The Endemic Species Flock of Labeobarbus spp. in L. Tana (Ethiopia) Threatened by Extinction: Implications for Conservation Management
Abstract
:1. Introduction
2. Materials and Methods
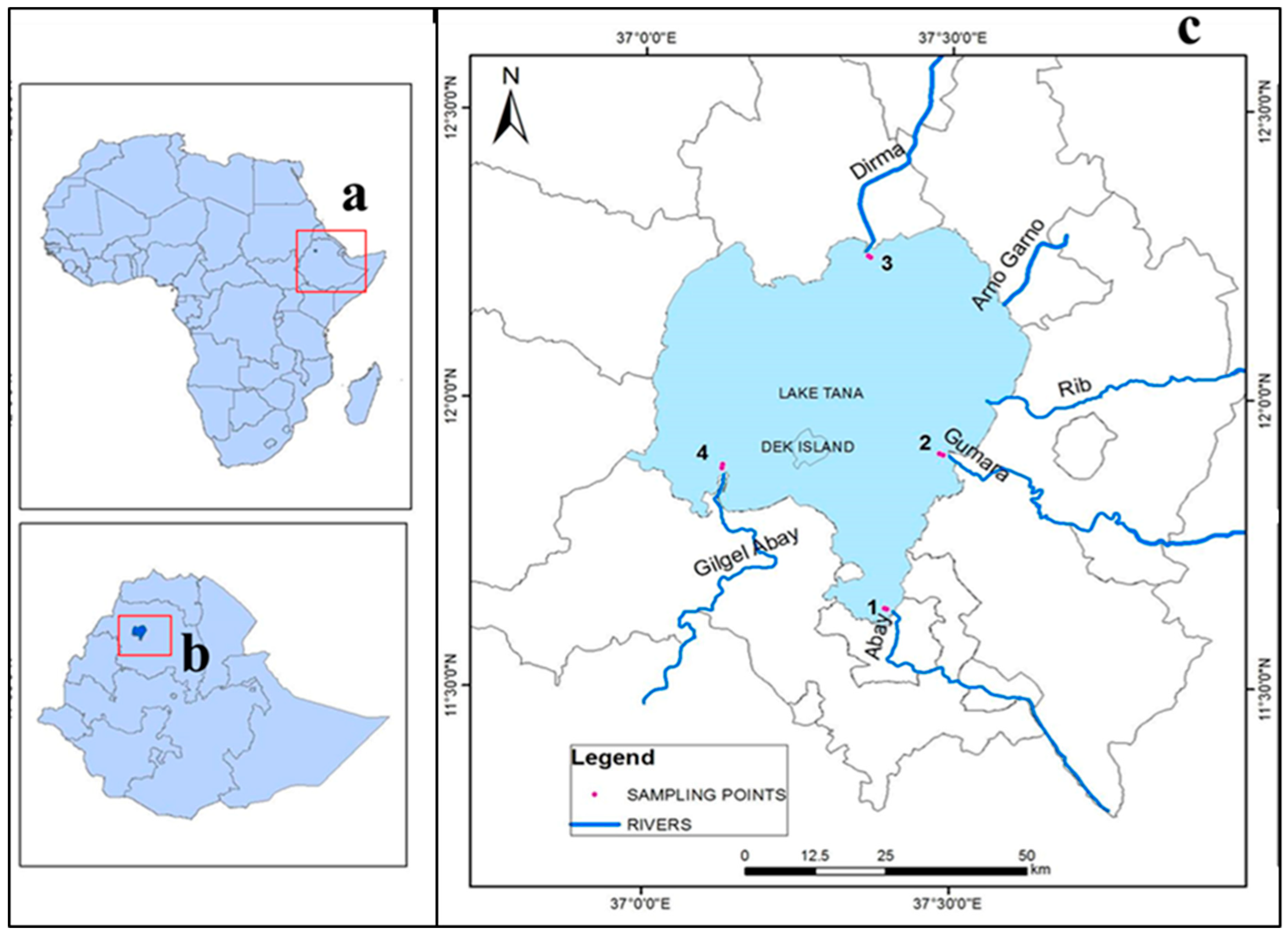
2.1. Study Area
2.2. Sampling Techniques
2.3. Data Analysis
3. Results
3.1. Abundance and Catch Distribution of Labeobarbus Species
3.1.1. Relative Abundance
3.1.2. Catch Per Unit Effort
3.2. Size Structure of Labeobarbus Species
3.2.1. Length Distribution
3.2.2. Size at Aaturity
4. Discussion
4.1. The Declining Stock of Labeobarbus Spp.
4.2. Insights into Size Distributions to Optimize Sustainable Fisheries Management
4.3. Management Strategies
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Species | Site | Total | ||||
|---|---|---|---|---|---|---|
| BD | GU | GO | KU | |||
| L. intermedius | Count | 373 (405)a | 514 (418)b | 300 (343)a | 267 (289)a | 1454 |
| Adjusted | −2.3 | 6.9 | −3.3 | −1.8 | ||
| L. tsanensis | Count | 242 (233)a | 216 (241)a | 217 (197)a | 162 (166)a | 837 |
| Adjusted | 0.8 | −2.1 | 1.8 | −0.4 | ||
| L. platydorsus | Count | 119 (173)a | 126 (179)a | 237 (147)b | 141 (124)c | 623 |
| Adjusted | −5.3 | −5.1 | 9.2 | 1.9 | ||
| L. megastoma | Count | 100 (94)a | 147 (97)b | 69 (80)a | 23 (67)c | 339 |
| Adjusted | 0.7 | 6.2 | −1.5 | −6.3 | ||
| L. brevicephalus | Count | 181 (124)a | 63 (128)b | 69 (105)b | 133 (89)a | 446 |
| Adjusted | 6.4 | −7.2 | −4.3 | 5.6 | ||
| L. crassibarbis | Count | 32 (41)a | 42 (42)a, b | 49 (35)b | 24 (29)a, b | 147 |
| Adjusted | −1.7 | 0.0 | 2.8 | −1.1 | ||
| L. nedgia | Count | 43 (27)a | 14 (28)b | 15 (23)b, c | 26 (20)a, c | 98 |
| Adjusted | 3.6 | −3.2 | −2.0 | 1.7 | ||
| L. gorgorensis | Count | 29 (22)a | 27 (22)a | 14 (18)a | 8 (16)a | 78 |
| Adjusted | 1.9 | 1.2 | −1.2 | −2.1 | ||
| L. truttiformis | Count | 9 (19)a, b | 37 (20)c | 5 (16)b | 17 (14)a, c | 68 |
| Adjusted | −2.7 | 4.7 | −3.2 | 1.1 | ||
| L. surkis | Count | 29 (20)a, b | 7 (21)c | 10 (17)b, c | 26 (14)a | 72 |
| Adjusted | 2.4 | −3.6 | −2.0 | 3.5 | ||
| L. longissimus | Count | 9 (8)a | 9 (8)a | 5 (7)a | 5 (6)a | 28 |
| Adjusted | 0.5 | 0.4 | −0.7 | −0.3 | ||
| L. macrophtalmus | Count | 9 (7)a | 8 (8)a | 5 (6)a | 4 (5)a | 26 |
| Adjusted | 0.8 | 0.2 | −0.5 | −0.6 | ||
| L. acutirostris | Count | 0 (4)a | 7 (4)a | 2 (3)a | 4 (3)a | 13 |
| Adjusted | −2.2 | 2.0 | −0.7 | 1,0 | ||
| L. gorguari | Count | 3 (1)a | 0 (1)a | 0 (1)a | 1 (1)a | 4 |
| Adjusted | 1.5 | −1.1 | −1.0 | 0.6 | ||
| L. daniellii | Count | 1 (1)a | 0 (1)a | 1 (1)a | 0 (1)a | 2 |
| Adjusted | 0.7 | −0.9 | 0.9 | −0.7 | ||
| Total | 1179 | 1217 | 998 | 841 | 4235 | |
Appendix B
| Species | n | a | b | P Value | FLmin | FL50% | 95%CI | ||
|---|---|---|---|---|---|---|---|---|---|
| Sex | Lower | Upper | |||||||
| L. intermedius | F | 361 | −5.0 | 0.2 | ** | 13.3 | 24.0 | 25.5 | 26.6 |
| M | 298 | −7.5 | 0.4 | ** | 13.5 | 17.3 | 16.5 | 17.9 | |
| L. tsanensis | F | 183 | −16.5 | 0.7 | ** | 14.2 | 22.3 | 21.9 | 22.9 |
| M | 125 | −7.6 | 0.4 | ** | 14.5 | 18.3 | 17.2 | 19.3 | |
| L. platydorsus | F | 108 | −17.7 | 0.7 | ** | 14.1 | 25.0 | 23.8 | 25.9 |
| M | 93 | −17.2 | 0.7 | ** | 14.2 | 23.6 | 22.6 | 25.2 | |
| L. megastoma | F | 39 | −11.4 | 0.4 | ** | 15.0 | 28.7 | 26.2 | 31.0 |
| M | 70 | −8.1 | 0.3 | ** | 16.2 | 25.4 | 23.2 | 27.3 | |
Appendix C
| Species | Mesh Size (Monofilament Gillnets) | Mesh Size (Multifilament Gillnets) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 cm | 6 cm | 6 cm | 8 cm | 10 cm | 12 cm | 14 cm | |||||||||||||||
| FL | TW | N | FL | TW | N | FL | TW | N | FL | TW | N | FL | TW | N | FL | TW | N | FL | TW | N | |
| L. intermedius | 10–25 | 11−413 | 438 | 13−42 | 30−1060 | 272 | 14−34 | 13−434.9 | 209 | 14.7−36 | 30−1255 | 357 | 18−52 | 80−2800 | 72 | 18.3−46 | 60−1625 | 82 | 22−65 | 150−4305 | 24 |
| L. tsanensis | 13−30 | 33−419 | 276 | 12−30 | 32−425 | 142 | 13−34 | 32−489 | 121 | 16−38 | 24−845 | 195 | 18−37 | 80−2755 | 60 | 20−43 | 82−1150 | 42 | 43 | 1325 | 1 |
| L. platydorsus | 13−26 | 31−232 | 150 | 12−28 | 40−315 | 99 | 12−29 | 17−305 | 88 | 16−47 | 45−1830 | 174 | 16−59 | 45−2685 | 68 | 21−52 | 147−2125 | 23 | 26−66 | 470−4095 | 21 |
| L. brevicephalus | 10−23 | 10−165 | 280 | 13−23 | 30−160 | 63 | 13−25 | 34−185 | 49 | 14−26 | 35−1026 | 53 | 28 | 340 | 1 | ||||||
| L. megastoma | 15−22 | 37−106 | 22 | 18−25 | 65−150 | 7 | 16−33 | 35−420 | 49 | 12−35 | 114−448 | 70 | 22−45 | 102−1070 | 112 | 27−57 | 170−2300 | 48 | 34−55 | 405−2175 | 31 |
| L. crassibarbis | 14−22 | 39–145 | 34 | 13–27 | 35–260 | 19 | 13–26 | 35–256 | 15 | 17–60 | 65–3660 | 42 | 27–55 | 215–2550 | 11 | 19–44 | 72–1485 | 18 | 37–53 | 1200–2330 | 8 |
| L. gorgorensis | 14–23 | 43–180 | 27 | 20–27 | 100–275 | 12 | 21–25 | 100–225 | 9 | 21–33 | 123–420 | 8 | 21–31 | 135–460 | 10 | 24–43 | 179–1300 | 8 | 33–40 | 556–1060 | 2 |
| L. longissimus | 15–16 | 38–49 | 3 | 21–29 | 145–390 | 5 | 21–33 | 180–625 | 8 | 21–45 | 115–1065 | 7 | 35–41 | 620–940 | 3 | 32–43 | 386–1010 | 2 | |||
| L. nedgia | 13–29 | 30–355 | 46 | 12–29 | 22–316 | 18 | 18–27 | 57–289 | 13 | 26–41 | 200–1060 | 9 | 21–55 | 133–3060 | 7 | 25–56 | 218–3190 | 4 | |||
| L. macrophtalmus | 15 | 51 | 1 | 19–20 | 95 | 2 | 21–28 | 120–279 | 8 | 23–36 | 158–645 | 10 | 31–37 | 266–525 | 5 | ||||||
| L. truttiformis | 16–17 | 46–57 | 7 | 21–25 | 120–580 | 9 | 23–31 | 219–445 | 13 | 27–40 | 245–1000 | 21 | 25–40 | 288–820 | 10 | 26–49 | 700–1820 | 8 | |||
| L. surkis | 14–26 | 35–413 | 51 | 17–21 | 74–125 | 4 | 17–25 | 80–238 | 13 | 18–22 | 90–161 | 4 | |||||||||
| L. gorguari | 18–21 | 78–121 | 2 | 20 | 122 | 1 | 40 | 980 | 1 | ||||||||||||
| L. daniellii | 24 | 155 | 1 | 31 | 345 | 1 | |||||||||||||||
| L. acutirostris | 21–24 | 95–150 | 2 | 26–28 | 215–285 | 5 | 26–31 | 270–340 | 3 | 37–39 | 710–780 | 2 | 54 | 2320 | 1 | ||||||
References
- Nagelkerke, L.A.; Sibbing, F.A. The large barbs (Barbus spp., Cyprinidae, Teleostei) of Lake Tana (Ethiopia), with a description of a new species, Barbus osseensis. Neth. J. Zool. 2000, 50, 179–214. [Google Scholar] [CrossRef]
- Palstra, A.P.; de Graaf, M.; Sibbing, F.A. Riverine spawning and reproductive segregation in a lacustrine cyprinid species flock, facilitated by homing? Anim. Biol. 2004, 54, 393–415. [Google Scholar]
- de Graaf, M.; Nentwich, E.D.; Osse, J.W.M.; Sibbing, F.A. Lacustrine spawning: Is this a new reproductive strategy among ‘large’ African cyprinid fishes? J. Fish Biol. 2005, 66, 1214–1236. [Google Scholar] [CrossRef]
- Anteneh, W.; Getahun, A.; Dejen, E.; Sibbing, F.A.; Nagelkerke, L.A.J.; De Graaf, M.; Wudneh, T.; Vijverberg, J.; Palstra, A.P. Spawning migrations of the endemic Labeobarbus (Cyprinidae, Teleostei) species of Lake Tana, Ethiopia: Status and threats. J. Fish Biol. 2012, 81, 750–765. [Google Scholar] [CrossRef] [PubMed]
- Gebremedhin, S.; Mingist, M.; Getahun, A.; Anteneh, W. Spawning migration of Labeobarbus spp. (Pisces: Cyprinidae) of Lake Tana to Arno-Garno river, Lake Tana sub-basin, Ethiopia. SINET Ethiop. J. Sci. 2012, 35, 95–106. [Google Scholar]
- Sibbing, F.A.; Nagelkerke, L.A.J. Resource partitioning by Lake Tana barbs predicted from fish morphometrics and prey characteristics. Rev. Fish Biol. Fish. 2001, 10, 393–437. [Google Scholar] [CrossRef]
- Gebremedhin, S.; Budusa, M.; Mingist, M.; Vijverberg, J. Determining factors for fishers’ income: The case of Lake Tana, Ethiopia. Int. J. Curr. Res. 2013, 5, 1182–1186. [Google Scholar]
- Gebremedhin, S.; Getahun, A.; Anteneh, W.; Bruneel, S.; Goethals, P. A Drivers-Pressure-State-Impact-Responses Framework to Support the Sustainability of Fish and Fisheries in Lake Tana, Ethiopia. Sustainability 2018, 10, 20. [Google Scholar] [CrossRef] [Green Version]
- Asfaw, K. Assessment of Reed Boat Fishery in the Northern Part of Lake Tana; Bahir Dar University: Bahir Dar, Ethiopia, 2013. [Google Scholar]
- Mingist, M.; Gebremedhin, S. Could sand mining be a major threat for the declining endemic Labeobarbus species of Lake Tana, Ethiopia? Singap. J. Trop. Geogr. 2016, 37, 195–208. [Google Scholar] [CrossRef]
- Gebremedhin, S.; Getahun, A.; Anteneh, W.; Gedif, B.; Gashu, B.; Tefera, B.; Berhanie, Z.; Alemaw, D. Effect of large weirs on abundance and diversity of migratory Labeobarbus species in tributaries of Lake Tana, Ethiopia. Afr. J. Aquat. Sci. 2017, 42, 367–373. [Google Scholar]
- de Graaf, M.; van Zwieten, P.A.M.; Machiels, M.A.M.; Lemma, E.; Wudneh, T.; Dejen, E.; Sibbing, F.A. Vulnerability to a small-scale commercial fishery of Lake Tana’s (Ethiopia) endemic Labeobarbus compared with African catfish and Nile tilapia: An example of recruitment-overfishing? Fish. Res. 2006, 82, 304–318. [Google Scholar] [CrossRef]
- Dejen, E.; Anteneh, W.; Vijverberg, J. The Decline of the Lake Tana (Ethiopia) Fisheries: Causes and Possible Solutions. Land Degrad. Dev. 2017, 28, 1842–1851. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, B.; de Graaf, M.; Nagelkerke, L.; Mingist, M.W.A. Assessment of motorized commercial gillnet fishery of the three commercially important fishes in Lake Tana. In Proceedings of the Ethiopian Fisheries and Aquatic Sciences Association (EFASA), Addis Ababa, Ethiopia, 14 March 2013. [Google Scholar]
- Getahun, A. Labeobarbus species. In The IUCN Red List of Threatened Species 2010; International Union for Conservation of Nature and Natural Resources: Gland, Switzerland, 2010. [Google Scholar]
- Snoeks, J.; Laleye, P.; Getahun, A.; Contreras-MacBeath, T. Labeobarbus macrophtalmus. In The IUCN Red List of Threatened Species; International Union for Conservation of Nature and Natural Resources: Gland, Switzerland, 2010. [Google Scholar]
- Mohr, P.A. The Geology of Ethiopia; Haile Selassie I University Press: Addis Ababa, Ethiopia, 1971. [Google Scholar]
- Nagelkerke, L.A.J. The Barbs of Lake Tana, Ethiopia: Morphological Diversity and Its Implications for Taxonomy, Trophic Resource Partitioning, and Fisheries. Ph.D. Thesis, Esperimental Animal Morphology and Cell Biology, Wageningen Agricultural University, Wageningen, The Netherlands, 1997. [Google Scholar]
- Kolding, J. Population dynamics and life-history styles of Nile tilapia, Oreochromis niloticus, in Ferguson’s Gulf, Lake Turkana, Kenya. Environ. Biol. Fishes 1993, 37, 25–46. [Google Scholar] [CrossRef]
- Siegel, V.; Sushin, V.; Damm, U. Catch per unit effort (CPUE) data from the early years of commercial krill fishing operations in the Atlantic sector of the Antarctic. ccAMLR Sci. 1998, 5, 31–50. [Google Scholar]
- King, M. Fisheries Biology, Assessment and Management; John Wiley & Sons: Chichester, UK, 2013. [Google Scholar]
- Ogle, D. FSA: Fisheries Stock Analysis, R package Version 0.6; Wiley: Hoboken, NJ, USA, 2015; p. 13. [Google Scholar]
- Vijverberg, J.; Dejen, E.; Getahun, A.; Nagelkerke, L.A.J. The composition of fish communities of nine Ethiopian lakes along a north-south gradient: Threats and possible solutions. Anim. Biol. 2012, 62, 315–335. [Google Scholar] [CrossRef] [Green Version]
- Nagelkerke, L.A.J.; Sibbing, F.A.; Osse, J.W.M. Morphological divergence during growth in the large barbs (Barbus spp) of Lake Tana, Ethiopia. Neth. J. Zool. 1995, 45, 431–454. [Google Scholar]
- de Graaf, M.; Machiels, M.A.M.; Wudneh, T.; Sibbing, F.A. Declining stocks of Lake Tana’s endemic Barbus species flock (Pisces, Cyprinidae): Natural variation or human impact? Biol. Conserv. 2004, 116, 277–287. [Google Scholar] [CrossRef]
- Gordon, D. Technological change and economies of scale in the history of Mweru-Luapula’s fishery (Zambia and Democratic Republic of Congo). FAO Fish. Tech. Pap. 2003, 426, 165–178. [Google Scholar]
- Neumann, R.; Allen, M. Size structure. In Analysis and Interpretation of Freshwater Fisheries Data; American Fisheries Society: Bethesda, MD, USA, 2007; pp. 375–421. [Google Scholar]
- Hunter, A.; Speirs, D.C.; Heath, M.R. Fishery-induced changes to age and length dependent maturation schedules of three demersal fish species in the Firth of Clyde. Fish. Res. 2015, 170, 14–23. [Google Scholar] [CrossRef] [Green Version]
- Lappalainen, A.; Saks, L.; Šuštar, M.; Heikinheimo, O.; Jürgens, K.; Kokkonen, E.; Kurkilahti, M.; Verliin, A.; Vetemaa, M. Length at maturity as a potential indicator of fishing pressure effects on coastal pikeperch (Sander lucioperca) stocks in the northern Baltic Sea. Fish. Res. 2016, 174, 47–57. [Google Scholar] [CrossRef]
- de Graaf, M.; Machiels, M.; Wudneh, T.; Sibbing, F. Length at maturity and gillnet selectivity of Lake Tana’s Barbus species (Ethiopia): Implications for management and conservation. Aquat. Ecosyst. Health Manag. 2003, 6, 325–336. [Google Scholar] [CrossRef]
- Nagelkerke, L.A.J.; Sibbing, F.A. Reproductive segregation among the Barbus intermedius complex of Lake Tana, Ethiopia. An example of intralacustrine speciation? J. Fish. Biol. 1996, 49, 1244–1266. [Google Scholar] [CrossRef]
- Mengistu, A.A. The Effect of Birbira Milletia Ferruginea (HOCHST.) Baker on Some Barbus Species (Cyprinidae TELEOSTEI) in Gumara River (Lake Tana), Ethiopia. Master’s Thesis, Addis Ababa University, Addis Ababa, Ethiopia, 2004. [Google Scholar]



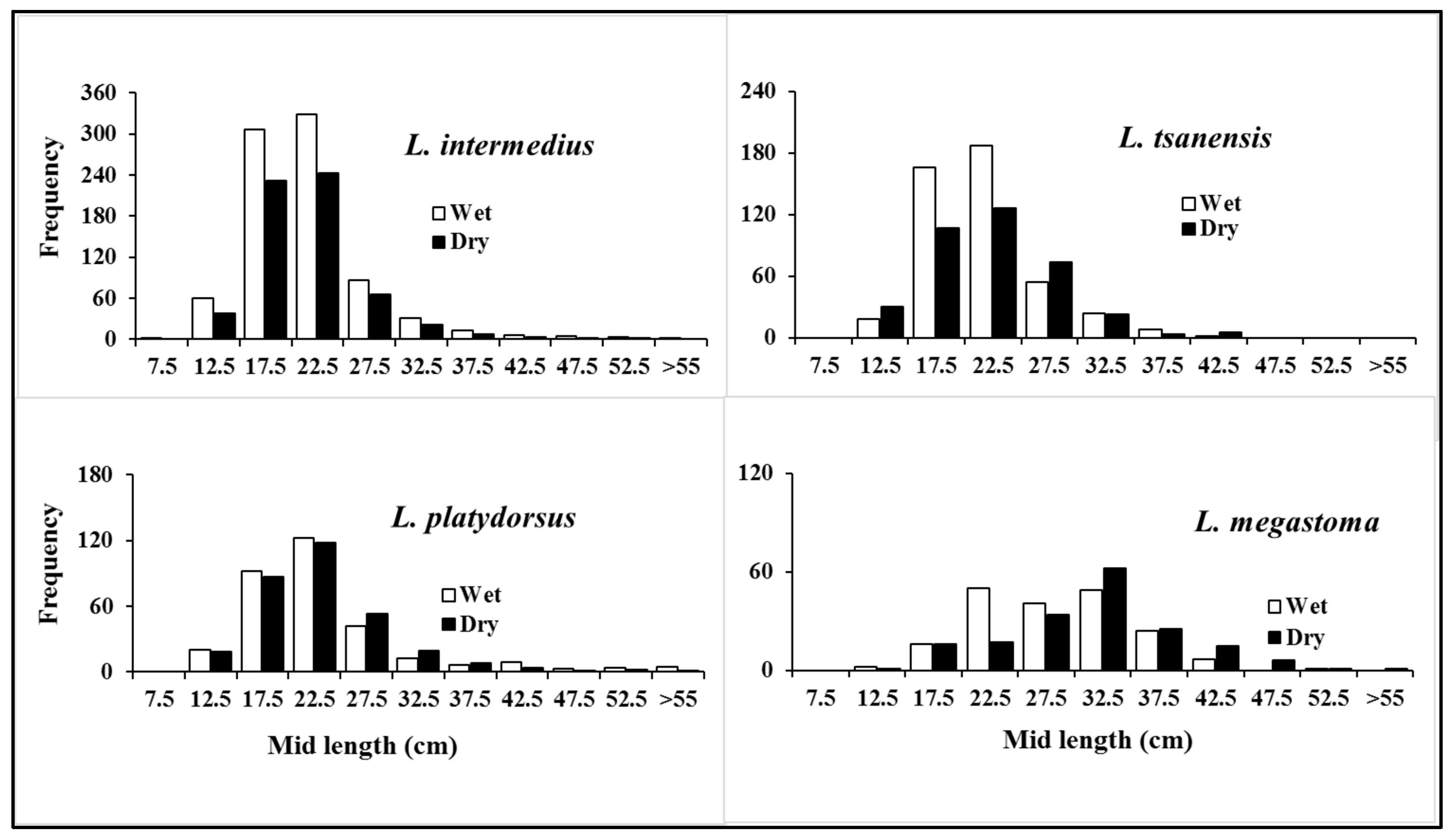

| Species | N | %N | W (kg) | %W | %F | IRI | %IRI |
|---|---|---|---|---|---|---|---|
| L. intermedius | 1454 | 34 | 241 | 27 | 100 | 6100 | 35 |
| L. tsanensis | 837 | 20 | 162 | 18 | 100 | 3800 | 22 |
| L. platydorsus | 623 | 15 | 171 | 19 | 100 | 3400 | 19 |
| L. megastoma | 339 | 8 | 131 | 15 | 71 | 1643 | 9 |
| L. brevicephalus | 446 | 11 | 31 | 3 | 57 | 800 | 5 |
| L. crassibarbis | 147 | 3 | 51 | 6 | 57 | 514 | 3 |
| L. nedgia | 98 | 2 | 28 | 3 | 86 | 429 | 2 |
| L. gorgorensis | 78 | 2 | 17 | 2 | 65 | 262 | 1 |
| L. surkis | 72 | 2 | 7 | 1 | 48 | 143 | 1 |
| L. truttiformis | 68 | 2 | 34 | 4 | 43 | 257 | 1 |
| L. longissimus | 28 | 1 | 11 | 1 | 38 | 76 | 0 |
| L. macrophtalmus | 26 | 1 | 8 | 1 | 43 | 86 | 0 |
| L. acutirostris | 13 | 0 | 6 | 1 | 24 | 31 | 0 |
| L. gorguari | 4 | 0 | 1 | 0 | 14 | 1 | 0 |
| L. dainellii | 2 | 0 | 1 | 0 | 5 | 0 | 0 |
| Total | 4235 | 899 | 17,542 | 100 |
| L. intermedius | L. tsanensis | |||||||
| Contrast | estimate | SE | t-ratio | P-value | estimate | SE | t-ratio | P-value |
| BD-GO | −0.29 | 5.29 | −0.06 | 0.999 | −3.75 | 5.57 | −0.67 | 0.907 |
| BD-GU | −12.83 | 5.29 | −2.43 | 0.089 | −13.75 | 5.57 | −2.47 | 0.081 |
| BD-KU | −1.88 | 5.29 | −0.35 | 0.985 | −1.17 | 5.57 | −0.21 | 0.997 |
| GO-GU | −12.54 | 5.29 | −2.37 | 0.099 | −10.00 | 5.57 | −1.80 | 0.291 |
| GO-KU | −1.58 | 5.29 | −0.30 | 0.991 | 2.58 | 5.57 | 0.46 | 0.967 |
| GU-KU | 10.96 | 5.29 | 2.07 | 0.180 | 12.58 | 5.57 | 2.26 | 0.125 |
| Dry-Wet | −11.80 | 3.71 | −3.17 | 0.003 | −4.67 | 4.31 | −1.08 | 0.29 |
| L. platydorsus | L. megastoma | |||||||
| BD-GO | −11.92 | 5.62 | −2.12 | 0.165 | −4.50 | 4.69 | −0.96 | 0.773 |
| BD-GU | −13.17 | 5.62 | −2.34 | 0.106 | −17.25 | 4.69 | −3.68 | 0.004 |
| BD-KU | −10.25 | 5.62 | −1.82 | 0.278 | 6.75 | 4.69 | 1.44 | 0.483 |
| GO-GU | −1.25 | 5.62 | −0.22 | 0.996 | −12.75 | 4.69 | −2.72 | 0.046 |
| GO-KU | 1.67 | 5.62 | 0.30 | 0.991 | 11.25 | 4.69 | 2.40 | 0.094 |
| GU-KU | 2.92 | 5.62 | 0.52 | 0.954 | 24.00 | 4.69 | 5.12 | 0.0001 |
| Dry-Wet | 0.50 | 4.21 | 0.12 | 0.906 | 3.92 | 4.16 | 0.94 | 0.35 |
| Site-Pairwise | Season-Pairwise | Estimate | R-Ratio | P-Value |
|---|---|---|---|---|
| BD-GO | Dry-Wet | −2.33 | −0.24 | 0.811 |
| BD-GU | Dry-Wet | 29.00 | 2.99 | 0.005 |
| BD-KU | Dry-Wet | 12.00 | 1.24 | 0.223 |
| GO-GU | Dry-Wet | 31.33 | 3.23 | 0.003 |
| GO-KU | Dry-Wet | 14.33 | 1.48 | 0.147 |
| GU-KU | Dry-Wet | −17.00 | −1.75 | 0.087 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gebremedhin, S.; Bruneel, S.; Getahun, A.; Anteneh, W.; Goethals, P. The Endemic Species Flock of Labeobarbus spp. in L. Tana (Ethiopia) Threatened by Extinction: Implications for Conservation Management. Water 2019, 11, 2560. https://doi.org/10.3390/w11122560
Gebremedhin S, Bruneel S, Getahun A, Anteneh W, Goethals P. The Endemic Species Flock of Labeobarbus spp. in L. Tana (Ethiopia) Threatened by Extinction: Implications for Conservation Management. Water. 2019; 11(12):2560. https://doi.org/10.3390/w11122560
Chicago/Turabian StyleGebremedhin, Shewit, Stijn Bruneel, Abebe Getahun, Wassie Anteneh, and Peter Goethals. 2019. "The Endemic Species Flock of Labeobarbus spp. in L. Tana (Ethiopia) Threatened by Extinction: Implications for Conservation Management" Water 11, no. 12: 2560. https://doi.org/10.3390/w11122560
APA StyleGebremedhin, S., Bruneel, S., Getahun, A., Anteneh, W., & Goethals, P. (2019). The Endemic Species Flock of Labeobarbus spp. in L. Tana (Ethiopia) Threatened by Extinction: Implications for Conservation Management. Water, 11(12), 2560. https://doi.org/10.3390/w11122560






