Removal of Organic Micropollutants in Wastewater Treated by Activated Sludge and Constructed Wetlands: A Comparative Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of AS and HSSF Systems
2.2. Sampling Strategy
2.3. Analytical Methods
2.3.1. Physicochemical and Water Quality Parameters
2.3.2. Determination of OMPs and Transformation Products by Biodegradation
2.3.3. Extractable Organic Matter
2.4. Statistics Analyses
3. Results and Discussion
3.1. Conventional Water Quality Parameters of Influent and Effluents from AS and HSSFs
3.2. Occurrence of OMPs in the Influent
3.3. Ocurrence of OMPs in Biosolids
3.4. Removal Efficiencies of OMPs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chamorro, S.; Hernández, V.; Matamoros, V.; Domínguez, C.; Becerra, J.; Vidal, G.; Piña, B.; Bayona, J.M. Chemical characterization of organic microcontaminant sources and biological effects in riverine sediments impacted by urban sewage and pulp mill discharges. Chemosphere 2013, 90, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Stuart, M.; Lapworth, D.; Crane, E.; Hart, A. Review of risk from potential emerging contaminants in UK groundwater. Sci. Total Environ. 2012, 416, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Correa, J.; Domínguez, V.; Martínez, M.; Vidal, G. Interaction between 2,4,6-TCP increasing concentration content in ECF bleached effluent and aerobic bacteria degradative activity. Environ. Int. 2003, 29, 459–465. [Google Scholar] [CrossRef]
- Dordio, A.; Carvalho, A.P.; Teixeira, D.M.; Dias, C.B.; Pinto, A.P. Removal of pharmaceuticals in microcosm constructed wetlands using Typha spp. and LECA. Bioresour. Technol. 2010, 101, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Vidal, G.; Becerra, J.; Hernández, V.; Decap, J.; Xavier, C.R. Anaerobic biodegradation of sterols contained in kraft mill effluents. J. Biosci. Bioeng. 2007, 104, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.L.; Wong, M.H. Pharmaceuticals and personal care products (PPCPs): A review on environmental contamination in China. Environ. Int. 2013, 59, 208–224. [Google Scholar] [CrossRef] [PubMed]
- Neumann, P.; González Vidal, G. Sequential ultrasound and low temperature thermal pretreatment: Process optimization and influence on sewage sludge solubilization, enzyme activity and anaerobic digestion. Bioresour. Technol. 2017, 234, 178–187. [Google Scholar] [CrossRef]
- Grandclément, C.; Seyssiecq, I.; Piram, A.; Wong-Wah-Chung, P.; Vanot, G.; Tiliacos, N.; Roch, N.; Doumenq, P. From the conventional biological wastewater treatment to hybrid processes, the evaluation of organic micropollutant removal: A review. Water Res. 2017, 111, 297–317. [Google Scholar] [CrossRef]
- Vidal, G.; Navia, R.; Levet, L.; Mora, M.L.; Diez, M.C. Kraft mill anaerobic effluent color enhancement by a fixed-bed adsorption system. Biotechnol. Lett. 2001, 23, 861–865. [Google Scholar] [CrossRef]
- Carballa, M.; Omil, F.; Lema, J.M.; Llompart, M.; García-Jares, C.; Rodríguez, I.; Gómez, M.; Ternes, T. Behavior of pharmaceuticals, cosmetics and hormones in a sewage treatment plant. Water Res. 2004, 38, 2918–2926. [Google Scholar] [CrossRef]
- Kosma, C.I.; Lambropoulou, D.A.; Albanis, T.A. Investigation of PPCPs in wastewater treatment plants in Greece: Occurrence, removal and environmental risk assessment. Sci. Total Environ. 2014, 466, 421–438. [Google Scholar] [CrossRef] [PubMed]
- Radjenović, J.; Petrović, M.; Barceló, D. Fate and distribution of pharmaceuticals in wastewater and sewage sludge of the conventional activated sludge (CAS) and advanced membrane bioreactor (MBR) treatment. Water Res. 2009, 43, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Ziylan, A.; Ince, N.H. The occurrence and fate of anti-inflammatory and analgesic pharmaceuticals in sewage and fresh water: Treatability by conventional and non-conventional processes. J. Hazard. Mater. 2011, 187, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Ávila, C.; Bayona, J.M.; Martín, I.; Salas, J.J.; García, J. Emerging organic contaminant removal in a full-scale hybrid constructed wetland system for wastewater treatment and reuse. Ecol. Eng. 2015, 80, 108–116. [Google Scholar] [CrossRef]
- Vera, I.; García, J.; Sáez, K.; Moragas, L.; Vidal, G. Performance evaluation of eight years experience from constructed wetlands systems in Catalonia as alternative treatment for small communities. Ecol. Eng. 2011, 37, 364–371. [Google Scholar] [CrossRef]
- Kadlec, R.H.; Wallace, S.D. Treatment Wetlands; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Hijosa-Valsero, M.; Reyes-Contreras, C.; Dominguez, C.; Bécares, E.; Bayona, J.M. Behaviour of pharmaceuticals and personal care products in constructed wetland compartments: Influent, effluent, pore water, substrate and plant roots. Chemosphere 2016, 145, 508–517. [Google Scholar] [CrossRef]
- Vymazal, J.; Březinová, T.D.; Koželuh, M.; Kule, L. Occurrence and removal of pharmaceuticals in four full-scale constructed wetlands in the Czech Republic—The first year of monitoring. Ecol. Eng. 2017, 98, 354–364. [Google Scholar] [CrossRef]
- Matamoros, V.; Arias, C.; Brix, H.; Bayona, J.M.; Arias, C.A. Preliminary screening of small-scale domestic wastewater treatment systems for removal of pharmaceutical and personal care products. Water Res. 2009, 43, 55–62. [Google Scholar] [CrossRef]
- Matamoros, V.; Salvadó, V. Evaluation of the seasonal performance of a water reclamation pond-constructed wetland system for removing emerging contaminants. Chemosphere 2012, 86, 111–117. [Google Scholar] [CrossRef]
- Matamoros, V.; Rodríguez, Y.; Albaigés, J. A comparative assessment of intensive and extensive wastewater treatment technologies for removing emerging contaminants in small communities. Water Res. 2016, 88, 777–785. [Google Scholar] [CrossRef]
- Verlicchi, P.; Zambello, E. How efficient are constructed wetlands in removing pharmaceuticals from untreated and treated urban wastewaters? A review. Sci. Total Environ. 2014, 470, 1281–1306. [Google Scholar] [CrossRef] [PubMed]
- Matamoros, V.; Bayona, J.M. Elimination of Pharmaceuticals and Personal Care Products in Subsurface Flow Constructed Wetlands. Environ. Sci. Technol. 2006, 40, 5811–5816. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Z.; Dong, H.; Zhu, B.; Qu, J.; Nie, Y. A comparison of various rural wastewater treatment processes for the removal of endocrine-disrupting chemicals (EDCs). Chemosphere 2013, 92, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Thomaidis, N.S.; Xu, J. Progress in the biological and chemical treatment technologies for emerging contaminant removal from wastewater: A critical review. J. Hazard. Mater. 2017, 323, 274–298. [Google Scholar] [CrossRef] [PubMed]
- Melvin, S.D.; Leusch, F.D. Removal of trace organic contaminants from domestic wastewater: A meta-analysis comparison of sewage treatment technologies. Environ. Int. 2016, 92, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda-Mardones, M.; Lopez, D.; Vidal, G. Methanogenic activity in the biomass from horizontal subsurface flow constructed wetlands treating domestic wastewater. Ecol. Eng. 2017, 105, 66–77. [Google Scholar] [CrossRef]
- López, D.; Fuenzalida, D.; Vera, I.; Rojas, K.; Vidal, G. Relationship between the removal of organic matter and the production of methane in subsurface flow constructed wetlands designed for wastewater treatment. Ecol. Eng. 2015, 83, 296–304. [Google Scholar] [CrossRef]
- American Public Health Association (APHA). Standard Methods for the Examination of Wastewater; APHA Publishing: Washington, DC, USA, 2005. [Google Scholar]
- Masi, F.; Martinuzzi, N. Constructed wetlands for the Mediterranean countries: Hybrid systems for water reuse and sustainable sanitation. Desalination 2007, 215, 44–55. [Google Scholar] [CrossRef]
- Henze, M.; Harremoës, P.; La Cour Jansen, J.; Arvin, E. Wastewater Treatment: Biological and Chemical Processes; Springer: Heidelberg, Germany, 2002. [Google Scholar]
- Leiva, A.M.; Núñez, R.; Gómez, G.; Lopez, D.; Vidal, G. Performance of ornamental plants in monoculture and polyculture horizontal subsurface flow constructed wetlands for treating wastewater. Ecol. Eng. 2018, 120, 116–125. [Google Scholar] [CrossRef]
- Burgos, V.; Araya, F.; Reyes-Contreras, C.; Vera, I.; Vidal, G. Performance of ornamental plants in mesocosm subsurface constructed wetlands under different organic sewage loading. Ecol. Eng. 2017, 99, 246–255. [Google Scholar] [CrossRef]
- Ye, F.; Li, Y. Enhancement of nitrogen removal in towery hybrid constructed wetland to treat domestic wastewater for small rural communities. Ecol. Eng. 2009, 35, 1043–1050. [Google Scholar] [CrossRef]
- Vymazal, J.; Kröpfelová, L. Multistage hybrid constructed wetland for enhanced removal of nitrogen. Ecol. Eng. 2015, 84, 202–208. [Google Scholar] [CrossRef]
- Verlicchi, P.; Al Aukidy, M.; Zambello, E. Occurrence of pharmaceutical compounds in urban wastewater: Removal, mass load and environmental risk after a secondary treatment—A review. Sci. Total Environ. 2012, 429, 123–155. [Google Scholar] [CrossRef]
- Lozano, N.; Rice, C.P.; Ramirez, M.; Torrents, A. Fate of Triclocarban, Triclosan and Methyltriclosan during wastewater and biosolids treatment processes. Water Res. 2013, 47, 4519–4527. [Google Scholar] [CrossRef] [PubMed]
- Ternes, T.A.; Joss, A.; Siegrist, H. Peer Reviewed: Scrutinizing Pharmaceuticals and Personal Care Products in Wastewater Treatment. Environ. Sci. Technol. 2004, 38, 392A–399A. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buser, H.-R.; Poiger, T.; Müller, M.D. Occurrence and Environmental Behavior of the Chiral Pharmaceutical Drug Ibuprofen in Surface Waters and in Wastewater. Environ. Sci. Technol. 1999, 33, 2529–2535. [Google Scholar] [CrossRef]
- Ferrando-Climent, L.; Collado, N.; Buttiglieri, G.; Gros, M.; Rodríguez-Roda, I.; Rodríguez-Mozaz, S.; Barceló, D. Comprehensive study of ibuprofen and its metabolites in activated sludge batch experiments and aquatic environment. Sci. Total Environ. 2012, 438, 404–413. [Google Scholar] [CrossRef]
- Clara, M.; Gans, O.; Windhofer, G.; Krenn, U.; Hartl, W.; Braun, K.; Scharf, S.; Scheffknecht, C. Occurrence of polycyclic musks in wastewater and receiving water bodies and fate during wastewater treatment. Chemosphere 2011, 82, 1116–1123. [Google Scholar] [CrossRef]
- Carranza-Diaz, O.; Schultze-Nobre, L.; Moeder, M.; Nivala, J.; Kuschk, P.; Koeser, H. Removal of selected organic micropollutants in planted and unplanted pilot-scale horizontal flow constructed wetlands under conditions of high organic load. Ecol. Eng. 2014, 71, 234–245. [Google Scholar] [CrossRef]
- Vymazal, J.; Kröpfelová, L. Removal of organics in constructed wetlands with horizontal sub-surface flow: A review of the field experience. Sci. Total Environ. 2009, 407, 3911–3922. [Google Scholar] [CrossRef]
- Zwiener, C.; Frimmel, F. Short-term tests with a pilot sewage plant and biofilm reactors for the biological degradation of the pharmaceutical compounds clofibric acid, ibuprofen, and diclofenac. Sci. Total Environ. 2003, 309, 201–211. [Google Scholar] [CrossRef]
- Alvarino, T.; Suarez, S.; Lema, J.; Omil, F. Understanding the removal mechanisms of PPCPs and the influence of main technological parameters in anaerobic UASB and aerobic CAS reactors. J. Hazard. Mater. 2014, 278, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Hijosa-Valsero, M.; Matamoros, V.; Pedescoll, A.; Martín-Villacorta, J.; Bécares, E.; García, J.; Bayona, J.M. Evaluation of primary treatment and loading regimes in the removal of pharmaceuticals and personal care products from urban wastewaters by subsurface-flow constructed wetlands. Int. J. Environ. Anal. Chem. 2011, 91, 632–653. [Google Scholar] [CrossRef]
- Avila, C.; Pedescoll, A.; Matamoros, V.; Bayona, J.M.; García, J. Capacity of a horizontal subsurface flow constructed wetland system for the removal of emerging pollutants: An injection experiment. Chemosphere 2010, 81, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Clara, M.; Strenn, B.; Gans, O.; Martinez, E.; Kreuzinger, N.; Kroiss, H. Removal of selected pharmaceuticals, fragrances and endocrine disrupting compounds in a membrane bioreactor and conventional wastewater treatment plants. Water Res. 2005, 39, 4797–4807. [Google Scholar] [CrossRef]
- Kimura, K.; Hara, H.; Watanabe, Y. Removal of pharmaceutical compounds by submerged membrane bioreactors (MBRs). Desalination 2005, 178, 135–140. [Google Scholar] [CrossRef]
- Adrian, N.R.; Suflita, J.M. Anaerobic biodegradation of halogenated and nonhalogenated N-, s-, and o-heterocyclic compounds in aquifer slurries. Environ. Toxicol. Chem. 1994, 13, 1551–1557. [Google Scholar] [CrossRef]
- Park, N.; Vanderford, B.J.; Snyder, S.A.; Sarp, S.; Kim, S.D.; Cho, J. Effective controls of micropollutants included in wastewater effluent using constructed wetlands under anoxic condition. Ecol. Eng. 2009, 35, 418–423. [Google Scholar] [CrossRef]
- Buerge, I.J.; Poiger, T.; Müller, M.D.; Buser, H.-R. Caffeine, an Anthropogenic Marker for Wastewater Contamination of Surface Waters. Environ. Sci. Technol. 2003, 37, 691–700. [Google Scholar] [CrossRef]
- Staples, C.A.; Dome, P.B.; Klečka, G.M.; Oblock, S.T.; Harris, L.R. A review of the environmental fate, effects, and exposures of bisphenol A. Chemosphere 1998, 36, 2149–2173. [Google Scholar] [CrossRef]
- Froehner, S.; Piccioni, W.; Machado, K.S.; Aisse, M.M. Removal Capacity of Caffeine, Hormones, and Bisphenol by Aerobic and Anaerobic Sewage Treatment. Water Air Soil Pollut. 2011, 216, 463–471. [Google Scholar] [CrossRef]
- Navia, R.; Levet, L.; Mora, M.L.; Vidal, G.; Díez, M.C. Allophanic Soil Adsorption System as a Bleached Kraft Mill Aerobic Effluent Post-Treatment. Water, Air, Soil Pollut. 2003, 148, 323–333. [Google Scholar] [CrossRef]
- Federle, T.W.; Kaiser, S.K.; Nuck, B.A. Fate and effects of triclosan in activated sludge. Environ. Toxicol. Chem. 2002, 21, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Ying, G.-G.; Yu, X.-Y.; Kookana, R.S. Biological degradation of triclocarban and triclosan in a soil under aerobic and anaerobic conditions and comparison with environmental fate modelling. Environ. Pollut. 2007, 150, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Bendz, D.; Paxéus, N.A.; Ginn, T.R.; Loge, F.J. Occurrence and fate of pharmaceutically active compounds in the environment, a case study: Höje River in Sweden. J. Hazard. Mater. 2005, 122, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Hijosa-Valsero, M.; Matamoros, V.; Sidrach-Cardona, R.; Martín-Villacorta, J.; Bécares, E.; Bayona, J.M. Comprehensive assessment of the design configuration of constructed wetlands for the removal of pharmaceuticals and personal care products from urban wastewaters. Water Res. 2010, 44, 3669–3678. [Google Scholar] [CrossRef] [PubMed]
- Polprasert, C.; Kittipongvises, S. Treatise on Water Science; Elsevier: Oxford, UK, 2011. [Google Scholar]
- Samaras, V.G.; Stasinakis, A.S.; Mamais, D.; Thomaidis, N.S.; Lekkas, T.D. Fate of selected pharmaceuticals and synthetic endocrine disrupting compounds during wastewater treatment and sludge anaerobic digestion. J. Hazard. Mater. 2013, 244, 259–267. [Google Scholar] [CrossRef]
- Matamoros, V.; Arias, C.; Brix, H.; Bayona, J.M. Removal of pharmaceuticalsand personal care products (PPCPs) from urban wastewater in a pilotvertical flow constructed wetland and a sand filter. Environ. Sci. Technol. 2007, 41, 8171–8177. [Google Scholar] [CrossRef]
- Reyes-Contreras, C.; Hijosa-Valsero, M.; Sidrach-Cardona, R.; Bayona, J.M.; Bécares, E. Temporal evolution in PPCP removal from urban wastewater by constructed wetlands of different configuration: A medium-term study. Chemosphere 2012, 88, 161–167. [Google Scholar] [CrossRef]
- Matamoros, M.; Hijosa-Valsero Bayona, J.M. Assessment of the pharmaceuticalactive compounds removal in wastewater treatment systems at enantiomeric level. Ibuprofen and naproxen. Chemosphere 2009, 75, 200–205. [Google Scholar] [CrossRef]
- Yeber, M.C.; Soto, C.; Riveros, R.; Navarrete, J.; Vidal, G. Optimization by factorial design of copper (II) and toxicity removal using a photocatalytic process with TiO2 as semiconductor. Chem. Eng. J. 2009, 152, 14–19. [Google Scholar] [CrossRef]


 ) and HSSFs (
) and HSSFs (  ). Error bars represent standard deviations.
). Error bars represent standard deviations.
 ) and HSSFs (
) and HSSFs (  ). Error bars represent standard deviations.
). Error bars represent standard deviations.
 ) and HSSFs (
) and HSSFs (  ). (Abbreviations used: ibuprofen (IBU); naproxen (NAP); diclofenac (DIC); methyl dihydrojasmonate (MDHJ); galaxolide (GAL); caffeine (CAF); carbamazepine (CAR); bisphenol-A (BIS-A); triclosan (TRI); 2-hydroxy ibuprofen (2OH-IBU); 1-hydroxy ibuprofen (1OH-IBU) and carboxy-ibuprofen (CAR-IBU)). Error bars represent standard deviations.
). (Abbreviations used: ibuprofen (IBU); naproxen (NAP); diclofenac (DIC); methyl dihydrojasmonate (MDHJ); galaxolide (GAL); caffeine (CAF); carbamazepine (CAR); bisphenol-A (BIS-A); triclosan (TRI); 2-hydroxy ibuprofen (2OH-IBU); 1-hydroxy ibuprofen (1OH-IBU) and carboxy-ibuprofen (CAR-IBU)). Error bars represent standard deviations.
 ) and HSSFs (
) and HSSFs (  ). (Abbreviations used: ibuprofen (IBU); naproxen (NAP); diclofenac (DIC); methyl dihydrojasmonate (MDHJ); galaxolide (GAL); caffeine (CAF); carbamazepine (CAR); bisphenol-A (BIS-A); triclosan (TRI); 2-hydroxy ibuprofen (2OH-IBU); 1-hydroxy ibuprofen (1OH-IBU) and carboxy-ibuprofen (CAR-IBU)). Error bars represent standard deviations.
). (Abbreviations used: ibuprofen (IBU); naproxen (NAP); diclofenac (DIC); methyl dihydrojasmonate (MDHJ); galaxolide (GAL); caffeine (CAF); carbamazepine (CAR); bisphenol-A (BIS-A); triclosan (TRI); 2-hydroxy ibuprofen (2OH-IBU); 1-hydroxy ibuprofen (1OH-IBU) and carboxy-ibuprofen (CAR-IBU)). Error bars represent standard deviations.
| OMPs | Uses | pKa | Log Kow | Log Koc | Log Dow | Water Solubility at 25 °C (mg/L) | Molecular Structure |
|---|---|---|---|---|---|---|---|
| Naproxen | Analgesic/ anti-inflammatory | 4.15 | 3.10 | 1.97 | 0.25 | 44.07 | 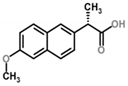 |
| Ibuprofen | 4.91 | 3.79 | 2.35 | 1.70 | 57.97 | 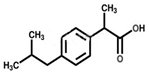 | |
| Diclofenac | 4.15 | 4.02 | 2.61 | 1.17 | 10.89 | 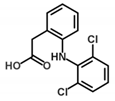 | |
| Carbamazepine | Anticonvulsant | n.a. | 2.25 | 2.28 | n.a. | 30.48 | 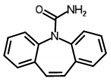 |
| Caffeine | Stimulant | −0.07 | 0.16 | 0.98 | −6.91 | 11,308 | 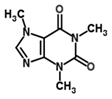 |
| Triclosan | Antibacterial/ antifungal | 7.90 | 4.66 | 3.93 | 3.66 | 9.30 | 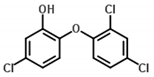 |
| Methyl dihydrojasmonate | Fragrances | n.a. | 2.98 | 2.70 | n.a. | 154.88 | 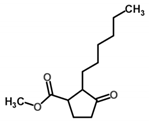 |
| Tonalide | n.a. | 6.35 | 4.72 | n.a. | 0.37 |  | |
| Galaxolide | n.a. | 6.26 | 4.10 | n.a. | 0.20 | 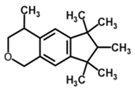 | |
| Bisphenol-A | Manufacture of plastics and epoxy resins | 8.73 | 3.64 | 3.10 | 3.63 | 146.15 | 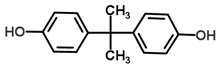 |
| 1-Hydroxy ibuprofen | Ibuprofen transformation products | 4.55 | 2.25 | 0.99 | −3.39 | 3192 |  |
| 2-Hydroxy ibuprofen | 4.63 | 2.29 | 1.01 | −3.31 | 2974 |  | |
| Carboxy-ibuprofen | 3.97 | 1.97 | 1.25 | −4.03 | 1453 |  | |
| Ibuprofen amide | n.a. | −2.75 | −1.03 | n.a. | 8083 | 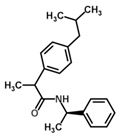 |
| Parameters | Influent | Effluents | |
|---|---|---|---|
| AS | HSSFs | ||
| pH | (7.95–8.81) | (6.89–7.17) | (7.05–7.75) |
| Temperature (°C) | 17 (15–20) | 17 (15–20) | 16 (15–20) |
| EC (mS/cm) | 3799 (2947–4534) | 1962 (1574–2293) | 3396 (2481–4293) |
| ORP (mV) | −229 (−291–−154) | 369 (300–457) | −281 (−241–(−341)) |
| DO (mg/L) | 0.53 (0.42–0.65) | 5.53 (4.25–7.32) | 0.24 (0.07–0.84) |
| COD (mg/L) | 274 (115–465) | 10.9 (4.6–18.5) | 74.4 (31.2–126.2) |
| BOD5 (mg/L) | 184 (48–372) | 0.4 (0.1–0.8) | 77.5 (20.2–156.8) |
| PO4 3−- P (mg/L) | 10 (5–15) | 1.8 (0.9–2.7) | 16 (14–18) |
| NH4+- N (mg/L) | 86 (31–190) | 21.9 (7.9–48.3) | 53.4 (19.2–118.0) |
| TC (mg/L) | 189 (162–210) | 50 (39–67) | 114 (97–137) |
| TOC (mg/L) | 83 (56–105) | 10 (7.66–15) | 20 (15–23) |
| TSS (mg/L) | 255 (76–565) | 5.6 (1.7–12.4) | 67.0 (20.0–148.5) |
| VSS (mg/L) | 193 (33–513) | 3.5 (0.6–9.4) | 37.6 (6.4–100.0) |
| OMPs (µg/L) | Influent | Effluents | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | Range | AS | HSSFs | |||||
| Mean | Median | Range | Mean | Median | Range | ||||
| Naproxen | 2.29 ± 0.72 | 2.43 | 1.94–3.69 | 0.010 ± 0.001 | 0.01 | 0.005–0.01 | 1.01 ± 0.26 | 1.14 | 0.76–1.39 |
| Ibuprofen | 7.75 ± 0.96 | 8.03 | 6.11–8.61 | 0.040 ± 0.003 | 0.03 | 0.03–0.04 | 2.62 ± 0.30 | 2.75 | 2.40–3.08 |
| Diclofenac | 1.03 ± 0.30 | 0.91 | 0.76–1.51 | 0.20 ± 0.04 | 0.19 | 0.16–0.26 | 0.28 ± 0.06 | 0.29 | 0.25–0.39 |
| Carbamazepine | 0.22 ± 0.05 | 0.21 | 0.19–0.31 | 0.16 ± 0.03 | 0.17 | 0.12–0.18 | 0.16 ± 0.02 | 0.15 | 0.15–0.20 |
| Caffeine | 7.33 ± 1.29 | 8.15 | 5.27–8.20 | 0.12 ± 0.01 | 0.12 | 0.10–0.13 | 0.57 ± 0.15 | 0.59 | 0.37–0.69 |
| Triclosan | 0.20 ± 0.06 | 0.19 | 0.15–0.30 | 0.020 ± 0.003 | 0.02 | 0.018–0.025 | 0.11 ± 0.04 | 0.11 | 0.06–0.14 |
| Methyl dihydrojasmonate | 3.49 ± 1.54 | 3.58 | 1.04–4.89 | 0.18 ± 0.01 | 0.18 | 0.16–0.19 | 0.53 ± 0.10 | 0.55 | 0.34–0.58 |
| Tonalide | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| Galaxolide | 1.41 ± 0.31 | 1.21 | 0.75–1.58 | 1.15 ± 0.18 | 1.11 | 0.97–1.39 | 0.73 ± 0.13 | 0.76 | 0.57–0.89 |
| Bisphenol-A | 0.16 ± 0.05 | 0.13 | 0.12–0.23 | 0.020 ± 0.002 | 0.018 | 0.016–0.02 | 0.07 ± 0.02 | 0.06 | 0.05–0.11 |
| 1-hydroxy ibuprofen | 8.32 ± 1.32 | 8.22 | 7.17–10.53 | <LOD | <LOD | <LOD | 3.26 ± 0.35 | 3.11 | 3.02–3.66 |
| 2-hydroxy ibuprofen | 29.24 ±9.87 | 29.86 | 26.38–50.45 | 0.78 ± 0.29 | 0.72 | 0.48–1.18 | 28 ± 2 | 27.77 | 24.83–29.55 |
| Carboxy-ibuprofen | 53.58 ± 7.94 | 55.65 | 42.50–60.52 | 0.21 ± 0.06 | 0.20 | 0.14–0.28 | 41.40 ± 2.34 | 40.35 | 38.98–44.21 |
| Ibuprofen amide | 0.56 ± 0.14 | 0.43 | 0.43–0.70 | <LOD | <LOD | <LOD | n.d. | n.d | n.d |
| OMPs (µg/L) | Influent | Effluents | |
|---|---|---|---|
| AS | HSSFs | ||
| Carbamazepine | 0.14 ± 0.05 | 0.05 ± 0.01 | 0.12 ± 0.03 |
| Triclosan | ± 0.01 | 0.003 ± 0.001 | <LOD |
| Tonalide | <LOD | <LOD | <LOD |
| Galaxolide | 0.55 ± 0.14 | 0.13 ± 0.04 | 0.37 ± 0.13 |
| Bisphenol–A | 0.02 ± 0.01 | 0.006 ± 0.001 | 0.010 ± 0.003 |
| Parameters | AS | HSSFs | ||
|---|---|---|---|---|
| Zone A | Zone B | Zone C | ||
| EOM (µg/kg) | 1912 ± 436 | 246 ± 16 | 225 ± 17 | 101 ± 5 |
| Triclosan (µg/kg) | 0.010 ± 0.002 | 0.0030 ± 0.0005 | <LOD | <LOD |
| Galaxolide (µg/kg) | 0.37 ± 0.03 | 0.020 ± 0.003 | 0.0070 ± 0.0002 | 0.0010 ± 0.0001 |
| Bisphenol-A (µg/kg) | 0.0010 ± 0.0001 | <LOD | <LOD | <LOD |
| 1-Hydroxy ibuprofen (µg/kg) | 0.110 ± 0.006 | 0.009 ± 0.001 | 0.006 ± 0.001 | <LOD |
| 2-Hydroxy ibuprofen (µg/kg) | 0.030 ± 0.004 | 0.0020 ± 0.0003 | 0.0010 ± 0.0002 | <LOD |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes Contreras, C.; López, D.; Leiva, A.M.; Domínguez, C.; Bayona, J.M.; Vidal, G. Removal of Organic Micropollutants in Wastewater Treated by Activated Sludge and Constructed Wetlands: A Comparative Study. Water 2019, 11, 2515. https://doi.org/10.3390/w11122515
Reyes Contreras C, López D, Leiva AM, Domínguez C, Bayona JM, Vidal G. Removal of Organic Micropollutants in Wastewater Treated by Activated Sludge and Constructed Wetlands: A Comparative Study. Water. 2019; 11(12):2515. https://doi.org/10.3390/w11122515
Chicago/Turabian StyleReyes Contreras, Carolina, Daniela López, Ana M. Leiva, Carmen Domínguez, Josep M. Bayona, and Gladys Vidal. 2019. "Removal of Organic Micropollutants in Wastewater Treated by Activated Sludge and Constructed Wetlands: A Comparative Study" Water 11, no. 12: 2515. https://doi.org/10.3390/w11122515
APA StyleReyes Contreras, C., López, D., Leiva, A. M., Domínguez, C., Bayona, J. M., & Vidal, G. (2019). Removal of Organic Micropollutants in Wastewater Treated by Activated Sludge and Constructed Wetlands: A Comparative Study. Water, 11(12), 2515. https://doi.org/10.3390/w11122515





