Spatiotemporal Dynamics of Submerged Aquatic Vegetation in a Deep Lake from Sentinel-2 Data
Abstract
:1. Introduction
2. Materials and Methods
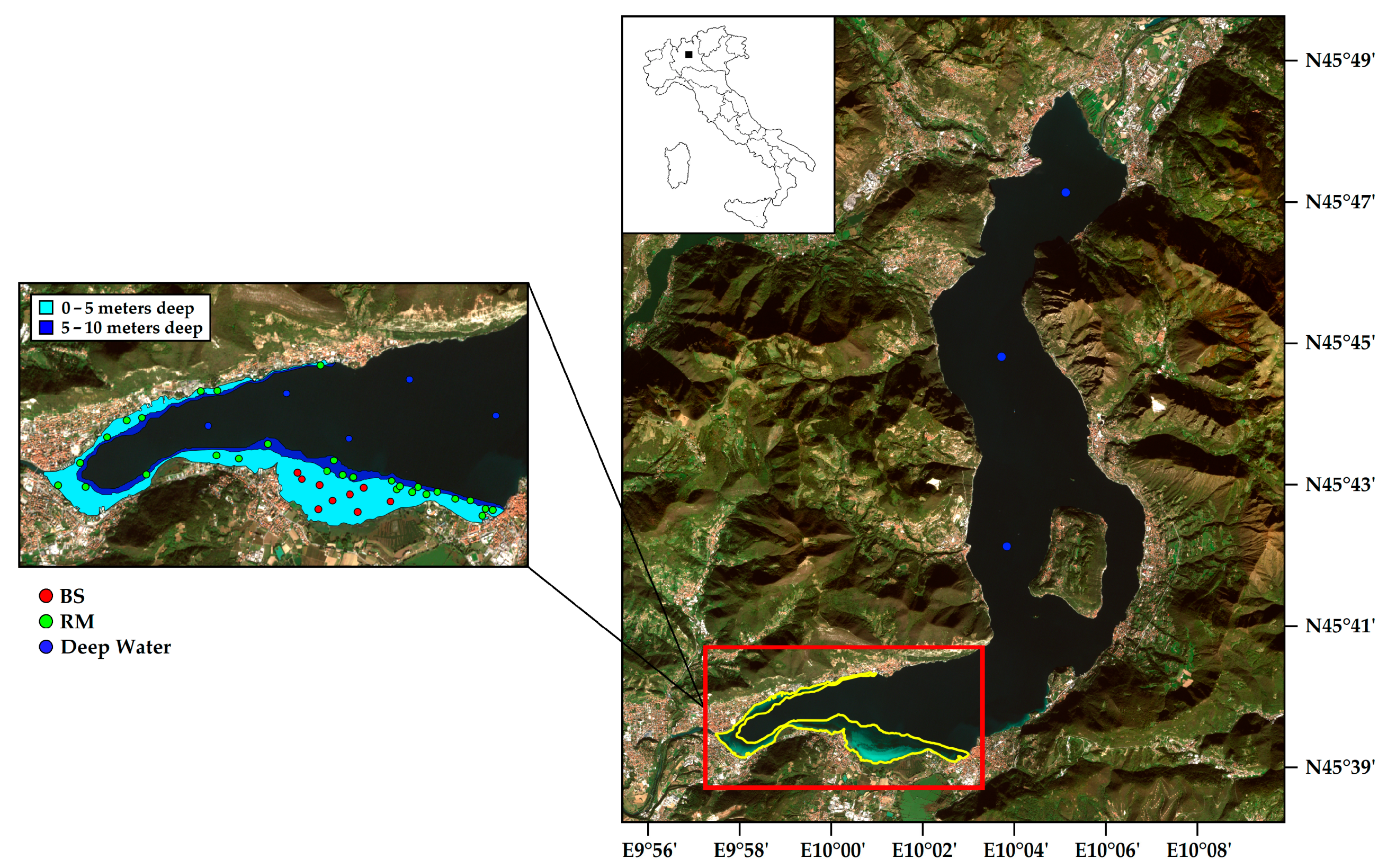
2.1. Study Area
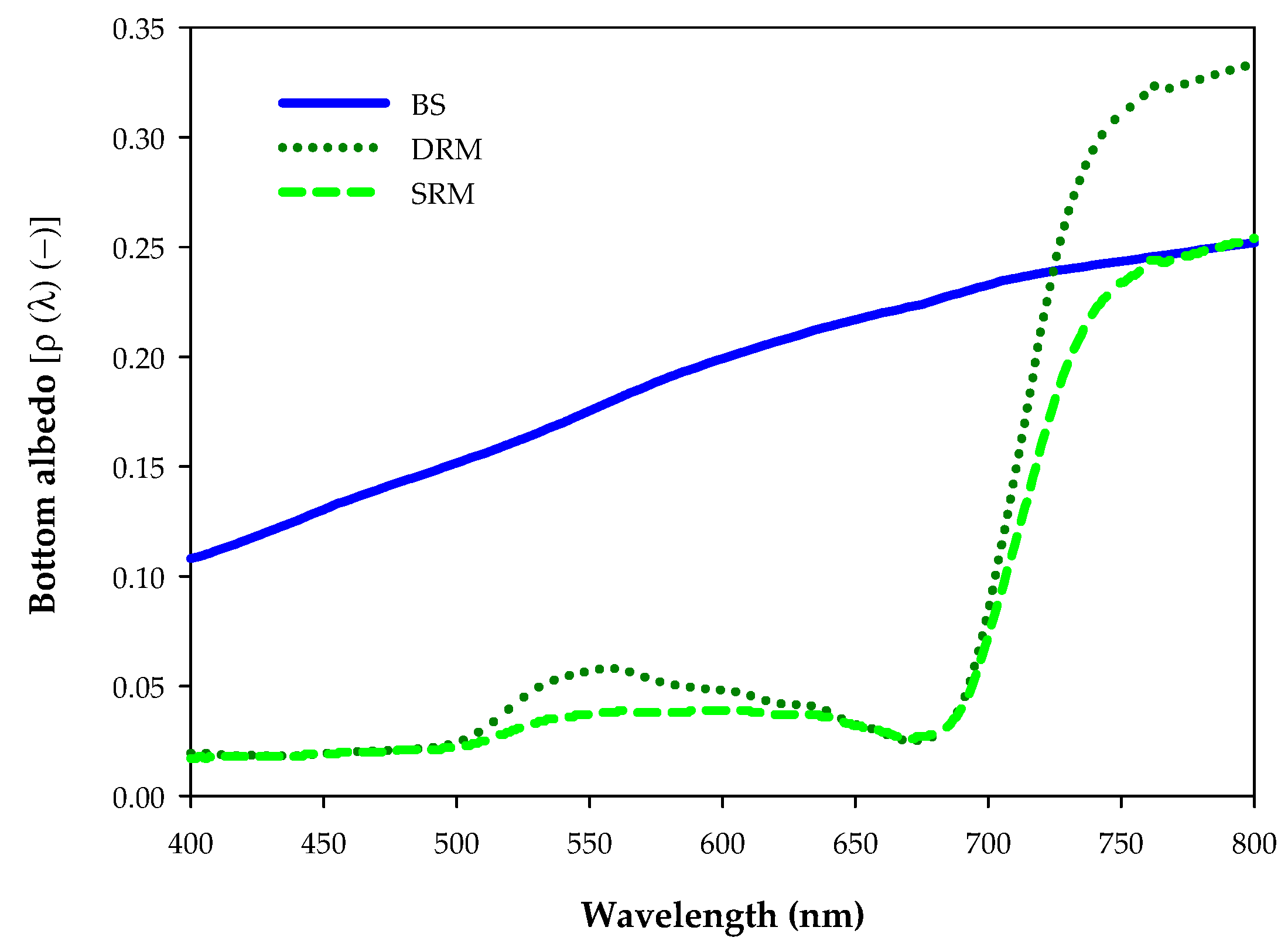
2.2. Image Processing
3. Results
3.1. Validation Results
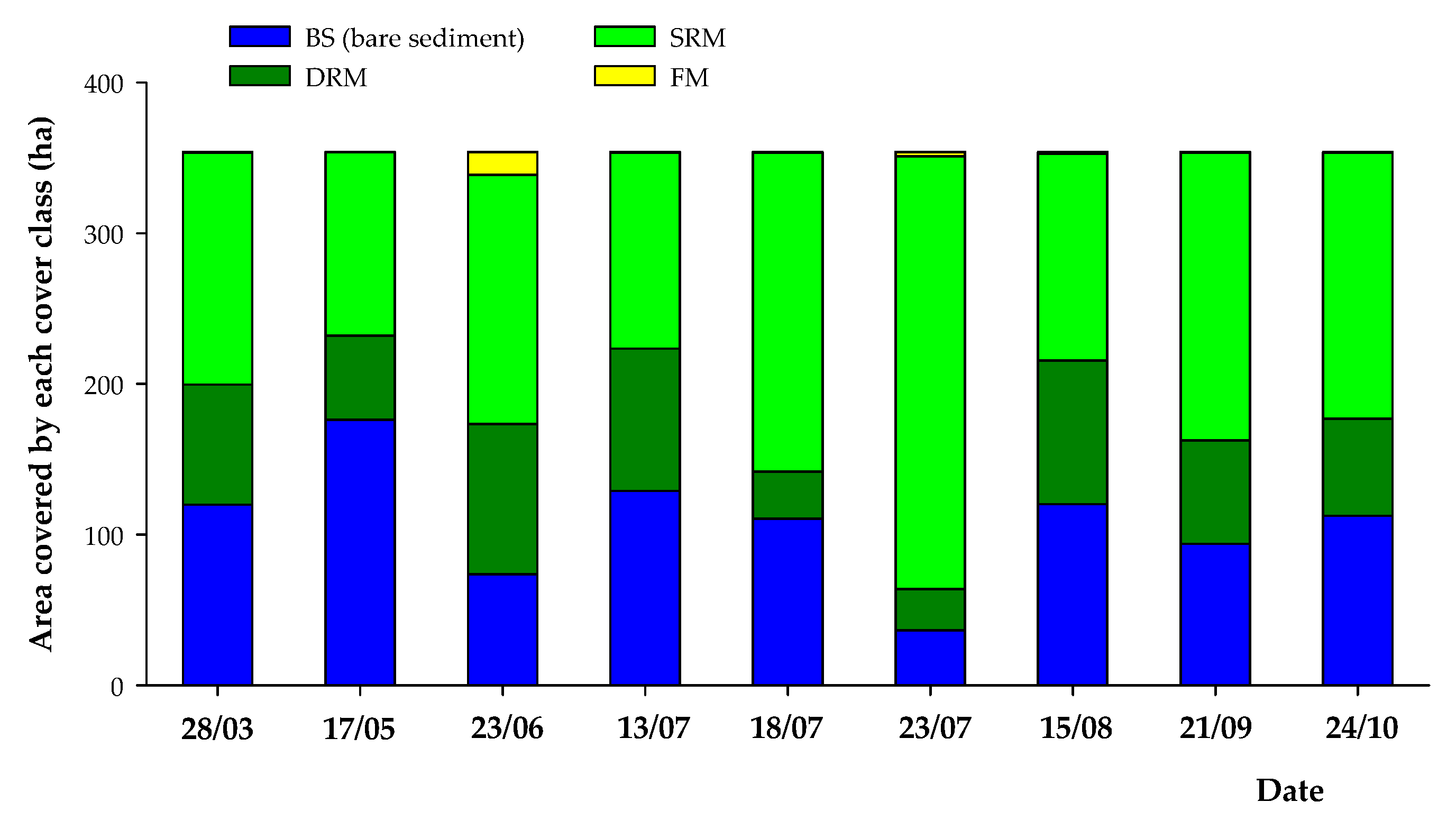
3.2. Intra-Annual (2017) Spatiotemporal Dynamics of SAV and Bare Sediment
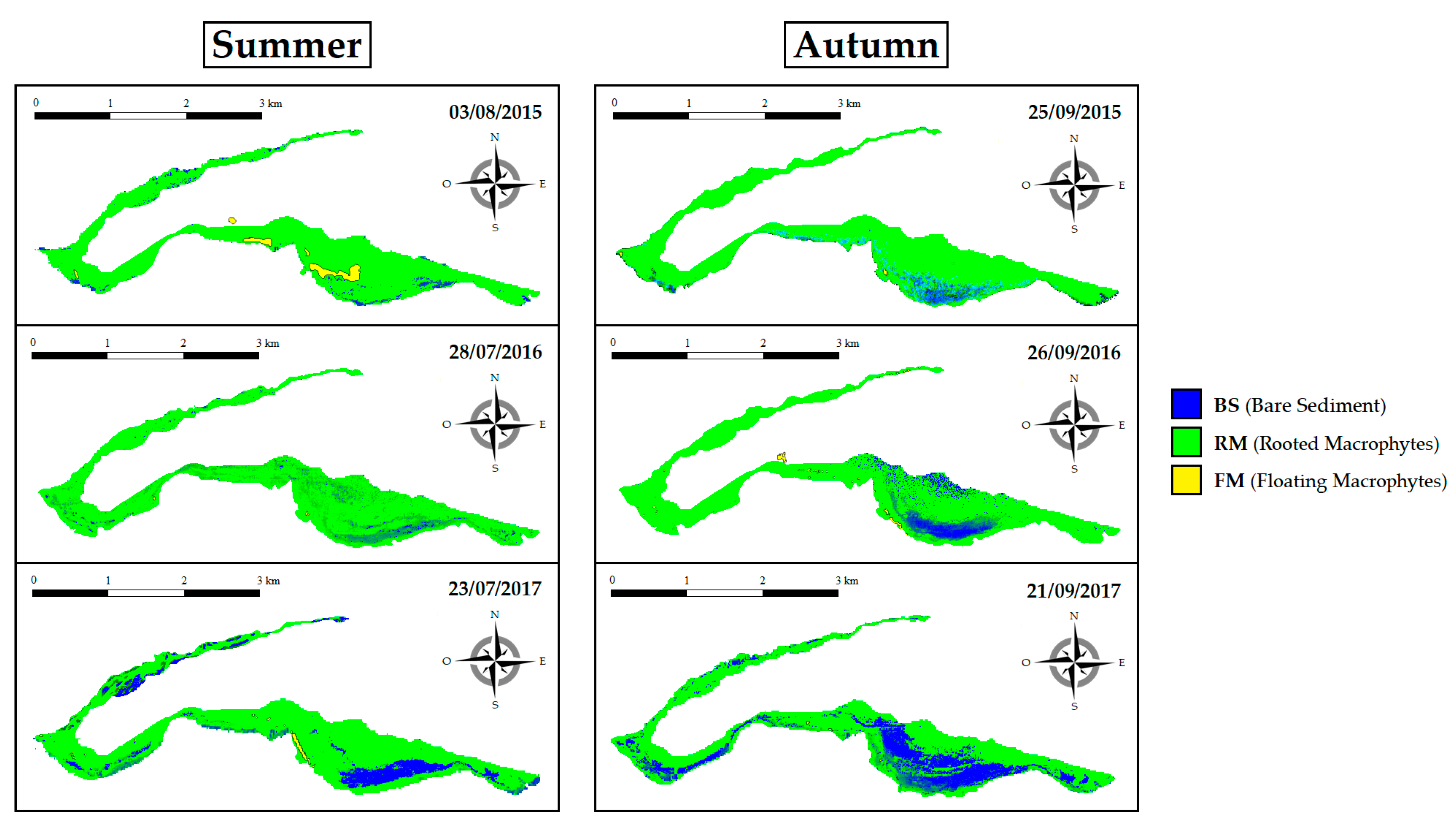
3.3. Spatiotemporal SAV Dynamics across Years (2015–2017)
3.4. Role of Bathymetry and Coastal Distance in SAV Dynamics
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Bolpagni, R.; Laini, A.; Azzella, M.M. Short-term dynamics of submerged aquatic vegetation diversity and abundance in deep lakes. Appl. Veg. Sci. 2016, 19, 711–723. [Google Scholar] [CrossRef]
- Azzella, M.M.; Bresciani, M.; Nizzoli, D.; Bolpagni, R. Aquatic vegetation in deep lakes: Macrophyte co-occurrence patterns and environmental determinants. J. Limnol. 2017, 76, 97–108. [Google Scholar] [CrossRef]
- Bouma, T.J.; De Vries, M.B.; Low, E.; Peralta, G.; Tánczos, I.C.; Van De Koppel, J.; Herman, P.M.J. Trade-offs related to ecosystem engineering: A case study on stiffness of emerging macrophytes. Ecology 2005, 86, 2187–2199. [Google Scholar] [CrossRef]
- Racchetti, E.; Bartoli, M.; Ribaudo, C.; Longhi, D.; Brito, L.E.Q.; Naldi, M.; Iacumin, P.; Viaroli, P. Short term changes in pore water chemistry in river sediments during the early colonization by Vallisneria spiralis. Hydrobiologia 2010, 652, 127–137. [Google Scholar] [CrossRef]
- Salgado, J.; Sayer, C.D.; Brooks, S.J.; Davidson, T.A.; Goldsmith, B.; Patmore, I.R.; Baker, A.G.; Okamura, B. Eutrophication homogenizes shallow lake macrophyte assemblages over space and time. Ecosphere 2018, 9, e02406. [Google Scholar] [CrossRef]
- Zhang, X.; Jeppesen, E.; Liu, X.; Qin, B.; Shi, K.; Zhou, Y.; Thomaz, S.M.; Deng, J. Global loss of aquatic vegetation in lakes. Earth-Sci. Rev. 2017, 173, 259–265. [Google Scholar] [CrossRef]
- Bolpagni, R.; Laini, A.; Stanzani, C.; Chiarucci, A. Aquatic plant diversity in Italy: Distribution, drivers and strategic conservation actions. Front. Plant Sci. 2018, 9, 116. [Google Scholar] [CrossRef] [PubMed]
- Hargeby, A.; Blindow, I.; Hansson, L.A. Shifts between clear and turbid states in a shallow lake: Multi-causal stress from climate, nutrients and biotic interactions. Archiv für Hydrobiologie 2004, 161, 433–454. [Google Scholar] [CrossRef]
- Azzella, M.M.; Bolpagni, R.; Oggioni, A. A preliminary evaluation of lake morphometric traits influence on the maximum colonization depth of aquatic plants. J. Limnol. 2014, 73, 1–7. [Google Scholar] [CrossRef]
- Azzella, M.M.; Rosati, L.; Iberite, M.; Bolpagni, R.; Blasi, C. Changes in aquatic plants in the Italian volcanic-lake system detected using current data and historical records. Aquat. Bot. 2014, 112, 41–47. [Google Scholar] [CrossRef]
- Kosten, S.; Kamarainen, A.; Jeppesen, E.; Van Nes, E.H.; Peeters, E.T.H.M.; Mazzeo, N.; Sass, L.; Hauxwell, J.; Hansel-welch, N.; Lauridsen, T.L.; et al. Climate-related differences in the dominance of submerged macrophytes in shallow lakes. Glob. Chang. Biol. 2009, 15, 2503–2517. [Google Scholar] [CrossRef]
- Alahuhta, J.; Kosten, S.; Akasaka, M.; Auderset, D.; Azzella, M.M.; Bolpagni, R.; Bove, C.P.; Chambers, P.A.; Chappuis, E.; Clayton, J.; et al. Global variation in the beta diversity of lake macrophytes is driven by environmental heterogeneity rather than latitude. J. Biogeogr. 2017, 44, 1758–1769. [Google Scholar] [CrossRef]
- Sand-Jensen, K.; Riis, T.; Vestergaard, O.; Larsen, S.E. Macrophyte decline in Danish lakes and streams over the past 100 years. J. Ecol. 2000, 88, 1030–1040. [Google Scholar] [CrossRef]
- Scheffer, M.; Carpenter, S.R. Catastrophic regime shifts in ecosystems: Linking theory to observation. Trends Ecol. Evol. 2003, 18, 648–656. [Google Scholar] [CrossRef]
- Thomaz, S.M.; Esteves, F.A.; Murphy, K.J.; dos Santos, A.M.; Caliman, A.; Guariento, R.D. Aquatic Macrophytes in the Tropics: Ecology of Populations and Communities, Impacts of Invasions and Human Use; UNESCO-EOLSS: Paris, France, 2011. [Google Scholar]
- Wade, P.M. The impact of human activity on the aquatic macroflora of Llangorse Lake, South Wales. Aquat. Conserv. Mar. Freshw. Ecosyst. 1999, 9, 441–459. [Google Scholar] [CrossRef]
- Zhang, M.; García Molinos, J.; Zhang, X.; Xu, J. Functional and taxonomic differentiation of macrophyte assemblages across the Yangtze River floodplain under human impacts. Front. Plant Sci. 2018, 9, 387. [Google Scholar] [CrossRef] [PubMed]
- Schallenberg, M.; Sorrell, B. Regime shifts between clear and turbid water in New Zealand lakes: Environmental correlates and implications for management and restoration. N. Z. J. Mar. Freshw. Res. 2009, 43, 701–712. [Google Scholar] [CrossRef]
- Bertrin, V.; Boutry, S.; Jan, G.; Ducasse, G.; Grigoletto, F.; Ribaudo, C. Effects of wind-induced sediment resuspension on distribution and morphological traits of aquatic weeds in shallow lakes. J. Limnol. 2017, 76, 84–96. [Google Scholar] [CrossRef]
- Keddy, P.A.; Reznicek, A.A. Great lakes vegetation dynamics: The role of fluctuating water levels and buried seeds. J. Great Lakes Res. 1986, 12, 25–36. [Google Scholar] [CrossRef]
- Wolkovich, E.M.; Cleland, E.E. The phenology of plant invasions: A community ecology perspective. Front. Ecol. Environ. 2011, 9, 287–294. [Google Scholar] [CrossRef]
- Sletvold, N.; Ågren, J. Climate-dependent costs of reproduction: Survival and fecundity costs decline with length of the growing season and summer temperature. Ecol. Lett. 2015, 18, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Malthus, T.J. Bio-optical modeling and remote sensing of aquatic macrophytes. Bio-opt. Model. Remote Sens. Inland Waters 2017, 263–308. [Google Scholar] [CrossRef]
- Hedley, J.D.; Roelfsema, C.; Brando, V.E.; Giardino, C.; Kutser, T.; Phinn, S.; Mumby, P.J.; Barrilero, O.; Laporte, J.; Koetz, B. Coral reef applications of Sentinel-2: Coverage, characteristics, bathymetry and benthic mapping with comparison to Landsat 8. Remote Sens. Environ. 2018, 216, 598–614. [Google Scholar] [CrossRef]
- Dekker, A.G.; Brando, V.E.; Anstee, J.M. Retrospective seagrass change detection in a shallow coastal tidal Australian lake. Remote Sens. Environ. 2005, 97, 415–433. [Google Scholar] [CrossRef]
- Dörnhöfer, K.; Oppelt, N. Remote sensing for lake research and monitoring–Recent advances. Ecol. Indic. 2016, 64, 105–122. [Google Scholar] [CrossRef]
- Luo, J.; Juhua, H.D.; Ronghua, M.; Xiuliang, J.; Fei, L.; Weiping, H.; Kun, S.; Wenjiang, H. Mapping species of submerged aquatic vegetation with multi-seasonal satellite images and considering life history information. Int. J. Appl. Earth Obs. Geoinf. 2017, 57, 154–165. [Google Scholar] [CrossRef]
- Dekker, A.G.; Phinn, S.R.; Anstee, J.; Bissett, P.; Brando, V.E.; Casey, B.; Fearns, P.; Hedley, J.; Klonowski, W.; Lee, Z.P.; et al. Intercomparison of shallow water bathymetry, hydro-optics, and benthos mapping techniques in Australian and Caribbean coastal environments. Limnol. Oceanogr. Methods 2011, 9, 396–425. [Google Scholar] [CrossRef]
- Lee, Z.; Carder, K.L.; Mobley, C.D.; Steward, R.G.; Patch, J.S. Hypespectral remote sensing for shallow waters: 2. Deriving bottom depths and water properties by optimization. Appl. Opt. 1999, 38, 3831–3843. [Google Scholar] [CrossRef]
- Lee, Z.; Carder, K.L.; Chen, R.F.; Peacock, T.G. Properties of the water column and bottom derived from Airborne Visible Infrared Imaging Specrometer (AVIRIS) data. J. Geophys. Res. 2001, 106, 11639–11651. [Google Scholar] [CrossRef]
- Petit, T.; Bajjouk, T.; Mouquet, P.; Rochette, S.; Vozel, B.; Delacourt, C. Hyperspectral remote sensing of coral reefs by semi-analytical model inversion–Comparison of different inversion setups. Remote Sens. Environ. 2017, 190, 348–365. [Google Scholar] [CrossRef]
- Fritz, C.; Kuhwald, K.; Schneider, T.; Geist, J.; Oppelt, N. Sentinel-2 for mapping the spatio-temporal development of submerged aquatic vegetation at Lake Starnberg (Germany). J. Limnol. 2019. [Google Scholar] [CrossRef]
- Zhou, G.; Ma, Z.; Sathyendranath, S.; Platt, T.; Jiang, C.; Sun, K. Canopy Reflectance Modeling of Aquatic Vegetation for Algorithm Development: Global Sensitivity Analysis. Remote Sens. 2018, 10, 837. [Google Scholar] [CrossRef]
- Pinardi, M.; Bresciani, M.; Villa, P.; Cazzaniga, I.; Laini, A.; Tóth, V.; Fadel, A.; Austoni, M.; Lami, A.; Giardino, C. Spatial and temporal dynamics of primary producers in shallow lakes as seen from space: Intra-annual observations from Sentinel-2A. Limnologica 2018, 72, 32–43. [Google Scholar] [CrossRef]
- Hedley, J.; Roelfsema, C.; Koetz, B.; Phinn, S. Capability of the Sentinel 2 mission for tropical coral reef mapping and coral bleaching detection. Remote Sens. Environ. 2012, 120, 145–155. [Google Scholar] [CrossRef]
- Barone, L.; Pilotti, M.; Valerio, G.; Balistrocchi, M.; Milanesi, L.; Chapra, S.; Nizzoli, N. Analysis of the residual nutrient load from a combined sewer system in a watershed of a deep Italian lake. J. Hydrol. 2019, in press. [Google Scholar] [CrossRef]
- Hutchinson, G.E. A Treatise on limnology. Geogr. Phys. Chem. 1957, 1. [Google Scholar] [CrossRef]
- Leoni, B.; Marti, C.L.; Imberger, J.; Garibaldi, L. Summer spatial variations in phytoplankton composition and biomass in surface waters of a warm-temperate, deep, oligo-holomictic lake: Lake Iseo, Italy. Inland Waters 2014, 4, 303–310. [Google Scholar] [CrossRef]
- Marti, C.L.; Imberger, J.; Garibaldi, L.; Leoni, B. Using time scales to characterize phytoplankton assemblages in a deep subalpine lake during the thermal stratification period: Lake Iseo, Italy. Water Resour. Res. 2015, 52, 1762–1780. [Google Scholar] [CrossRef]
- Garibaldi, L.; Anzani, A.; Marieni, A.; Leoni, B.; Mosello, R. Studies on the phytoplankton of the deep subalpine Lake Iseo. J. Limnol. 2003, 62, 177–189. [Google Scholar] [CrossRef]
- Leoni, B. Zooplankton predators and preys: Body size and stable isotope to investigate the pelagic food web in a deep lake (Lake Iseo, Northern Italy). J. Limnol. 2017, 76, 85–93. [Google Scholar] [CrossRef]
- Valerio, G.; Pilotti, M.; Barontini, S.; Leoni, B. Sensitivity of the multiannual thermal dynamics of a deep pre-alpine lake to climatic change. Hydrol. Process. 2015, 29, 767–779. [Google Scholar] [CrossRef]
- Rogora, M.; Buzzi, F.; Dresti, C.; Leoni, B.; Lepori, F.; Mosello, R.; Patelli, M.; Salmaso, N. Climatic effects on vertical mixing and deep-water oxygen content in the subalpine lakes in Italy. Hydrobiologia 2018, 1–18. [Google Scholar] [CrossRef]
- Bolpagni, R.; Azzella, M.M.; Agostinelli, C.; Beghi, A.; Bettoni, E.; Brusa, G.; De Molli, C.; Formenti, R.; Galimberti, F.; Cerabolini, B.E.L. Integrating the Water Framework Directive into the Habitats Directive: Analysis of distribution patterns of lacustrine EU habitats in lakes of Lombardy (northern Italy). J. Limnol. 2017, 76 (Suppl. 1), 75–83. [Google Scholar] [CrossRef]
- Vermote, E.F.; Tanré, D.; Deuze, J.L.; Herman, M.; Morcette, J.J. Second simulation of the satellite signal in the solar spectrum, 6S: An overview. IEEE Trans. Geosci. Remote Sens. 1997, 35, 675–686. [Google Scholar] [CrossRef]
- Vermote, E.F.T.D.; Tanré, D.; Deuzé, J.L.; Herman, M.; Morcrette, J.J.; Kotchenova, S.Y. Second simulation of a satellite signal in the solar spectrum-vector (6SV). 6S User Guide Version 2006, 3, 1–55. [Google Scholar]
- AERONET Station of the Archaeological Museum of Sirmione (BS, Italy). Available online: https://aeronet.gsfc.nasa.gov/new_web/photo_db_v3/Sirmione_Museo_GC.html (accessed on 10 January 2019).
- Bresciani, M.; Cazzaniga, I.; Austoni, M.; Sforzi, T.; Buzzi, F.; Morabito, G.; Giardino, C. Mapping phytoplankton blooms in deep subalpine lakes from Sentinel-2A and Landsat-8. Hydrobiologia 2018, 824, 197–214. [Google Scholar] [CrossRef]
- Giardino, C.; Candiani, G.; Bresciani, M.; Lee, Z.; Gagliano, S.; Pepe, M. BOMBER: A tool for estimating water quality and bottom properties from remote sensing images. Comput. Geosci. 2012, 45, 313–318. [Google Scholar] [CrossRef]
- Giardino, C.; Bresciani, M.; Cazzaniga, I.; Schenk, K.; Rieger, P.; Braga, F.; Matta, E.; Brando, V.E. Evaluation of multi-resolution satellite sensors for assessing water quality and bottom depth of Lake Garda. Sensors 2014, 14, 24116–24131. [Google Scholar] [CrossRef]
- Bresciani, M.; Giardino, C.; Lauceri, R.; Matta, E.; Cazzaniga, I.; Pinardi, M.; Lami, A.; Austoni, M.; Viaggiu, E.; Congestri, R.; et al. Earth observation for monitoring and mapping of cyanobacteria blooms. Case studies on five Italian lakes. J. Limnol. 2017, 76, 127–139. [Google Scholar] [CrossRef]
- Bresciani, M.; Bolpagni, R.; Braga, F.; Oggioni, A.; Giardino, C. Retrospective assessment of macrophytic communities in southern Lake Garda (Italy) from in situ and MIVIS (Multispectral Infrared and Visible Imaging Spectrometer) data. J. Limnol. 2012, 71, 180–190. [Google Scholar] [CrossRef]
- Fritz, C.; Schneider, T.; Geist, J. Seasonal variation in spectral response of submerged aquatic macrophytes: A case study at Lake Starnberg (Germany). Water 2017, 9, 527. [Google Scholar] [CrossRef]
- Yadav, S.; Yoneda, M.; Tamura, M.; Susaki, J.; Ishikawa, K.; Yamashiki, Y. A satellite-based assessment of the distribution and biomass of submerged aquatic vegetation in the optically shallow basin of Lake Biwa. Remote Sens. 2017, 9, 966. [Google Scholar] [CrossRef]
- Lawniczak-Malińska, M.; Ptak, M.; Celewicz, S.; Choiński, A. Impact of Lake Morphology and Shallowing on the Rate of Overgrowth in Hard-Water Eutrophic Lakes. Water 2018, 10, 1827. [Google Scholar] [CrossRef]
- Sand-Jensen, K.; Pedersen, N.L.; Thorsgaard, I.; Moeslund, B.; Borum, J.; Brodersen, K.P. 100 years of vegetation decline and recovery in Lake Fure, Denmark. J. Ecol. 2008, 96, 260–271. [Google Scholar] [CrossRef]
- Hilt, S.; Alirangues Nunez, M.M.; Bakker, E.S.; Blindow, I.; Davidson, T.; Gillefalk, M.; Hansson, L.A.; Janse, J.H.; Janssen, A.B.G.; Jeppesen, E.; et al. Response of submerged macrophytes to external and internal restoration measures of temperate shallow lakes. Front. Plant Sci. 2018, 9, 194. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, E.; Roelofs, J.G.M. Degraded softwater lakes: Possibilities for restoration. Restor. Ecol. 2001, 9, 155–166. [Google Scholar] [CrossRef]
- Silveira, M.J.; Harthman, V.C.; Michelan, T.S.; Souza, L.A. Anatomical development of roots of native and nonnative submerged aquatic macrophytes in different sediment types. Aquat. Bot. 2016, 133, 24–27. [Google Scholar] [CrossRef]
- Pilotti, M.; Simoncelli, S.; Valerio, G. A simple approach to the evaluation of the actual water renewal time of natural stratified lakes. Water Resour. Res. 2014, 50, 2830–2849. [Google Scholar] [CrossRef]
- Soana, E.; Bartoli, M. Seasonal variation of radial oxygen loss in Vallisneria spiralis L.: An adaptive response to sediment redox? Aquat. Bot. 2013, 104, 228–232. [Google Scholar] [CrossRef]



| In Situ | |||||
|---|---|---|---|---|---|
| RM | BS | Deep Water | Producer’s Accuracy | ||
| Classified | RM | 29 | 1 | 1 | 93.5% |
| BS | 7 | 100.0% | |||
| Deep Water | 1 | 8 | 88.9% | ||
| User’s Accuracy | 100.0% | 77.8% | 88.9% | 93.6% | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghirardi, N.; Bolpagni, R.; Bresciani, M.; Valerio, G.; Pilotti, M.; Giardino, C. Spatiotemporal Dynamics of Submerged Aquatic Vegetation in a Deep Lake from Sentinel-2 Data. Water 2019, 11, 563. https://doi.org/10.3390/w11030563
Ghirardi N, Bolpagni R, Bresciani M, Valerio G, Pilotti M, Giardino C. Spatiotemporal Dynamics of Submerged Aquatic Vegetation in a Deep Lake from Sentinel-2 Data. Water. 2019; 11(3):563. https://doi.org/10.3390/w11030563
Chicago/Turabian StyleGhirardi, Nicola, Rossano Bolpagni, Mariano Bresciani, Giulia Valerio, Marco Pilotti, and Claudia Giardino. 2019. "Spatiotemporal Dynamics of Submerged Aquatic Vegetation in a Deep Lake from Sentinel-2 Data" Water 11, no. 3: 563. https://doi.org/10.3390/w11030563
APA StyleGhirardi, N., Bolpagni, R., Bresciani, M., Valerio, G., Pilotti, M., & Giardino, C. (2019). Spatiotemporal Dynamics of Submerged Aquatic Vegetation in a Deep Lake from Sentinel-2 Data. Water, 11(3), 563. https://doi.org/10.3390/w11030563






