Characteristics of Heterotrophic Nitrifying and Aerobic Denitrifying Arthrobacter nicotianae D51 Strain in the Presence of Copper
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain and Culture Medium Preparation
2.2. Identification of Strain D51
2.3. Shake Flask Experiments
2.4. Assessment for Gas Detection
2.5. Analytical Methods and Statistical Analysis
3. Results
3.1. Identification of Strain D51
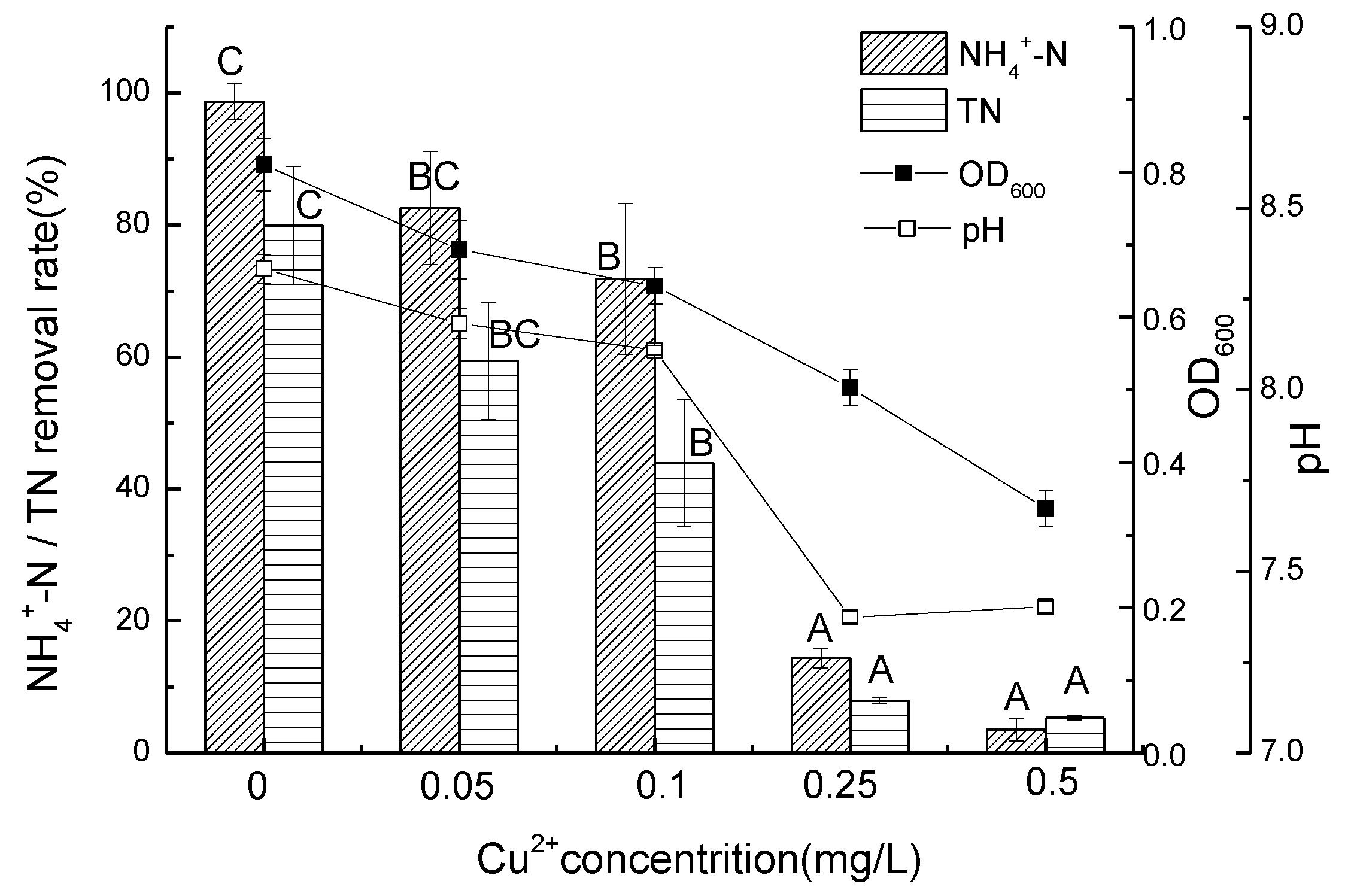
3.2. Ammonium Removal Performance of Strain D51 with Different Dosages of Added Cu2+
3.3. Nitrate Nitrogen Removal Performance of Strain D51 with Different Dosages of Added Cu2+
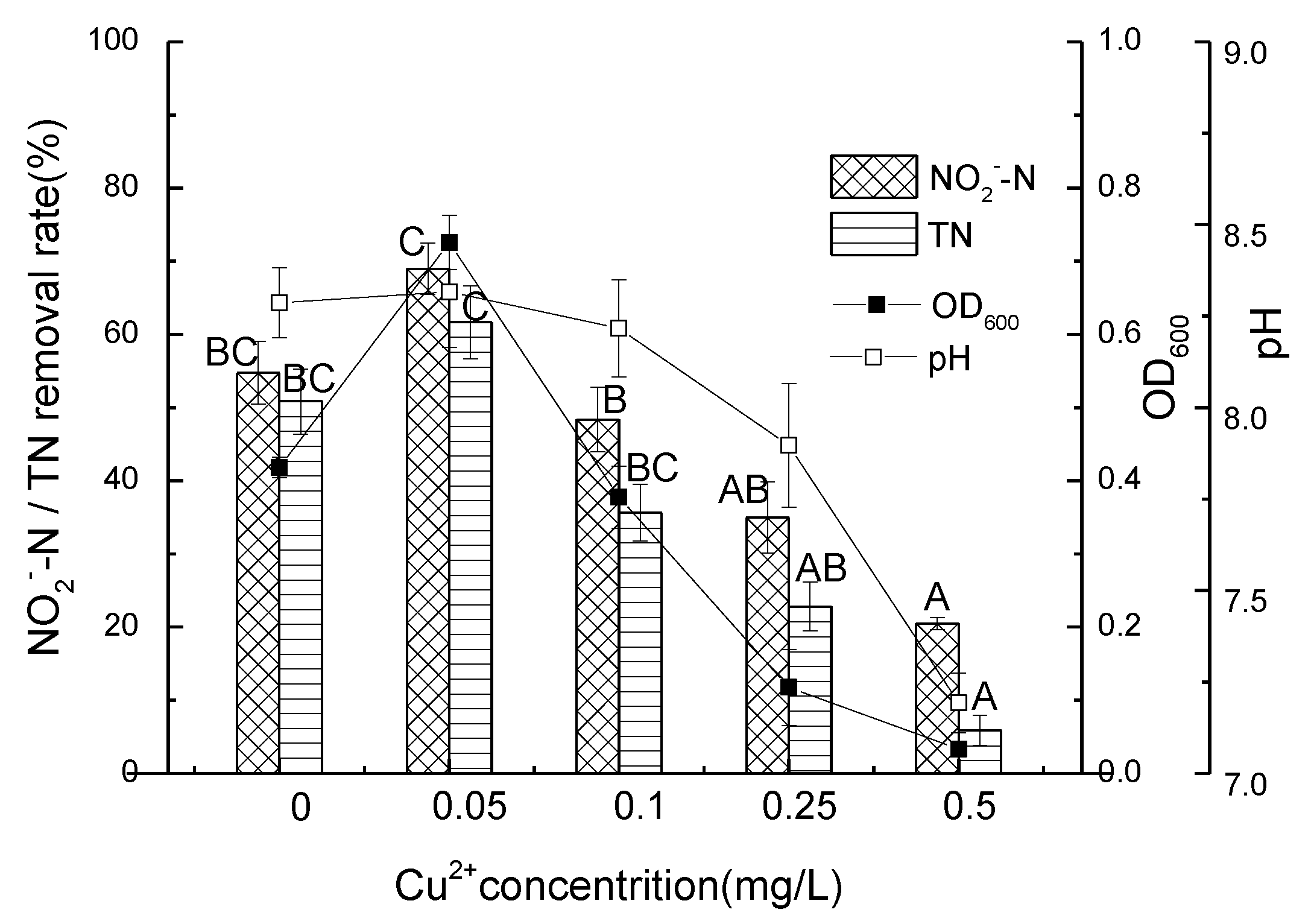
3.4. Nitrite Nitrogen Removal Performance of Strain D51 with Different Dosages of Added Cu2+
3.5. Assessment for Gas Detection
4. Discussion
5. Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- Kim, J.K.; Park, K.J.; Cho, K.S.; Nam, S.W.; Park, T.J.; Bajpai, R. Aerobic nitrification–denitrification by heterotrophic Bacillus strains. Bioresour. Technol. 2005, 96, 1897–1906. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.Z.; Zhu, G.B. Biological nitrogen removal with nitrification and denitrification via nitrite pathway. Appl. Microbiol. Biotechnol. 2006, 73, 15–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robertson, L.A.; Kuenen, J.G. Combined heterotrophic nitrification and aerobic denitrification in Thiosphaera pantotropha and other bacteria. Antonie. Van. Leeuwenhoek. 1990, 57, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.L.; Liu, Y.; Ai, G.M.; Miao, L.L.; Zheng, H.Y.; Liu, Z.P. The characteristics of a novel heterotrophic nitrification aerobic denitrification bacterium, Bacillus methylotrophicus strain L7. Bioresour. Technol. 2012, 108, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.M.; Fang, H.D.; Su, B.; Chen, J.F.; Lin, J.M. Characterization of a halophilic heterotrophic nitrification–aerobic denitrification bacterium and its application on treatment of saline wastewater. Bioresour. Technol. 2015, 179, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.P.; Wang, S.M.; Zhang, D.W.; Zhou, L.X. Isolation and nitrogen removal characteristics of an aerobic heterotrophic nitrifying-denitrifying bacterium, Bacillus subtilis A1. Bioresour. Technol. 2011, 102, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Wang, H.Y.; Yang, K. Tolerance of an aerobic denitrifier (Pseudomonas stutzeri) to high O2 concentrations. Biotechnol. Lett. 2014, 36, 719–722. [Google Scholar] [CrossRef] [PubMed]
- Khardenavis, A.A.; Kapley, A.; Purohit, H.J. Simultaneous nitrification and denitrification by diverse Diaphorobacter sp. Appl. Microbiol. Biotechnol. 2007, 77, 403–409. [Google Scholar] [CrossRef] [PubMed]
- O’Loughlin, E.J.; Sims, G.K.; Traina, S.J. Biodegradation of 2-methyl, 2-ethyl, and 2-hydroxypyridine by an arthrobacter sp. isolated from subsurface sediment. Biodegradation 1999, 10, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Verstraete, W.; Alexander, M. Heterotrophic nitrification by Arthrobacter sp. J. Bacteriol. 1972, 110, 955–961. [Google Scholar] [PubMed]
- Fu, F.L.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yu, J.J.; Jia, X.Y.; Jin, R.C. Enhancement of anammox performance by Cu (II), Ni (II) and Fe (III) supplementation. Chemosphere 2014, 117, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Altas, L. Inhibitory effect of heavy metals on methane-producing anaerobic granular sludge. J. Hazard. Mater. 2009, 162, 1551–1556. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Q.; Zhang, Z.Z.; Guo, Q.; Chen, Q.Q.; Jin, R.C.; Jia, X.Y. Variation in the performance and sludge characteristics of anaerobic ammonium oxidation inhibited by copper. Sep. Purif. Technol. 2015, 142, 108–115. [Google Scholar] [CrossRef]
- Feng, B.; Fang, Z.; Hou, J.C.; Ma, X.; Huang, Y.L.; Huang, L.Q. Effects of heavy metal wastewater on the anoxic/aerobic-membrane bioreactor bioprocess and membrane fouling. Bioresour. Technol. 2013, 142, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.Y.; Kazuichi, I. Evaluation of inhibitory effects of heavy metals on anaerobic ammonium oxidation (anammox) by continuous feeding tests. Appl. Microbiol. Biotechnol. 2014, 98, 6965–6972. [Google Scholar] [CrossRef] [PubMed]
- Felgate, H.; Giannopoulos, G.; Sullivan, M.J.; Gates, A.J.; Clarke, T.A.; Baggs, E.; Rowley, G.; Richardson, D.J. The impact of copper, nitrate and carbon status on the emission of nitrous oxide by two species of bacteria with biochemically distinct denitrification pathways. Environ. Microbiol. 2012, 14, 1788–1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ochoa-Herrera, V.; León, G.; Banihani, Q.; Jim, A.; Reyes Sierra-Alvarez, F. Toxicity of copper (II) ions to microorganisms in biological wastewater treatment systems. Sci. Total Environ. 2011, 412, 380–385. [Google Scholar] [CrossRef] [PubMed]
- He, T.X.; Li, Z.L.; Sun, Q.; Xu, Y.; Ye, Q. Heterotrophic nitrification and aerobic denitrification by Pseudomonas tolaasii Y-11 without nitrite accumulation during nitrogen conversion. Bioresour. Technol. 2016, 200, 493–499. [Google Scholar] [CrossRef] [PubMed]
- He, T.X.; Ye, Q.; Sun, Q.; Cai, X.; Ni, J.P.; Li, Z.L.; Xie, D.T. Removal of nitrate in simulated water at low temperature by a novel psychrotrophic and aerobic bacterium, Pseudomonas taiwanensis Strain J. Biomed. Res. Int. 2018. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Deng, S.P.; Acosta-Martínez, V.; Katsaliro, E. Characterization of redox-related soil microbial communities along a river floodplain continuum by fatty acid methyl ester (FAME) and 16S rRNA genes. Appl. Soil Ecol. 2008, 40, 499–509. [Google Scholar] [CrossRef]
- Pratt, B.; Riesen, R.; Johnston, C.G. PLFA analyses of microbial communities associated with PAH-contaminated riverbank sediment. Microb. Ecol. 2012, 64, 680–691. [Google Scholar] [CrossRef] [PubMed]
- Tsuruta, T.; Umenai, D.; Hatano, T.; Hirajima, T.; Sasaki, K. Screening micro-organisms for cadmium absorption from aqueous solution and cadmium absorption properties of Arthrobacter nicotianae. Biosci. Biotechnol. Biochem. 2014, 78, 1791–1796. [Google Scholar] [CrossRef] [PubMed]
- Hatano, T.; Tsuruta, T. Removal and recovery of chromium (III) from aqueous chromium (III) using Arthrobacter nicotianae cells. Adv. Microbiol. 2017, 7, 487–497. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Li, A.; Gao, J. Biodegradation of pentachloronitrobenzene by Arthrobacter nicotianae DH19. Lett. Appl. Microbiol. 2015, 61, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Ribitsch, D.; Winkler, S.; Gruber, K.; Karl, W.; Wehrschütz-Sigl, E.; Eiteljörg, I.; Schratl, P.; Remler, P.; Stehr, R.; Bessler, C.; et al. Engineering of choline oxidase from Arthrobacter nicotianae for potential use as biological bleach in detergents. Appl. Microbiol. Biotechnol. 2010, 87, 1743–1752. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Zhang, L.P.; Kang, Z.; Chen, J.; Du, J.C. High-level production of creatine amidinohydrolase from Arthrobacter nicotianae 23710 in escherichia coli. Appl. Biochem. Biotechnol. 2015, 175, 2564–2573. [Google Scholar] [CrossRef] [PubMed]
- Su, J.J.; Yeh, K.S.; Tseng, P.W. A Strain of Pseudomonas sp. isolated from piggery wastewater treatment systems with heterotrophic nitrification capability in Taiwan. Curr. Microbiol. 2006, 53, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Liu, T.; Liu, G.; Zhou, J.; Huang, J.; Wang, A. Simultaneous heterotrophic nitrification and aerobic denitrification by the marine origin bacterium Pseudomonas sp. ADN-42. Appl. Biochem. Biotechnol. 2015, 175, 2000–2011. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; He, Y.L.; Zhang, X.F. Nitrogen removal capability through simultaneous heterotrophic nitrification and aerobic denitrification by Bacillus sp. LY. Environ. Technol. 2010, 31, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, W.C.; Feng, Y.; Zhu, X.H.; Zhou, H.Z.; Tan, Z.L.; Li, X.D. Impact resistance of different factors on ammonia removal by heterotrophic nitrification–aerobic denitrification bacterium Aeromonas sp. HN-02. Bioresour. Technol. 2014, 167, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Gui, M.Y.; Chen, Q.; Ma, T.; Zheng, M.S.; Ni, J.R. Effects of heavy metals on aerobic denitrification by strain Pseudomonas stutzeri PCN-1. Appl. Microbiol. Biotechnol. 2017, 101, 1717–1727. [Google Scholar] [CrossRef] [PubMed]
- Qiao, N.; Gao, B.; Zhang, L.Y.; Chen, R.J. Factors affecting the denitrification property of heterotrophic nitrification–aerobic denitrifier. Fresenius. Environ. Bull. 2012, 21, 3773–3778. [Google Scholar]
- Kundu, P.; Pramanik, A.; Dasgupta, A.; Mukherjee, S.; Mukherjee, J. Simultaneous heterotrophic nitrification and aerobic denitrification by chryseobacterium sp. R31 isolated from a battoir wastewater. Biomed. Res. Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.X.; Yang, L.; Liang, X. The characteristics of a novel heterotrophic nitrifying and aerobic denitrifying bacterium, Acinetobacter junii YB. Bioresour. Technol. 2014, 171, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Angar, Y.; Kebbouche-Gana, S.; Djelali, N.E.; Khemili-Talbi, S. Novel approach for the ammonium removal by simultaneous heterotrophic nitrification and denitrification using a novel bacterial species co-culture. World J. Microbiol. Biotechnol. 2016, 32, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Yi, L.H.; Hughes, J.; Xiao, F.Z. Heterotrophic nitrogen removal by a newly isolated Acinetobacter calcoaceticus HNR. Bioresour. Technol. 2010, 101, 5194–5200. [Google Scholar] [CrossRef] [PubMed]
- He, T.X.; Li, Z.L.; Xie, D.T.; Sun, Q.; Xu, Y.; Ye, Q.; Ni, J.P. Simultaneous nitrification and denitrification with different mixed nitrogen loads by a hypothermia aerobic bacterium. Biodegradation 2018, 29, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Nies, D.H. Microbial heavy-metal resistance. Appl. Microbiol. Biotechnol. 1999, 51, 730–750. [Google Scholar] [CrossRef] [PubMed]
- Epstein, E. Health issues related to beneficial use of biosolids. Proc. Water Environ. Fed. 2001, 79–87. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Q.Q.; Jiang, X.Y.; Hu, H.Y.; Shi, M.L.; Jin, R.C. Insight into the short- and long-term effects of Cu (II) on denitrifying biogranules. J. Hazard. Mater. 2016, 304, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Black, A.; Hsu, P.C.L.; Hamonts, K.E.; Clough, T.J.; Condon, L.M. Influence of copper on expression of nirS, norB and nosZ and the transcription and activity of NIR, NOR and N2OR in the denitrifying soil bacteria Pseudomonas stutzer. Microb. Biotechnol. 2016, 9, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.Y.; Chen, Y.G.; Chen, H.; Li, X.; Peng, Y.Z.; Wang, S.Y. Minimizing nitrous oxide in biological nutrient removal from municipal wastewater by controlling copper ion concentrations. Appl. Microbiol. Biotechnol. 2013, 97, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.F.; Li, C.Q.; Ramesh, B.; Hu, N. Cloning, purifcation and characterization of novel Cu-containing nitrite reductase from the Bacillus frmus GY-49. World J. Microbiol. Biotechnol. 2018, 34, 1–10. [Google Scholar] [CrossRef] [PubMed]



| Library | Sim Index | Name |
|---|---|---|
| TSBA6 6.21 | 0.504 | Arthrobacter aurescens |
| 0.438 | Bacillus viscosus | |
| CLIN6 6.20 | 0.219 | Kocuria varians |
| 0.185 | Micrococcus luteus GC subgroup A | |
| 0.170 | Bacillus megaterium | |
| 0.168 | Bacillus circulans GC subgroup B |
| Nitrogen Resource | Control Groups (mg·L−1) | Experimental Groups (mg·L−1) | Net Production (mg·L−1) |
|---|---|---|---|
| NH4+-N | 0.0009 | 0.0280 | 0.0271 |
| NO3−-N | 0.0140 | 0.0284 | 0.0144 |
| NO2−-N | 0.0160 | 0.2323 | 0.2163 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, X.; Li, K.; He, T.; Wang, Y.; Zhang, X.; Xie, E.; Ding, N.; Li, Z. Characteristics of Heterotrophic Nitrifying and Aerobic Denitrifying Arthrobacter nicotianae D51 Strain in the Presence of Copper. Water 2019, 11, 434. https://doi.org/10.3390/w11030434
Cai X, Li K, He T, Wang Y, Zhang X, Xie E, Ding N, Li Z. Characteristics of Heterotrophic Nitrifying and Aerobic Denitrifying Arthrobacter nicotianae D51 Strain in the Presence of Copper. Water. 2019; 11(3):434. https://doi.org/10.3390/w11030434
Chicago/Turabian StyleCai, Xi, Kaili Li, Tengxia He, Yaxin Wang, Xue Zhang, Enyu Xie, Ningning Ding, and Zhenlun Li. 2019. "Characteristics of Heterotrophic Nitrifying and Aerobic Denitrifying Arthrobacter nicotianae D51 Strain in the Presence of Copper" Water 11, no. 3: 434. https://doi.org/10.3390/w11030434
APA StyleCai, X., Li, K., He, T., Wang, Y., Zhang, X., Xie, E., Ding, N., & Li, Z. (2019). Characteristics of Heterotrophic Nitrifying and Aerobic Denitrifying Arthrobacter nicotianae D51 Strain in the Presence of Copper. Water, 11(3), 434. https://doi.org/10.3390/w11030434





