visualized by the concomitant reductions in the fluorescent DOM compositional indicators (FDOM). In our study, these fluorescence parameters displayed net average decreases of around 40%, starting from phase 2. The fluorescence signals of type A and C denote the presence of humic-like substances, and the fluorophores of the type T are indicative of the occurrence of aromatic amino acids (i.e., proteins) and/or undegraded polyphenols in the sample [34]. The former are then proxies of more recalcitrant DOM, whereas the signal of type T indicated a fresh-like (i.e., labile) DOM. In our case, the ratio between the fluorescence peaks of type A and type T decreased slightly along the studied period, from mean values in phases 1 and 4 of 8.2 and 6.4, respectively, which would have accordingly indicate greater aromatic content in the initial DOM pool.
Both of the wetlands promoted notable decreases of bacterial abundances, which thus indicate a good capability to act as biofilters. The physical retention of the bacteria through the filtration substrate may explain these reductions, as well as the low survival rates of the allochthonous microbes that were entering with the wastewater. These might have included pathogens as well as the bacteria from the activated sludge, which had a low functional relation with the wetland environment [35]. Additionally, the retention of bacteria by filtration can be improved with the presence of plants in the wetland [36], which likely occurred in our studied system. Nevertheless, the reduction of the total bacterial numbers in the studied CW reached up to 80% in some periods, which indicates removal efficiencies that were comparable to other subsurface flow constructed wetlands [37]. Interestingly, and despite this net reduction in the bacterial numbers, the wetland performance enhanced the microbial activity of the remaining or newly growing community, as indicated by the increase of the proportion of the bacterial cells with a high DNA content [20,38]. This demonstrates the high degradative capacity of the pollutants by the wetland’s microbiota. Several studies indicated that microbial activity is higher in the subsurface (vertical flow) systems compared to the surface flow systems [39], which agrees with observations that showed how the wetland design mainly determines the activity and structure of the microbial assemblage, rather than the characteristics of the wastewater influent [40]. This is a promising finding in our study, as it indicates that the microbial community that was leaving the wetland was not largely influenced by the composition of its inlet, but it could still play its role in pollutants removal. Future research would then be related to the molecular and functional diversity of the microbial community involved in the tertiary treatment.
The role of aquatic plants in constructed wetlands has been widely studied [9,41], although the knowledge on the relative relevance of the species used has always been more limited [42]. Vymazal [13] reported an exhaustive revision of the aquatic plants that are used in constructed wetlands, some of them, such as the species of genera Typha, Scirpus, Phragmites, Juncus, and Eleocharis, being widely used. Typha latifolia, which grew in our Wetland 2, is one of the most used plants in constructed wetlands, commonly presenting a high efficiency in inorganic nutrient and organic matter removal [29]. However, H. nodiflorum, the riparian plant covering Wetland 1 in our study, was not mentioned in this detailed revision, neither in successive studies, so we could assume that our study represented the first that was conducted with this species in these types of constructed wetlands. Thus, our contributions implied an important step for the basic knowledge to be applied when constructing wetlands for wastewater treatment. H. nodiflorum had been only used sporadically for the reduction of heavy metals in soils [43] and its antimicrobial activity has also been reported [44]. However, it was never demonstrated so far as being usable in treatment wetlands, and even less was known on its capacity for offering good efficiencies under the more problematic winter periods, which enhances the importance of our results. The biomass production of H. nodiflorum in the vertical flow Wetland 1 was greater than that of T. latifolia. This might have responded to a lower capacity of T. latifolia to adapt to the lack of water occurring in the unsaturated vertical system [45]. Additionally, H. nodiflorum is a wild plant that resists moderate pollution levels and relatively cold temperatures, thus being appropriate for the environmental context where the studied CW system is located. In this sense, there is a growing evidence in the importance of selecting native plants species for constructed wetlands to maximize the removal and purification of effluents [10,15] in such way that these benefits could be compromised if the selection of the plants is not appropriate, particularly in monoculture systems [45]. Here we demonstrated how a native plant such as H. nodiflorum, which is well adapted to the climate and local conditions, could reach adequate levels of efficiency for its use in these types of wetlands, with operational advantages and cost reduction. This plant might then have a relevant role in the naturalization of treated waters, especially during periods when most of the plants that are typically used in treatment wetlands cannot be fully operative.
5. Conclusions
In summary, the CW system using different types of subsurface flow, as well as the combination of native riparian plants with others that are more commonly used in treatment wetlands, demonstrated an efficient role in the further removal of pollutants, as well in the equalization of the physical, chemical, and biological features to those of the receiving stream. The organic matter content was reduced, and the remaining content was partially transformed to less bioactive molecules. The concentrations of inorganic nutrients, both nitrogen and phosphorus, were notably reduced, with ammonium being partially nitrified and the resulting nitrate being partially denitrified, and the phosphorus showing lower but also noticeable reductions during some periods. Nitrification is relevant for the naturalization of effluents from urban wastewaters [32,46]. Both of the wetlands were also relatively efficient in removing suspended solids, whereas a strong reduction in the bacterial load was also performed by these CWs. The remaining bacteria, however, presented a high activity index, thus maintaining a high degradative capacity that explained the pollutant-removal capacity of the CW system. However, the relative contribution in the reduction of the pollution loads and the renaturalization of water changed among the wetlands and within the operational phases. While the Typha latifolia wetland was less active in purification during winter, the wetland that was covered by the riparian plant H. nodiflorum performed well under low temperatures, and thus a serial design of contiguous wetlands, of both riparian plants and T. latifolia, could offer a better performance in the naturalization of the WWTP effluents through the year. Hence, these results showed how the combination of several types of plants on wetlands t disposed in series could be complementary in order to reach the naturalization targets of the WWTP effluents at different seasons, achieving the reduction of several pollutants. The sequential chronology of the periods in which each wetland operates optimally, because of the different vegetative cycles of the plant that is used and their contrasting operability under different temperature regimes, enhances the utility of complementing the elected combination of the types of subsurface flow and plant cover, allowing the water naturalization and pollutants removal prior to the water disposal remaining all over the year. In general, the reduction of the pollutants to almost natural levels, as well as the assimilation of other physical, chemical, and biological features to those of the natural environments, alleviates the impacts on the rivers where effluents are poured. In relative terms, the flow of these effluents is important in basins with a chronical water deficit, as it is the case of the Mediterranean small streams, where these discharges, when not well naturalized, could have negative effects on the sediments, water, and biota downstream the WWTP. The importance of this study lay in the novelty of the use of native riparian plants in treatment wetlands in combination with other classically used plants, and their contribution to the improvement of the quality and naturalization of treated wastewater after conventional intensive processes.
Supplementary Materials
The following are available online at http://www.mdpi.com/2073-4441/10/6/717/s1, Figure S1: Percent variations in different water features after passing Wetland 1 (left), Wetland 2 (centre) and overall constructed wetland (right), compared to each of their inlets at each of the operational phases (P). The yield in the removal of pollutants or change in the variable was calculated as a percentage comparing the value of each variable in the wetland inlet to that in the wetland outlet. (A) pH, (B) electrical conductivity, (C) dissolved oxygen, (D) alkalinity, and (E) suspended solids. Data represent the mean (bar) and the standard deviation (whisker). Dashed line represents 100 %, this is, equal concentration in the inlet as in the outlet. Wetland 1 was inoperative during Phase 3, whereas Wetland 2 was inoperative during Phase 2. n.a. = data not available. Figure S2: Percent variations in different water features after passing Wetland 1 (left), Wetland 2 (center) and overall constructed wetland (right), compared to each of their inlets at each of the operational phases (P). The yield in the removal of pollutants or change in the variable was calculated as a percentage comparing the value of each variable in the wetland inlet to that in the wetland outlet. (A) FDOM-A (humic and fulvic acids), (B) FDOM-C (humic and fulvic acids), (C) FDOM-T (proteins), (D) CDOM of high molecular weight (S350-400), (E) CDOM of low molecular weight (S275-295), and (F) Sr ratio (S275-295/S350-400). Data represent the mean (bar) and the standard deviation (whisker). Dashed line represents 100 %, this is, equal concentration in the inlet as in the outlet. Wetland 1 was inoperative during Phase 3, whereas Wetland 2 was inoperative during Phase 2. n.a. = data not available, Table S1: Meteorological conditions during the different studied phases, obtained from the nearest meteorological station (Chulilla), as well as plant status during the studied phase.
Author Contributions
Conceptualization, A.C., A.P., C.R., A.C.S., C.F., and M.P.; Methodology, A.C., A.P., C.R., D.M., J.M.-L., H.E. and M.P.; Software, A.P., C.R.; Validation, A.C., A.P., C.R., and M.P.; Formal Analysis, A.C., A.P., C.R., A.C.S., C.F., and M.P.; Investigation, A.C., A.P., C.R., D.M., J.M.-L., A.C.S., C.F., and M.P.; Resources, A.C., M.P., T.M. and G.F. Data Curation, A.P., C.R., and M.P.; Writing-Original Draft Preparation, A.C.; Writing-Review & Editing, A.C., A.P., C.R., A.C.S., C.F., and M.P.; Visualization, A.C. and M.P.; Supervision, A.C., M.P., T.M. and G.F.; Project Administration, A.C., M.P., T.M. and G.F.; Funding Acquisition: A.C., M.P., T.M. and G.F.
Funding
This research was partly funded by the Generalitat Valenciana (IVACE, Instituto Valenciano de Competitividad Empresarial) and by the European Regional Development Fund (through the ERDF Operational Programme 2014–2020 for Valencian Community), IMIDCA/2017/12 (program ‘Proyectos de I + D en Cooperación—PIDCOP-CV’), project: ‘Advanced study of constructed wetlands for the improvement of the quality of their effluents and wise management to mitigate climate change’.
Acknowledgments
The authors are indebted to EPSAR (Entidad Pública de Saneamiento de Aguas Residuales de la Comunidad Valenciana), as well as to Diputación de Valencia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Camacho, A.; Peinado, R.; Santamans, A.C.; Picazo, A. Functional ecological patterns and the effect of anthropogenic disturbances on a recently restored Mediterranean coastal lagoon. Needs for a sustainable restoration. Estuar. Coast. Mar. Sci. 2012, 114, 105–117. [Google Scholar] [CrossRef]
- Ferriol, C.; Miracle, M.R.; Vicente, E. Effects of nutrient addition, recovery thereafter and the role of macrophytes in nutrient dynamics of a Mediterranean shallow lake: A mesocosm experiment. Mar. Freshw. Res. 2017, 68, 506–518. [Google Scholar] [CrossRef]
- Camacho, A.; de la Hera, A.; Manzano, M.; Martí-Cardona, B.; Stephan, R.M. Assessment and valuation of wetlands services for their consideration into decision-making. In Management and Protection of Mediterranean Groundwater-Related Coastal Wetlands and Their Services; Manzano, M., Ed.; UNESCO-IHP in the Frame of the UNEP-MAP/GEF: Paris, France, 2015; 214p. [Google Scholar]
- Vymazal, J. Constructed wetlands for treatment of industrial wastewaters: A review. Ecol. Eng. 2014, 73, 724–751. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, J.; Ngo, H.H.; Guo, W.; Hu, Z.; Liang, S.; Fan, J.; Liu, H. A review on the sustainability of constructed wetlands for wastewater treatment: Design and operation. Bioresour. Technol. 2015, 175, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Camacho, A. Indicadores para la evaluación del estado ecológico de los humedales. In Los Humedales Mediterráneos: El contexto Ambiental y Social. Reflexiones para su Estudio y Gestión Eficaz; Viñals, M.J., Blasco, D., Morant, M., Eds.; Fundación Biodiversidad: Madrid, Spain, 2011; 266p. [Google Scholar]
- Dotro, G.; Langergraber, G.; Molle, P.; Nival, J.; Puigagut, J.; Stein, O.; Von Sperling, M. Treatment Wetlands. Biological Wastewater Treatment Series; IWA Publishing: London, UK, 2017. [Google Scholar]
- Vymazal, J. Constructed wetlands for wastewater treatment: A Review. In Proceedings of the Taal 2007: The 12th World Lake Conference, Jaipur, Rajasthan, India, 28 October 2007–2 November 2007; Sengupta, M., Dalwani, R., Eds.; Jaipur, India, 2008; pp. 965–980. [Google Scholar]
- Shelef, O.; Gross, A.; Rachmilevitch, S. Role of plants in a constructed wetland: Current and new perspectives. Water 2013, 5, 405–419. [Google Scholar] [CrossRef]
- Brisson, J.; Chazarenc, F. Maximizing pollutant removal in constructed wetlands: Should we pay more attention to macrophyte species selection? Sci. Total Environ. 2009, 407, 3923–3930. [Google Scholar] [CrossRef] [PubMed]
- Vymazal, J. The use of hybrid constructed wetlands for wastewater treatment with special attention to nitrogen removal: A review of a recent development. Water Res. 2013, 47, 4795–4811. [Google Scholar] [CrossRef] [PubMed]
- Camacho, A.; Picazo, A.; Rochera, C.; Santamans, A.C.; Morant, D.; Miralles-Lorenzo, J.; Castillo-Escrivà, A. Methane emissions in Spanish saline lakes: Current rates, temperature and salinity responses, and evolution under different climate change scenarios. Water 2017, 9, 659. [Google Scholar] [CrossRef]
- Vymazal, J. Emergent plants used in free water surface constructed wetlands: A review. Ecol. Eng. 2013, 61, 582–592. [Google Scholar] [CrossRef]
- Rodriguez, M.; Brisson, J. Does the combination of two plant species improve removal efficiency in treatment wetlands? Ecol. Eng. 2016, 91, 302–309. [Google Scholar] [CrossRef]
- Guittonny-Philippe, A.; Petit, M.E.; Masotti, V.; Monnier, Y.; Malleret, L.; Coulomb, B.; Combroux, I.; Baumberger, T.; Viglione, J.; Laffont-Schwob, I. Selection of wild macrophytes for use in constructed wetlands for phytoremediation of contaminant mixtures. J. Environ. Manag. 2015, 147, 108–123. [Google Scholar] [CrossRef] [PubMed]
- APHA-AWWA-WEF. Standard Methods for the Examination of Water and Wastewater, 21th ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Helms, J.R.; Stubbins, A.; Ritchie, J.C.; Minor, E.C.; Kieber, D.J.; Mopper, K. Absorption spectral slopes and slope ratios as indicators of molecular weight, source, and photobleaching of chromophoric dissolved organic matter. Limnol. Oceanogr. 2008, 53, 955–969. [Google Scholar] [CrossRef]
- Coble, P.G. Characterization of marine and terrestrial DOM in seawater using excitation emission matrix spectroscopy. Mar. Chem. 1996, 51, 325–346. [Google Scholar] [CrossRef]
- Rizzo, C.; Rappazzo, A.C.; Michaud, L.; De Domenico, E.; Rochera, C.; Camacho, A.; Lo Giudice, A. Efficiency in hydrocarbon degradation and biosurfactant production by Joostella sp. A8 when grown in pure culture and consortia. J. Environ. Sci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Lebaron, P.; Servais, P.; Agogue, H.; Courties, C.; Joux, F. Does the high nucleic acid content of individual bacterial cells allow us to discriminate between active cells and inactive cells in aquatic systems? Appl. Environ. Microbiol. 2001, 67, 1775–1782. [Google Scholar] [CrossRef] [PubMed]
- Gasith, A.; Resh, V.H. Streams in Mediterranean climate regions: Abiotic influences and biotic responses to predictable seasonal events. Annu. Rev. Ecol. Syst. 1999, 30, 51–81. [Google Scholar] [CrossRef]
- Rueda, J.; Camacho, A.; Mezquita, F.; Hernández, R.; Roca, J.R. Effect of episodic and regular sewage discharges on the water chemistry and macroinvertebrate fauna of a Mediterranean stream. Water Air Soil Pollut. 2002, 140, 425–444. [Google Scholar] [CrossRef]
- Wakelin, S.A.; Colloff, M.J.; Kookana, R.S. Effect of wastewater treatment plant effluent on microbial function and community structure in the sediment of a freshwater stream with variable seasonal flow. Appl. Environ. Microbiol. 2008, 74, 2659–2668. [Google Scholar] [CrossRef] [PubMed]
- Comte, K.; Fayolle, S.; Roux, M. Quantitative and qualitative variability of epiphytic algae on one Apiaceae (Apium nodiflorum L.) in a karstic river (Southeast of France). Hydrobiologia 2005, 543, 37–53. [Google Scholar] [CrossRef]
- Gagnon, V.; Chazarenc, F.; Kõiv, M.; Brisson, J. Effect of plant species on water quality at the outlet of a sludge treatment wetland. Water Res. 2012, 46, 5305–5315. [Google Scholar] [CrossRef] [PubMed]
- Huertas, E.; Folch, M.; Salgot, M.; Gonzalvo, I.; Passarell, C. Constructed wetlands effluent for streamflow augmentation in the Besòs River (Spain). Desalination 2006, 188, 141–147. [Google Scholar] [CrossRef]
- Molle, P.; Prost-Boucle, S.; Lienard, A. Potential for total nitrogen removal by combining vertical flow and horizontal flow constructed wetlands: A full-scale experiment study. Ecol. Eng. 2008, 34, 23–29. [Google Scholar] [CrossRef]
- Luederitz, V.; Eckert, E.; Lange-Weber, M.; Lange, A.; Gersberg, R.M. Nutrient removal efficiency and resource economics of vertical flow and horizontal flow constructed wetlands. Ecol. Eng. 2001, 18, 157–171. [Google Scholar] [CrossRef]
- Ciria, M.P.; Solano, M.L.; Soriano, P. Role of macrophyte Typha latifolia in a constructed wetland for wastewater treatment and assessment of its potential as a biomass fuel. Biosyst. Eng. 2005, 92, 535–544. [Google Scholar] [CrossRef]
- Chung, A.K.C.; Wu, Y.; Tam, N.Y.; Wong, M.H. Nitrogen and phosphate mass balance in a sub-surface flow constructed wetland for treating municipal wastewater. Ecol. Eng. 2008, 32, 81–89. [Google Scholar] [CrossRef]
- Camacho, A.; Murueta, N.; Blasco, E.C.; Santamans, A.; Picazo, A. Hydrology-driven macrophyte dynamics determines the ecological functioning of a model Mediterranean temporary lake. Hydrobiologia 2016, 774, 93–107. [Google Scholar] [CrossRef]
- Vymazal, J. Removal of nutrients in various types of constructed wetlands. Sci. Total Environ. 2007, 380, 48–65. [Google Scholar] [CrossRef] [PubMed]
- Atlas, R.M.; Bartha, R. Microbial Ecology, 4th ed.; Benjamin Cummings: Menlo Park, CA, USA, 1998. [Google Scholar]
- Hansen, A.M.; Kraus, T.E.C.; Pellerin, B.A.; Fleck, J.A.; Downing, B.D.; Bergamaschi, B.A. Optical properties of dissolved organic matter (DOM): Effects of biological and photolytic degradation. Limnol. Oceanogr. 2016, 61, 1015–1032. [Google Scholar] [CrossRef]
- Vymazal, J. Horizontal sub-surface flow and hybrid constructed wetlands systems for wastewater treatment. Ecol. Eng. 2005, 25, 478–490. [Google Scholar] [CrossRef]
- Vacca, G.; Wand, H.; Nikolausz, M.; Kuschk, P.; Kästner, M. Effect of plants and filter materials on bacteria removal in pilot-scale constructed wetlands. Water Res. 2005, 39, 1361–1373. [Google Scholar] [CrossRef] [PubMed]
- Green, M.B.; Griffin, P.; Seabridge, J.K.; Dhobie, D. Removal of bacteria in subsurface flow wetlands. Water Sci. Technol. 1997, 35, 109–116. [Google Scholar] [CrossRef]
- Gasol, J.M.; Zweifel, U.L.; Peters, F.; Fuhrman, J.A.; Hagstrom, A. Significance of size and nucleic acid content heterogeneity as measured by flow cytometry in natural planktonic bacteria. Appl. Environ. Microbiol. 1999, 65, 4475–4483. [Google Scholar] [PubMed]
- Truu, M.; Juhanson, J.; Truu, J. Microbial biomass, activity and community composition in constructed wetlands. Sci. Total Environ. 2009, 13, 3958–3971. [Google Scholar] [CrossRef] [PubMed]
- Adrados, B.; Sánchez, O.; Arias, C.A.; Becares, E.; Garrido, L.; Mas, J.; Brix, H.; Morato, J. Microbial communities from different types of natural wastewater treatment systems: Vertical and horizontal flow constructed wetlands and biofilters. Water Res. 2014, 55, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Gersberg, R.M.; Elkins, B.V.; Lyon, S.R.; Goldman, C.R. Role of aquatic plants in wastewater treatment by artificial wetlands. Water Res. 1986, 20, 363–368. [Google Scholar] [CrossRef]
- Brix, H. Do macrophytes play a role in constructed treatment wetlands? Water Sci. Technol. 1997, 35, 11–17. [Google Scholar] [CrossRef]
- Vlyssides, A.; Barampouti, E.M.; Mai, S. Heavy metal removal from water resources using the aquatic plant Apium nodiflorum. Commun. Soil Sci. Plant Anal. 2005, 36, 1075–1081. [Google Scholar] [CrossRef]
- Menghini, L.; Leporini, LC.; Tirillini, B.; Epifano, F.; Genovese, S. Chemical composition and inhibitory activity against Helicobacter pylori of the essential oil of Apium nodiflorum (Apiaceae). J. Med. Food 2010, 13, 228–230. [Google Scholar] [CrossRef] [PubMed]
- Leto, C.; Tuttolomondo, T.; La Bella, S.; Leone, R.; Licata, M. Effects of plant species in a horizontal subsurface flow constructed wetland–phytoremediation of treated urban wastewater with Cyperus alternifolius L. and Typha latifolia L. in the West of Sicily (Italy). Ecol. Eng. 2013, 61, 282–291. [Google Scholar] [CrossRef]
- Platzer, C. Design recommendations for subsurface flow constructed wetlands for nitrification and denitrification. Water Sci. Technol. 1999, 40, 257–263. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).