Quantification of Uncertainties from Image Processing and Analysis in Laboratory-Scale DNAPL Release Studies Evaluated by Reflective Optical Imaging
Abstract
:1. Introduction
2. Delineation of an IPA Framework for DNAPL Release Studies
2.1. General Considerations for Optical Imaging
2.2. Conceptualization of a Basic IPA Framework for DNAPL Release Studies
3. Experimental Investigations for IPA Framework Evaluation
3.1. Fluids, Porous Media and Dyes
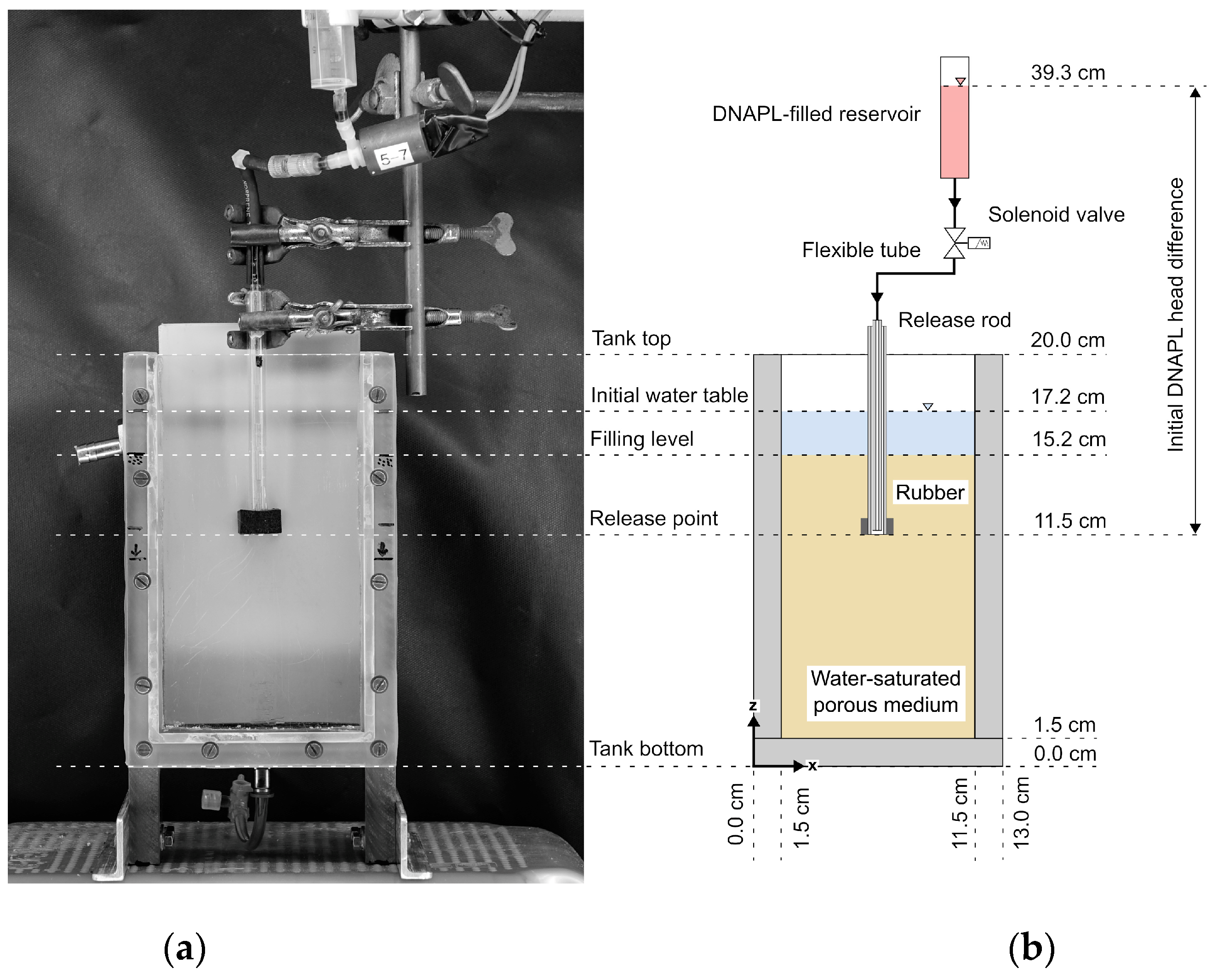
3.2. Tank Setup for Investigating DNAPL Release in Different Porous Media
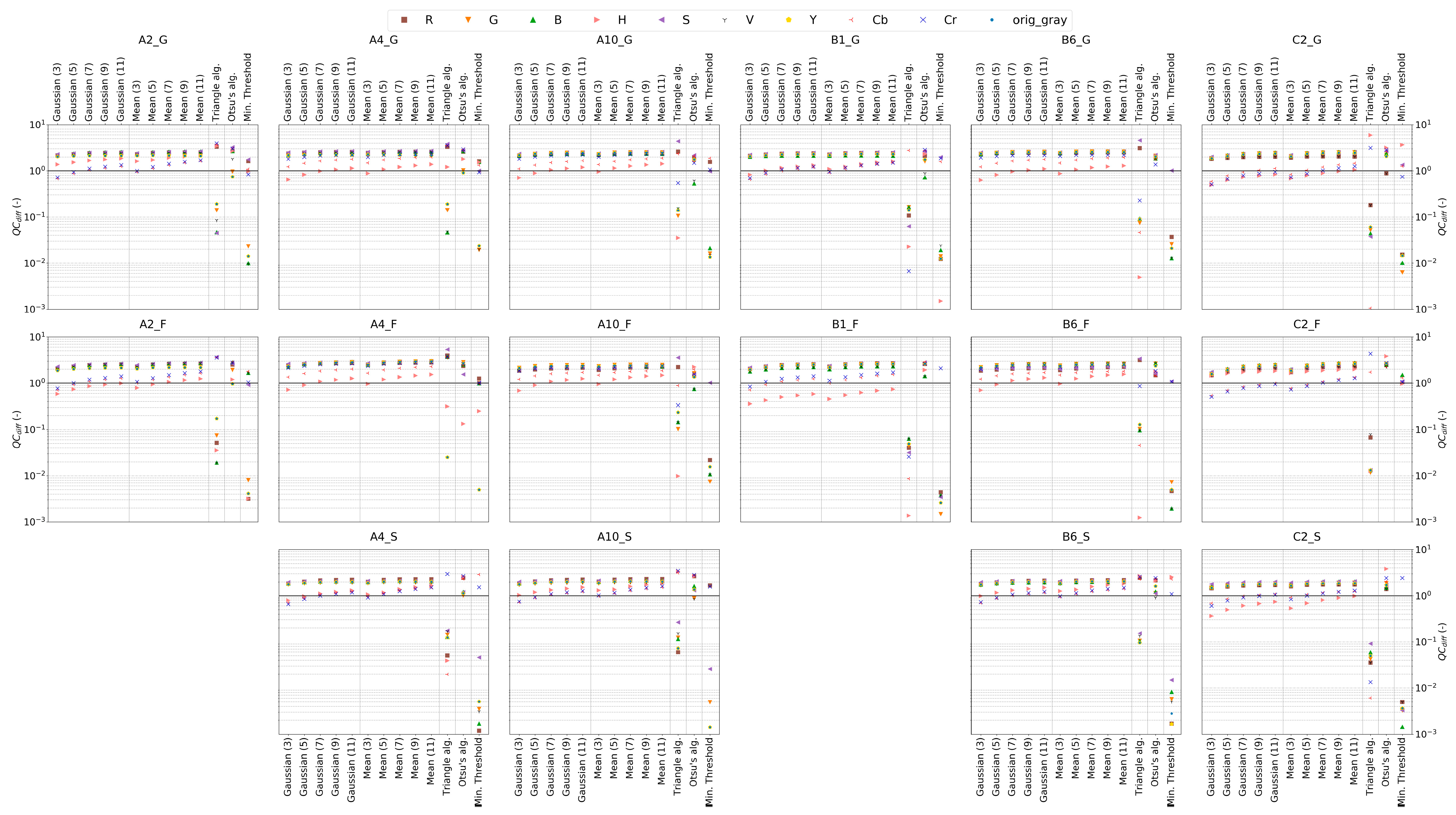
3.3. Measures for IPA Framework Evaluation
4. Results and Discussion
4.1. Evaluation of Dye Configurations and their Suitability for DNAPL Release Studies
4.2. Delineation of Uncertainties related to Individual IPA Framework Steps Applied to Experimental DNAPL Release Scenarios
4.2.1. Correction of Temporal Illumination Fluctuation
4.2.2. Color Model Change and Binary Conversion Algorithms
4.2.3. Correction of Spatially Non-Uniform Illumination and Background Exclusion
4.2.4. Delineation of Optimized IPA Framework Configuration for each Porous Media Type
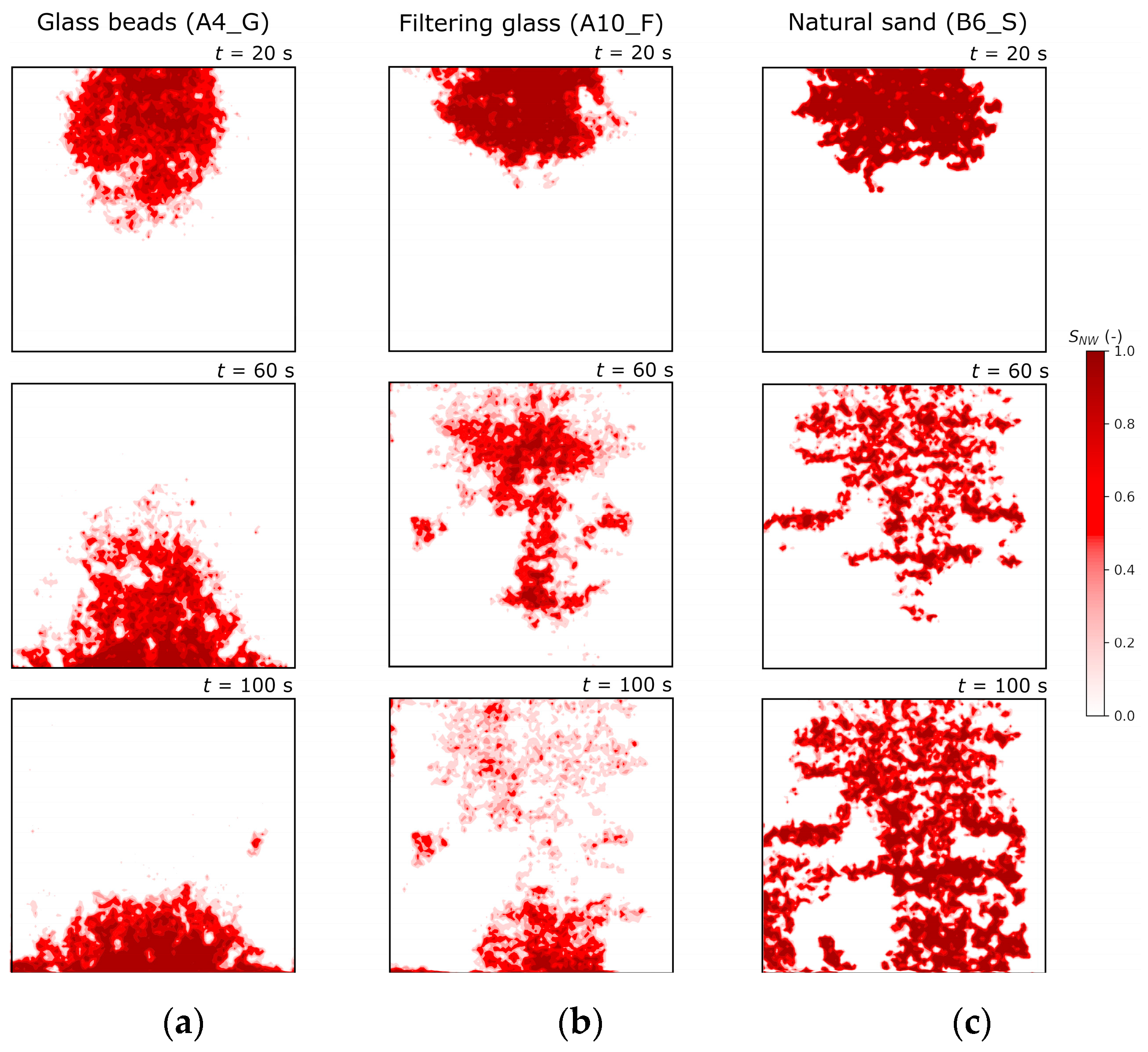
4.2.5. Calculation of DNAPL Saturation and Comparison for the three Porous Media Types
5. Conclusions
5.1. Summary
5.2. Limitations and Outlook
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Aim | IPA Step | Exemplary References | |
|---|---|---|---|
| NAPL-Related | Other | ||
| Correction of temporal illumination fluctuations | Image normalization using black and white cards or grey cards | [56] | [66,76] |
| Correction using white card or gray card or reference areas | [29,30] | [60,77,78] | |
| Standardization of image | - | [69,79] | |
| Color model change to uncover required information | Conversion to HSI or YCbCr | [42,49,68,80] | - |
| Acquisition of monochromatic image and uncover required information (e.g., to identify peaks) | Recording gray-scale image | [56] | [71,76,81] |
| Using single band of RGB or HSI | [34,49,80] | [56,62,66,68,76,78,79] | |
| Converting to gray scale | [48] | [82,83] | |
| Correction of spatially non-uniform illumination | Correction using a reference image, either flat-field image or without tracer and then dividing, subtracting or normalizing to the image | - | [62,84,85] |
| Converting by using adaptive thresholding algorithm using percent distance between peaks | [30] | - | |
| Contrast enhancement | Gamma correction | - | [77] |
| Color balance adjustment | [70] | - | |
| Adjustment of luminescence level | [70] | - | |
| CLAHE with Rayleigh distribution | - | [71] | |
| Reduction to area of interest, i.e., cut image to relevant size and to exclude background such as tank set-up | Cropping image | [34,56,70] | [37,71,72,83] |
| Reduction of optical noise | Median filter | - | [66,79,82] |
| Image smoothing using 7 × 7 filter window, filter is just applied if a specific threshold is exceeded | - | [85] | |
| Acquiring a reflection image, which is a map of incident disturbances based on the soil particles for correcting surface roughness and varying optical properties of the soil | - | [85] | |
| Morphological opening operation | - | [71,72] | |
| Wavelet de-noising | - | [71] | |
| Image subtraction with reference image (generally image without Tracer) | - | [62,84] | |
| Background elimination | Image subtraction with reference image (generally image without Tracer) | [48] | [37,66] |
| Background leveling | - | [79] | |
| Thresholding based on fuzzy partition and tsallis entropy | - | [71] | |
| Adaption to representative elementary volume | Averaging RGB values for, e.g., 2 × 2 pixel | - | [37] |
| Identifying object of interest | Defining mean threshold for background and object of interest | - | [72,85] |
| De-blurring image | Unsharpness filtering | - | [71] |
| Acquisition of binary image | Converting to black and white | [70] | - |
| Converting to b/w using specific threshold | [34] | [71] | |
| Converting by using adaptive thresholding algorithm using percent distance between peaks | [30] | - | |
| Delineate NAPL saturation | Mesh overlay | [34] | - |
| ID | Type | Aqueous Phase Dye | DNAPL Phase Dye | Non-Horizontal Phase Interface | Gradient Interface | Poor Color Contrast between Phases | Poor Color Contrast between Fluid and Sediment | Sorption to Sediment | Photo-Degradation | Not Suitable |
|---|---|---|---|---|---|---|---|---|---|---|
| A1_G | Dual | Indigo carmine | Oil-Red-O | Yes | Yes | No | Not tested | Not tested | No | Yes |
| A1_F | Not tested | Not tested | Yes | |||||||
| A1_S | Not tested | Not tested | Yes | |||||||
| A2_G | Dual | Indigo carmine | Sudan IV | No | No | No | No | Not tested | No | No |
| A2_F | No | Not tested | No | |||||||
| A2_S | Not tested | Not tested | Yes | |||||||
| A3_G | Dual | Brilliant Blue FCF | Oil-Red-O | Yes | Yes | No | Not tested | Not tested | No | Yes |
| A3_F | Not tested | Not tested | Yes | |||||||
| A3_S | Not tested | Not tested | Yes | |||||||
| A4_G | Dual | Brilliant Blue FCF | Sudan IV | No | No | No | No | Not tested | No | No |
| A4_F | No | Not tested | No | |||||||
| A4_S | No | Not tested | No | |||||||
| A5_G | Dual | Rhodamine-B | Sudan Blue | No | No | Yes | Not tested | Not tested | No | Yes |
| A5_F | Not tested | Not tested | Yes | |||||||
| A5_S | Not tested | Not tested | Yes | |||||||
| A6_G | Dual | Red pen ink | Sudan Blue | Yes | No | No | Not tested | Not tested | No | Yes |
| A6_F | Not tested | Not tested | Yes | |||||||
| A6_S | Not tested | Not tested | Yes | |||||||
| A7_G | Dual | Blue pen ink | Oil-Red-O | No | Yes | No | Not tested | Not tested | No | Yes |
| A7_F | Not tested | Not tested | Yes | |||||||
| A7_S | Not tested | Not tested | Yes | |||||||
| A8_G | Dual | Blue pen ink | Sudan IV | No | Yes | No | Not tested | Not tested | No | Yes |
| A8_F | Not tested | Not tested | Yes | |||||||
| A8_S | Not tested | Not tested | Yes | |||||||
| A9_G | Dual | Green pen ink | Oil-Red-O | Yes | Yes | No | Not tested | Not tested | No | Yes |
| A9_F | Not tested | Not tested | Yes | |||||||
| A9_S | Not tested | Not tested | Yes | |||||||
| A10_G | Dual | Green pen ink | Sudan IV | No | No | No | No | Not tested | No | No |
| A10_F | No | Not tested | No | |||||||
| A10_S | No | Not tested | No | |||||||
| A11_G | Dual | Green pen ink | Sudan Blue | No | No | Yes | Not tested | Not tested | No | Yes |
| A11_F | Not tested | Not tested | Yes | |||||||
| A11_S | Not tested | Not tested | Yes | |||||||
| B1_G | Water | Indigo carmine | - | No | No | No | No | No | No | No |
| B1_F | No | No | No | |||||||
| B1_S | Yes | No | Yes | |||||||
| B2_G | Water | Brilliant Blue FCF | - | Yes | Yes | No | No | No | No | Yes |
| B2_F | No | No | Yes | |||||||
| B2_S | No | No | Yes | |||||||
| B3_G | Water | Rhodamine-B | - | Yes | Yes | No | No | No | No | Yes |
| B3_F | No | No | Yes | |||||||
| B3_S | No | No | Yes | |||||||
| B4_G | Water | Red pen ink | - | No | No | No | Yes | Yes | No | Yes |
| B4_F | Yes | Yes | Yes | |||||||
| B4_S | Yes | Yes | Yes | |||||||
| B5_G | Water | Blue pen ink | - | No | Yes | No | Yes | Yes | No | Yes |
| B5_F | Yes | Yes | Yes | |||||||
| B5_S | Yes | Yes | Yes | |||||||
| B6_G | Water | Green pen ink | - | No | No | No | No | No | No | No |
| B6_F | No | No | No | |||||||
| B6_S | No | No | No | |||||||
| C1_G | DNAPL | - | Oil-Red-O | Yes | No | No | No | No | No | Yes |
| C1_F | No | No | Yes | |||||||
| C1_S | No | No | Yes | |||||||
| C2_G | DNAPL | - | Sudan IV | No | No | No | No | No | No | No |
| C2_F | No | No | No | |||||||
| C2_S | No | No | No | |||||||
| C3_G | DNAPL | - | Sudan Blue | No | No | Yes | Yes | No | No | Yes |
| C3_F | No | No | Yes | |||||||
| C3_S | Yes | No | Yes |
References
- Mackay, D.M.; Cherry, J.A. Groundwater contamination: Pump-and-treat remediation. Environ. Sci. Technol. 1989, 23, 630–636. [Google Scholar] [CrossRef]
- European Commission (EC). Regulation No. 850/2004 of the European Parliament and of the Council of 29 April 2004 on Persistent Organic Pollutants and Amending Directive 79/117/EEC. 2004, p. 31. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02004R0850-20120710&from=EN (accessed on 23 August 2019).
- Engelmann, C.; Händel, F.; Binder, M.; Yadav, P.K.; Dietrich, P.; Liedl, R.; Walther, M. The fate of DNAPL contaminants in non-consolidated subsurface systems—Discussion on the relevance of effective source zone geometries for plume propagation. J. Hazard. Mat. 2019, 375, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Kueper, B.H.; Stroo, H.F.; Vogel, C.M.; Ward, C.H. (Eds.) Chlorinated Solvent Source Zone Remediation; Springer: New York, NY, USA; Heidelberg, Germany; Dordrecht, The Netherlands; London, UK, 2014; p. 713. [Google Scholar]
- Agaoglu, B.; Copty, N.K.; Scheytt, T.; Hinkelmann, R. Interphase mass transfer between fluids in subsurface formations: A review. Adv. Water Resour. 2015, 79, 162–194. [Google Scholar] [CrossRef]
- Powers, S.E.; Nambi, I.M.; Curry, G.W., Jr. Non-aqueous phase liquid dissolution in heterogeneous systems: Mechanisms and a local equilibrium modeling approach. Water Resour. Res. 1998, 34, 3293–3302. [Google Scholar] [CrossRef]
- Brusseau, M.L.; Hatton, J.; DiGuiseppi, W. Assessing the impact of source-zone remediation efforts at the contaminant-plume scale through analysis of contaminant mass discharge. J. Contam. Hydrol. 2011, 126, 130–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiedemeier, T.; Rifai, H.; Newell, C.; Wilson, J. Natural Attenuation of Fuels and Chlorinated Solvents in the Subsurface; John Wiley & Sons, Inc.: Hoboken, NY, USA, 1999; p. 619. [Google Scholar]
- Falta, R.W.; Basu, N.; Rao, P.S.C. Assessing impacts of partial mass depletion in DNAPL source zones: II, coupling source strength functions to plume evolution. J. Contam. Hydrol. 2005, 79, 45–66. [Google Scholar] [CrossRef]
- Fure, A.D.; Jawitz, J.W.; Annable, M.D. DNAPL source depletion: Linking architecture and flux response. J. Contam. Hydrol. 2006, 85, 118–140. [Google Scholar] [CrossRef]
- Liedl, R.; Valocchi, A.J.; Dietrich, P.; Grathwohl, P. Finiteness of steady state plumes. Water Resour. Res. 2005, 41, 1–8. [Google Scholar] [CrossRef]
- Brusseau, M.L.; Nelson, N.T.; Zhang, Z.; Blue, J.E.; Rohrer, J.; Allen, T. Source-zone characterization of a chlorinated-solvent contaminated superfund site in Tuscon, AZ. J. Contam. Hydrol. 2007, 90, 21–40. [Google Scholar] [CrossRef]
- Sookhak Lari, K.; Davis, G.B.; Rayner, J.L.; Bastow, T.P.; Puzon, G.J. Natural Source zone depletion of LNAPL: A critical review supporting modelling approaches. Water Res. 2019, 157, 630–646. [Google Scholar] [CrossRef]
- Werth, C.J.; Zhang, C.; Brusseau, M.L.; Oostrom, M.; Baumann, T. A review of non-invasive imaging methods and applications in contaminant hydrogeology research. J. Contam. Hydrol. 2010, 113, 1–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cnudde, V.; Boone, M.N. high-resolution X-ray computed tomography in geosciences: A review of the current technology and applications. Earth-Sci. Rev. 2013, 123, 1–17. [Google Scholar] [CrossRef]
- Power, C.; Gerhard, J.I.; Tsourlos, P.; Soupios, P.; Simyrdanis, K.; Karaoulis, M. Improved time-lapse electrical resistivity tomography monitoring of dense non-aqueous phase liquids with surface-to-horizontal borehole arrays. J. Appl. Geosci. 2015, 112, 1–13. [Google Scholar] [CrossRef]
- Alesse, B.; Orlando, L.; Palladini, L. Non-invasive lab test in the monitoring of vadose zone contaminated by light non-aqueous phase liquid. Geophys. Prosp. 2019. [Google Scholar] [CrossRef]
- Zhang, C.; Yoon, H.; Werth, C.J.; Valocchi, A.J.; Basu, N.B.; Jawitz, J.W. Evaluation of simplified mass transfer models to simulate the impacts of source zone architecture on nonaqueous phase liquid dissolution in heterogeneous porous media. J. Contam. Hydrol. 2008, 102, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Mazáč, O.; Kelly, W.E.; Landa, I. A hydrogeophysical model for relations between electrical and hydraulic properties of aquifers. J. Hydrol. 1985, 79, 1–19. [Google Scholar] [CrossRef]
- Dietrich, P.; Fechner, T.; Whittaker, J. Anmerkungen zur Interpretation tomographischer Messungen. In Proceedings of the DGG-Mitteilungen Sonderband II/1999–5. DGG-Seminar “Umweltgeophysik”, Neustadt, Germany, April 1998; pp. 13–21. [Google Scholar]
- Huang, W.E.; Smith, C.C.; Lerner, D.N.; Thornton, S.F.; Oram, A. Physical modelling of solute transport in porous media: Evaluation of an imaging technique using UV excited fluorescent dye. Water Res. 2002, 36, 1843–1853. [Google Scholar] [CrossRef]
- Peng, Z.; Duwig, C.; Delmas, P.; Gaudet, J.P.; Gastelum Strozzi, A.; Charrier, P.; Denis, H. Visualization and Characterization of Heterogeneous Water Flow in Double-Porosity Media by Means of X-ray Computed Tomography. Transp. Porous Med. 2015, 110, 543–564. [Google Scholar] [CrossRef]
- Maas, H.-G.; Hampel, U. Photogrammetric Techniques in Civil Engineering Material Testing and Structure Monitoring. Photogram. Eng. Rem. Sens. 2006, 1, 39–45. [Google Scholar] [CrossRef]
- Konz, M.; Ackerer, P.; Younes, A.; Huggenberger, P.; Zechner, E. Two-dimensional stable-layered laboratory-scale experiments for testing density-coupled flow models. Water Resour. Res. 2009, 45, W02404. [Google Scholar] [CrossRef]
- Robinson, G.; Ahmed, A.A.; Hamill, G.A. Experimental saltwater intrusion in coastal aquifers using automated image analysis: Applications to homogeneous aquifers. J. Hydrol. 2016, 538, 304–313. [Google Scholar] [CrossRef] [Green Version]
- Stoeckl, L.; Walther, M.; Graf, T. A new numerical benchmark of a freshwater lens. Water Resour. Res. 2016, 52, 2474–2489. [Google Scholar] [CrossRef] [Green Version]
- Stoeckl, L.; Walther, M.; Morgan, L.K. Physical and Numerical Modelling of Post-Pumping Seawater Intrusion. Geofluids 2019, 7191370. [Google Scholar] [CrossRef]
- Cohen, R.M.; Bryda, A.P.; Shaw, S.T.; Spalding, C.P. Evaluation of Visual Methods to Detect NAPL in Soil and Water. Groundwater Monit. Remed. 1992, 12, 132–141. [Google Scholar] [CrossRef]
- Glass, R.J.; Conrad, S.H.; Peplinski, W. Gravity-destabilized nonwetting phase invasion in macroheterogeneous porous media: Experimental observations of invasion dynamics and scale analysis. Water Resour. Res. 2000, 36, 3121–3137. [Google Scholar] [CrossRef] [Green Version]
- Zhong, L.; Mayer, A.; Glass, R.J. Visualization of surfactant-enhanced nonaqueous phase liquid mobilization and solubilization in a two-dimensional micromodel. Water Resour. Res. 2001, 37, 523–537. [Google Scholar] [CrossRef] [Green Version]
- Oostrom, M.; Dane, J.H.; Wietsma, T.W. A Review of Multidimensional, Multifluid, Intermediate-Scale Experiments: Flow Behavior, Saturation Imaging, and Tracer Detection and Quantification. Vadose Zone J. 2007, 6. [Google Scholar] [CrossRef]
- Page, J.W.E.; Soga, K.; Illangasekare, T. The significance of heterogeneity on mass flux from DNAPL source zones: An experimental investigation. J. Contam. Hydrol. 2007, 94, 215–234. [Google Scholar] [CrossRef]
- Bob, M.M.; Brooks, M.C.; Mravik, S.C.; Wood, L. A modified light transmission visualization method for DNAPL saturation measurements in 2-D models. Adv. Water Resour. 2008, 31, 727–742. [Google Scholar] [CrossRef]
- Luciano, A.; Viotti, P.; Papini, M.P. Laboratory investigation of DNAPL migration in porous media. J. Hazard. Mat. 2010, 176, 1006–1017. [Google Scholar] [CrossRef]
- Luciano, A.; Viotti, P.; Papini, M.P. On Morphometric Properties of DNAPL Sources: Relating Architecture to Mass Reduction. Water Air Soil Pollut. 2012, 223, 2849–2864. [Google Scholar] [CrossRef]
- Luciano, A.; Manchini, G.; Torretta, V.; Viotti, P. An empirical model for the evaluation of the dissolution rate from a DNAPL-contaminated area. Environ. Sci. Pollut. Res. 2018, 25, 33992–34004. [Google Scholar] [CrossRef] [PubMed]
- Citarella, D.; Cupola, F.; Tanda, M.G.; Zanini, A. Evaluation of dispersivity coefficients by means of a laboratory image analysis. J. Contam. Hydrol. 2015, 172, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Kashuk, S.; Mercurio, S.R.; Iskander, M. Visualization of dyed NAPL concentration in transparent porous media using color space components. J. Contam. Hydrol. 2014, 162–163, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kashuk, S.; Mercurio, S.R.; Iskander, M. Methodology for Optical Imaging of NAPL 3D Distribution in Transparent Porous Media. Geotech. Test. J. 2015, 38, 603–619. [Google Scholar] [CrossRef]
- Pan, Y.; Yang, J.; Jia, Y.; Xu, Z. Experimental study on non-aqueous phase liquid multiphase flow characteristics and controlling factors in heterogeneous porous media. Environ. Earth Sci. 2016, 75. [Google Scholar] [CrossRef]
- Wu, M.; Cheng, Z.; Wu, J.; Wu, J. Estimation of representative elementary volume for DNAPL saturation and DNAPL-water interfacial areas in 2D heterogeneous porous media. J. Hydrol. 2017, 549, 12–26. [Google Scholar] [CrossRef]
- Rahman, N.A.; Foong, L.K.; Lewis, R.W.; Nazir, R. Laboratory Investigation of LNAPL Migration in Double-Porosity Soil Under Fractured Condition Using Digital Image Analysis. Transp. Porous Med. 2018, 125, 521–542. [Google Scholar] [CrossRef]
- Gonzales, R.C.; Woods, R.E. Digital Image Processing, 2nd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2002; p. 793. [Google Scholar]
- Singh, P.; Garg, A.K. Non Uniform Background Removal using Morphology based Structuring Element for Particle Analysis. Int. J. Comp. Appl. 2011, 33, 11–16. [Google Scholar]
- Robinson, G.; Hamill, G.A.; Ahmed, A.A. Automated image analysis for experimental investigations of salt water intrusion in coastal aquifers. J. Hydrol. 2015, 530, 350–360. [Google Scholar] [CrossRef] [Green Version]
- Detwiler, R.L.; Pringle, S.E.; Glass, R.J. Measurement of fracture aperture fields using transmitted light: An evaluation of measurement errors and their influence on simulations of flow and transport through a single fracture. Water Resour. Res. 1999, 35, 2605–2617. [Google Scholar] [CrossRef]
- Abdoulhalik, A.; Ahmed, A.A. Transience of seawater intrusion and retreat in response to incremental water-level variations. Hydrol. Proc. 2018, 32, 2721–2733. [Google Scholar] [CrossRef]
- Alazaiza, M.Y.D.; Ngien, S.K.; Copty, N.; Bob, M.M.; Kamaruddin, S.A. Assessing the influence of infiltration on the migration of light non-aqueous phase liquid in double-porosity soil media using a light transmission visualization method. Hydrogeol. J. 2019, 27, 581–593. [Google Scholar] [CrossRef]
- Gerhard, J.I.; Kueper, B.H. Capillary pressure characteristics necessary for simulating DNAPL infiltration, redistribution, and immobilization in saturated porous media. Water Resour. Res. 2003, 39, 1212. [Google Scholar] [CrossRef]
- Rezanezhad, F.; Vogel, H.-J.; Roth, K. Experimental study of fingered flow through initially dry sand. Hydrol. Earth Syst. Sci. Discuss. EGU 2006, 3, 2595–2620. [Google Scholar] [CrossRef] [Green Version]
- Konz, M.; Ackerer, P.; Huggenberger, P.; Veit, C. Comparison of light transmission and reflection techniques to determine concentrations in flow tank experiments. Exp. Fluids 2009, 47, 85. [Google Scholar] [CrossRef]
- Sabnis, R.W. Handbook of Biological Dyes and Stains: Synthesis and Industrial Applications; John Wiley & Sons: Hoboken, NJ, USA, 2010; p. 544. [Google Scholar]
- Cápiro, N.L.; Stafford, B.P.; Rixey, W.G.; Bedient, P.B.; Alvarez, P.J.J. Fuel-grade ethanol transport and impacts to groundwater in a pilot-scale aquifer tank. Water Res. 2007, 41, 656–664. [Google Scholar] [CrossRef]
- Nsir, K.; Schäfer, G.; di Chiara Roupert, R.; Razakarisoa, O.; Toussaint, R. Laboratory experiments on DNAPL fingering in water-saturated porous media. Int. J. Multiph. Flow 2012, 40, 83–92. [Google Scholar] [CrossRef]
- Jawitz, J.W.; Annable, M.D.; Rao, P.S.C. Miscible fluid displacement stability in unconfined porous media: Two-dimensional flow experiments and simulations. J. Contam. Hydrol. 1998, 31, 211–230. [Google Scholar] [CrossRef]
- Kechavarzi, C.; Soga, K.; Wiart, P. Multispectral image analysis method to determine dynamic fluid saturation distribution in two-dimensional three-fluid phase flow laboratory experiments. J. Contam. Hydrol. 2000, 46, 265–293. [Google Scholar] [CrossRef]
- Brusseau, M.; Nelson, N.T.; Oostrom, M.; Zhang, Z.; Johnson, G.R.; Wietsma, T.W. Influence of Heterogeneity and Sampling Method on Aqueous Concentrations Associated with NAPL Dissolution. Environ. Sci. Technol. 2000, 34, 3657–3664. [Google Scholar] [CrossRef]
- Ghanem, A.; Soerens, T.S.; Adel, M.M.; Thoma, G.J. Investigation of Fluorescent Dyes as Partitioning Tracers for Subsurface Nonaqueous Phase Liquid (NAPL) Characterization. J. Environ. Eng. 2003, 129, 740. [Google Scholar] [CrossRef]
- Käss, W. Lehrbuch der Hydrogeologie, Band 9: Geologische Markierungstechnik (Textbook of geohydrological marking and tracing techniques, vol. 9, 2nd edn.); Borntraeger: Berlin, Germany, 2004; p. 557. [Google Scholar]
- Barns, G.L.; Wilson, R.D.; Thornton, S.F. Fluorescent dye imaging of the volume sampled by single well forced-gradient tracer tests evaluated in a laboratory-scale aquifer physical model. J. Contam. Hydrol. 2012, 128, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Barns, G.L.; Thornton, S.F.; Wilson, R.D. Identification of small-scale low and high permeability layers using single well forced-gradient tracer tests: Fluorescent dye imaging and modelling at the laboratory scale. J. Contam. Hydrol. 2015, 172, 84–99. [Google Scholar] [CrossRef] [PubMed]
- Ilankoon, I.M.S.K.; Neethling, S.J. Inter-particle liquid spread pertaining to heap leaching using UV fluorescence based image analysis. Hydrometallurgy 2019, 183, 175–185. [Google Scholar] [CrossRef]
- Davis, S.N.; Thompson, G.M.; Bentley, H.W.; Stiles, G. Ground-Water Tracers—A Short Review. Groundwater 1980, 18, 14–23. [Google Scholar] [CrossRef]
- Tuck, D.M.; Iversen, G.M.; Pirkle, W.A. Organic dye effects on dense nonaqueous phase liquids (DNAPL) entry pressure in water saturated porous media. Water Resour. Res. 2003, 39, 1207. [Google Scholar] [CrossRef]
- Stoeckl, L.; Houben, G. Flow dynamics and age stratification of freshwater lenses: Experiments and modeling. J. Hydrol. 2012, 458–459, 9–15. [Google Scholar] [CrossRef]
- Belfort, B.; Weill, S.; Lehmann, F. Image analysis method for the measurement of water saturation in a two-dimensional experimental flow tank. J. Hydrol. 2017, 550, 343–354. [Google Scholar] [CrossRef] [Green Version]
- PSF (2018) Python Software Foundation. Python Language Reference, version 3.6. Available online: http://www.python.org (accessed on 29 October 2019).
- Simantiraki, F.; Aivalioti, M.; Gidarakos, E. Implementation of an image analysis technique to determine LNAPL infiltration and distribution in unsaturated porous media. Desalination 2009, 248, 705–715. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Q.; Hu, J. Adaptive enhancement for nonuniform illumination images via nonlinear mapping. J. Electron. Imag. 2017, 26, 053012. [Google Scholar] [CrossRef] [Green Version]
- Halihan, T.; Sefa, V.; Sale, T.; Lyverse, M. Mechanism for detecting NAPL using electrical resistivity imaging. J. Contam. Hydrol. 2017, 205, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Riaño, A.B.; Rodriguez, I.H.; Bannwart, A.C.; Rodriguez, O.M.H. Film thickness measurement in oil-water pipe flow using image processing technique. Exp. Therm. Fluid Sci. 2015, 68, 330–338. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, Y.; Liu, H.; Gao, H. In-situ water level measurement using NIR-imaging video camera. Flow Meas. Inst. 2019, 67, 95–106. [Google Scholar] [CrossRef]
- Otsu, N. A threshold selection method from fray-level histograms. IEEE Trans. Syst. Man. Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- Van Valkenburg, M.E.; Annable, M. Mobilization and entry of DNAPL pools into finer sand media by cosolvents: Two-dimensional chamber studies. J. Contam. Hydrol. 2002, 59, 211–230. [Google Scholar] [CrossRef]
- Kamon, M.; Endo, K.; Kawabata, J.; Inui, T.; Katsumi, T. Two-dimensional DNAPL migration affected by groundwater flow in unconfined aquifer. J. Hazard. Mat. 2004, 110, 1–12. [Google Scholar] [CrossRef]
- Bridge, J.; Banwart, S.; Heathwaite, A. Noninvasive Quantitative Measurement of Colloid Transport in Mesoscale Porous Media Using Time Lapse Fluorescence Imaging. Environ. Sci. Technol. 2006, 40, 5930–5936. [Google Scholar] [CrossRef]
- Rahman, M; Jose, S.; Nowak, W.; Cirpka, O. Experiments on vertical transverse mixing in a large-scale heterogeneous model aquifer. J. Contam. Hydrol. 2005, 80, 130–148. [Google Scholar] [CrossRef]
- Konz, M.; Ackerer, P.; Meier, E.; Huggenberger, P.; Zechner, E.; Gechter, D. On the measurement of solute concentrations in 2-D flow tank experiments. Hydrol. Earth Sys. Sci. 2008, 12, 727–738. [Google Scholar] [CrossRef] [Green Version]
- McNeil, J.D.; Oldenborger, G.A.; Schincariol, R.A. Quantitative imaging of contaminant distributions in heterogeneous porous media laboratory experiments. J. Contam. Hydrol. 2006, 84, 36–54. [Google Scholar] [CrossRef] [PubMed]
- Darnault, C.J.G.; Throop, J.A.; DiCarlo, D.A.; Rimmer, A.; Steenhuis, T.S.; Parlange, J.-Y. Visualization by light transmission of oil and water contents in transient two-phase flow fields. J. Contam. Hydrol. 1998, 31, 337–348. [Google Scholar] [CrossRef]
- Rossi, M.; Lehmann, P.; Ursino, N.; Ippisch, O.; Flühler, H. Solute mixing during imbibition and drainage in a macroscopically heterogeneous medium. Water Resour. Res. 2007, 43, 1–13. [Google Scholar] [CrossRef]
- Zhang, Q.; Volker, R.E.; Lockington, D.A. Experimental investigation of contaminant transport in coastal groundwater. Adv. Environ. Res. 2002, 6, 229–237. [Google Scholar] [CrossRef]
- Madhania, S.; Muharam, Y.; Winardi, S.; Purwanto, W.W. Mechanism of molasses–water mixing behavior in bioethanol fermenter. Experiments and CFD modeling. Energy Rep. 2019, 5, 454–461. [Google Scholar] [CrossRef]
- Bridge, J.W.; Bannwart, S.A.; Heathwaite, A.L. High-Resolution Measurement of Pore Saturation and Colloid Removal Efficiency in Quartz Sand Using Fluorescence Imaging. Environ. Sci. Technol. 2007, 41, 8288–8294. [Google Scholar] [CrossRef]
- Aeby, P.; Schultze, U.; Braichotte, D.; Bundt, M.; Moser-Boroumand, F.; Wydler, H.; Flühler, H. Fluorescence Imaging of Tracer Distributions in Soil Profiles. Environ. Sci. Technol. 2001, 35, 753–760. [Google Scholar] [CrossRef]




| Variables | Glass Beads (G) (1 mm) | Filtering Glass (F) (1…2 mm) | Natural Sand (S) (1…2 mm) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Trial Number | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 |
| Fully water-saturated hydraulic conductivity (× 10−3 m/s) | 6.13 | 6.26 | 5.81 | 8.75 | 7.53 | 6.87 | 3.17 | 4.41 | 2.89 |
| Porosity (-) | 0.393 | 0.401 | 0.391 | 0.445 | 0.435 | 0.436 | 0.371 | 0.381 | 0.359 |
| Bulk density (× 103 kg/m3) | 1.59 | 1.51 | 1.51 | 1.36 | 1.39 | 1.40 | 1.58 | 1.59 | 1.61 |
| Grain density (× 103 kg/m3) | 2.47 | 2.52 | 2.48 | 2.45 | 2.46 | 2.48 | 2.51 | 2.57 | 2.51 |
| Dye | Manufacturer | Amount |
|---|---|---|
| Indigo carmine 1 | Co. Sigma-Aldrich GmbH | 2 mg |
| Brilliant Blue FCF 1 | Co. Supelco™ Analytical | 5 mg |
| Rhodamine-B 1 | Co. Sigma-Aldrich GmbH | 5 mg |
| Red pen ink (4001 TP/6 Pink) 1 | Co. Pelikan | 1.0 mL |
| Blue pen ink (4001 TP/6 Royal blue) 1 | Co. Pelikan | 1.0 mL |
| Green pen ink (4001 TP/6 Dark green) 1 | Co. Pelikan | 0.5 mL |
| Oil-Red-O (dissolved in 0.5% propylene glycol) 2 | Co. Sigma-Aldrich GmbH | 4 mL |
| Sudan IV 2 | Co. Alfa Aesar | 5 mg |
| Sudan Blue 2 | Co. abcr GmbH | 10 mg |
| Scenario Class | Porous Medium | Dye Configuration | |||
|---|---|---|---|---|---|
| Glass Beads (G) | Filtering Glass (F) | Natural Sand (S) | DNAPL Phase | Aqueous Phase | |
| Dual color | A2_G | A2_F | - | Sudan IV | Indigo carmine |
| A4_G | A4_F | A4_S | Sudan IV | Brilliant Blue FCF | |
| A10_G | A10_F | A10_S | Sudan IV | Green pen ink | |
| Water color | B1_G | B1_F | - | - | Indigo carmine |
| B6_G | B6_F | B6_S | - | Green pen ink | |
| DNAPL color | C2_G | C2_F | C2_S | Sudan IV | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Engelmann, C.; Schmidt, L.; Werth, C.J.; Walther, M. Quantification of Uncertainties from Image Processing and Analysis in Laboratory-Scale DNAPL Release Studies Evaluated by Reflective Optical Imaging. Water 2019, 11, 2274. https://doi.org/10.3390/w11112274
Engelmann C, Schmidt L, Werth CJ, Walther M. Quantification of Uncertainties from Image Processing and Analysis in Laboratory-Scale DNAPL Release Studies Evaluated by Reflective Optical Imaging. Water. 2019; 11(11):2274. https://doi.org/10.3390/w11112274
Chicago/Turabian StyleEngelmann, Christian, Luisa Schmidt, Charles J. Werth, and Marc Walther. 2019. "Quantification of Uncertainties from Image Processing and Analysis in Laboratory-Scale DNAPL Release Studies Evaluated by Reflective Optical Imaging" Water 11, no. 11: 2274. https://doi.org/10.3390/w11112274
APA StyleEngelmann, C., Schmidt, L., Werth, C. J., & Walther, M. (2019). Quantification of Uncertainties from Image Processing and Analysis in Laboratory-Scale DNAPL Release Studies Evaluated by Reflective Optical Imaging. Water, 11(11), 2274. https://doi.org/10.3390/w11112274






