Seasonal Fluxes of Dissolved Nutrients in Streams of Catchments Dominated by Swidden Agriculture in the Maya Forest of Belize, Central America
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
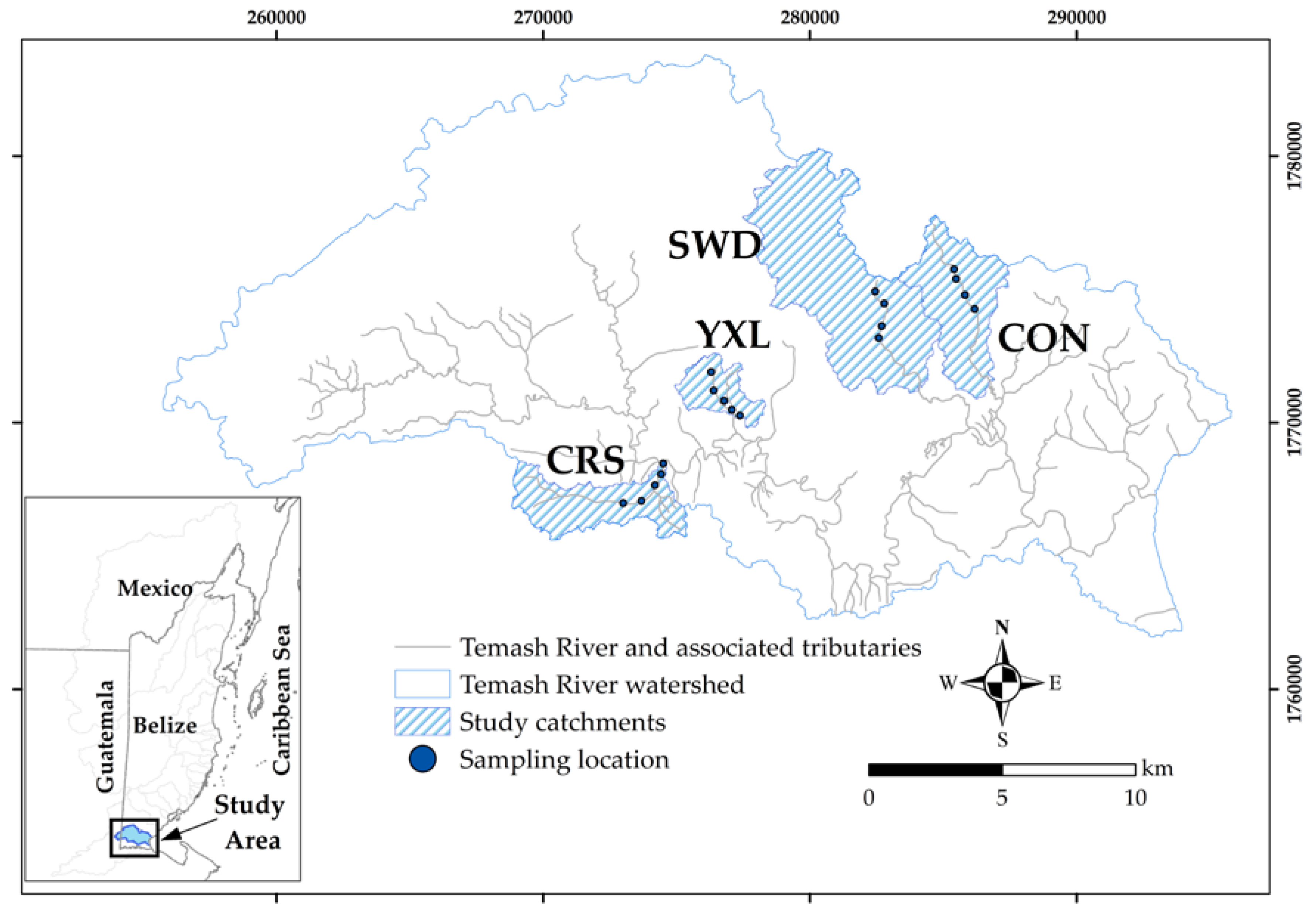
2.2. Catchment Selection, Delineation, and Characterization
2.3. Land Use and Land Cover Analysis
2.4. Annual Rainfall Pattern
2.5. Instantaneous Discharge
2.6. Sample Collection and Laboratory Analysis
2.7. Nutrient Fluxes and Annualized Nutrient Export
2.8. Statistical Analyses
3. Results
3.1. Catchment Characteristics and Land Cover Change
3.2. Precipitation and Seasonal Stream Discharge
3.3. Seasonal and Longitudinal Variation of In-Stream Nutrient Concentrations
3.4. Nutrient Fluxes and Annual Export
4. Discussion
4.1. Seasonal Hydrologic Changes and Nutrient Pulses
4.2. Swidden, Land Cover Change, and Nutrient Dynamics
4.3. Nutrient Exports
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tortajada, C.; Landry, C.; Rodriguez, E.M.; Meinzen-Dick, R.; Moellendorf, S.; Porras, I.; Ratner, B.; Shea, A.; Swallow, B.; Thomich, T.; et al. Freshwater Ecosystem Services. In Millennium Ecosystem Assessment: Current State & Trends Assessment; Island Press: Washington, DC, USA, 2005; pp. 213–254. [Google Scholar]
- Lewis, W.M. Physical and Chemical Features of Tropical Flowing Waters. In Tropical Stream Ecology; Elsevier: Amsterdam, The Netherlands, 2008; pp. 1–21. ISBN 978-0-12-088449-0. [Google Scholar]
- Amarasekera, K.N.; Lee, R.F.; Williams, E.R.; Eltahir, E.A.B. ENSO and the natural variability in the flow of tropical rivers. J. Hydrol. 1997, 200, 24–39. [Google Scholar] [CrossRef]
- Hunt, R.J.; Jardine, T.D.; Hamilton, S.K.; Bunn, S.E. Temporal and spatial variation in ecosystem metabolism and food web carbon transfer in a wet-dry tropical river. Freshw. Biol. 2012, 57, 435–450. [Google Scholar] [CrossRef]
- Sala, O.E.; Chapin, F.S.; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global Biodiversity Scenarios for the Year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef]
- McClain, M.E. The application of ecohydrological principles for better water resources management in South America. In The Ecohydrology of South American Rivers and Wetlands; IAHS Special Publication No. 6; IAHS Press: Wallingford, UK, 2002. [Google Scholar]
- Castello, L.; Macedo, M.N. Large-scale degradation of Amazonian freshwater ecosystems. Glob. Chang. Biol. 2016, 22, 990–1007. [Google Scholar] [CrossRef]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.-I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.-H.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef]
- Biggs, T.W.; Dunne, T.; Martinelli, L.A. Natural controls and human impacts on stream nutrient concentrations in a deforested region of the Brazilian Amazon basin. Biogeochemistry 2004, 68, 227–257. [Google Scholar] [CrossRef]
- Downing, J.A.; McClain, M.; Twilley, R.; Melack, J.M.; Elser, J.; Rabalais, N.N.; Lewis, W.M.; Turner, R.E.; Corredor, J.; Soto, D.; et al. The impact of accelerating land-use change on the N-cycle of tropical aquatic ecosystems: Current conditions and projected changes. Biogeochemistry 1999, 46, 109–148. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Caraco, N.F.; Correll, D.L.; Howarth, R.W.; Sharpley, A.N.; Smith, V.H. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol. Appl. 1998, 8, 559–568. [Google Scholar] [CrossRef]
- Caraco, N.F.; Cole, J.J. Human Impact on Nitrate Export: An Analysis Using Major World Rivers. Ambio 1999, 28, 167–170. [Google Scholar]
- Tappin, A.D. An Examination of the Fluxes of Nitrogen and Phosphorus in Temperate and Tropical Estuaries: Current Estimates and Uncertainties. Estuar. Coast. Shelf Sci. 2002, 55, 885–901. [Google Scholar] [CrossRef]
- Neill, C.; Deegan, L.A.; Thomas, S.M.; Cerri, C.C. Deforestation for pasture alters nitrogen and phosphorus in small Amazonian streams. Ecol. Appl. 2001, 11, 1817–1828. [Google Scholar] [CrossRef]
- Thomas, S.M.; Neill, C.; Deegan, L.A.; Krusche, A.V.; Ballester, V.M.; Victoria, R.L. Influences of land use and stream size on particulate and dissolved materials in a small Amazonian stream network. Biogeochemistry 2004, 68, 135–151. [Google Scholar] [CrossRef]
- Markewitz, D.; Davidson, E.; Moutinho, P.; Nepstad, D. Nutrient Loss and Redistribution After Forest Clearing on a Highly Weathered Soil in Amazonia. Ecol. Appl. 2004, 14, 177–199. [Google Scholar] [CrossRef]
- Figueiredo, R.O.; Markewitz, D.; Davidson, E.A.; Schuler, A.E.; Dos S Watrin, O.; de Souza Silva, P. Land-use effects on the chemical attributes of low-order streams in the eastern Amazon. J. Geophys. Res. Biogeosci. 2010, 115. [Google Scholar] [CrossRef] [Green Version]
- Nóbrega, R.L.B.; Guzha, A.C.; Lamparter, G.; Amorim, R.S.S.; Couto, E.G.; Hughes, H.J.; Jungkunst, H.F.; Gerold, G. Impacts of land-use and land-cover change on stream hydrochemistry in the Cerrado and Amazon biomes. Sci. Total Environ. 2018, 635, 259–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neill, C.; Jankowski, K.; Brando, P.M.; Coe, M.T.; Deegan, L.A.; Macedo, M.N.; Riskin, S.H.; Porder, S.; Elsenbeer, H.; Krusche, A.V. Surprisingly modest water quality impacts from expansion and intensification of large-scale commercial agriculture in the Brazilian Amazon-Cerrado Region. Trop. Conserv. Sci. 2017, 10, 1940082917720669. [Google Scholar] [CrossRef]
- Riskin, S.H.; Neill, C.; Jankowski, K.; Krusche, A.V.; McHorney, R.; Elsenbeer, H.; Macedo, M.N.; Nunes, D.; Porder, S. Solute and sediment export from Amazon forest and soybean headwater streams. Ecol. Appl. 2017, 27, 193–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myers, N. Tropical forests: Present status and future outlook. Clim. Chang. 1991, 19, 3–32. [Google Scholar] [CrossRef]
- Houghton, R.A. The worldwide extent of land-use change. BioScience 1994, 44, 305–313. [Google Scholar] [CrossRef]
- Laurance, W.F.; Sayer, J.; Cassman, K.G. Agricultural expansion and its impacts on tropical nature. Trends Ecol. Evol. 2014, 29, 107–116. [Google Scholar] [CrossRef]
- van Vliet, N.; Mertz, O.; Heinimann, A.; Langanke, T.; Pascual, U.; Schmook, B.; Adams, C.; Schmidt-Vogt, D.; Messerli, P.; Leisz, S.; et al. Trends, drivers and impacts of changes in swidden cultivation in tropical forest-agriculture frontiers: A global assessment. Glob. Environ. Chang. 2012, 22, 418–429. [Google Scholar] [CrossRef]
- Attiwill, P.M. The disturbance of forest ecosystems: The ecological basis for conservative management. For. Ecol. Manag. 1994, 63, 247–300. [Google Scholar] [CrossRef]
- Turner, B.L., II; Villar, S.C.; Foster, D.; Geoghegan, J.; Keys, E.; Klepeis, P.; Lawrence, D.; Mendoza, P.M.; Manson, S.; Ogneva-Himmelberger, Y.; et al. Deforestation in the southern Yucatan peninsular region: An integrative approach. For. Ecol. Manag. 2001, 154, 353–370. [Google Scholar] [CrossRef]
- Brenner, M.; Rosenmeier, M.F.; Hodell, D.A.; Curtis, J.H. Paleolimnology of the Maya Lowlands: Long-term perspectives on interactions among climate, environment, and humans. Anc. Mesoam. 2002, 13, 141–157. [Google Scholar] [CrossRef]
- Buck, D.G.; Esselman, P.C.; Villafranco, J. Monitoring land use changes along riparian corridors in lowland tropical watersheds: Application of human impact mapping and estimation of local stress intensity. Mesoamericana 2011, 15, 51–62. [Google Scholar]
- Chicas, S.D.; Omine, K.; Ford, J.B. Identifying erosion hotspots and assessing communities’ perspectives on the drivers, underlying causes and impacts of soil erosion in Toledo’s Rio Grande Watershed: Belize. Appl. Geogr. 2016, 68, 57–67. [Google Scholar] [CrossRef]
- Esselman, P.; Jiang, S.; Peller, H.; Buck, D.; Wainwright, J. Landscape drivers and social dynamics shaping microbial contamination risk in three Maya communities in Southern Belize, Central America. Water 2018, 10, 1678. [Google Scholar] [CrossRef]
- Lee, M.D.; Stednick, J.D.; Gilbert, D.M. Belize Environmental Water Quality Monitoring—Final Report and Annexes; Natural Resources Management and Protection Project; USAID: Belize City, Belize, 1995; p. 298.
- Meerman, J.C.; Herrera, P.; Howe, A. Rapid Ecological Assessment: Sarstoon Temash National Park, Toledo District, Belize; Sartstoon Temash Institute for Indigenous Management: Toledo, Belize, 2003; p. 73. [Google Scholar]
- Wright, A.C.S.; Romney, D.H.; Arbuckle, R.H. Land in British Honduras: Report of the Land Use Survey Team; Colonial Research Publication; HMSO: London, UK, 1959.
- U.S. Geological Survey Earth Explorer. 2017. Available online: https://earthexplorer.usgs.gov (accessed on 1 June 2018).
- Wainwright, J.; Jiang, S.; Mercer, K.; Liu, D. The political ecology of a highway through Belize’s forested borderlands. Environ. Plan. A 2015, 47, 833–849. [Google Scholar] [CrossRef]
- Wainwright, J.; Jiang, S.; Liu, D. Deforestation and the world-as-representation: The Maya forest of southern Belize. In Land Change Science, Political Ecology, and Sustainability: Synergies and Divergences; Brannstrom, C., Vadjunec, J., Eds.; Routledge: New York, NY, USA, 2013; pp. 169–190. [Google Scholar]
- The MathWorks. MATLAB 2018a; The MathWorks, Inc.: Natick, MA, USA, 2018. [Google Scholar]
- Chang, C.C.; Lin, C.J. A library for support vector machines. ACM Trans. Intell. Syst. Technol. 2011, 2, 1–27. [Google Scholar] [CrossRef]
- Marengo, J.A.; Liebmann, D.; Kousky, V.E.; Filizola, N.P.; Wainer, I.C. Onset and end of the rainy season in the Brazlian Amazon Basin. J. Clim. 2001, 14, 833–852. [Google Scholar] [CrossRef]
- Gore, J.A. Discharge measurements and streamflow analysis. In Methods in Stream Ecology; Elsevier: London, UK, 2006; pp. 51–78. [Google Scholar]
- Grayson, R.B.; Gippel, C.J.; Finlayson, B.L.; Hart, B.T. Catchment-wide impacts on water quality: The use of “snapshot” sampling during stable flow. J. Hydrol. 1997, 199, 121–134. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 17th ed.; American Public Health Association: Washington, DC, USA, 1989. [Google Scholar]
- Meerman, J.C.; Sabido, W. Central American Ecosystems Map-Belize (Vol. 1); Program for Belize: Belize City, Belize, 2001; p. 58. [Google Scholar]
- McDowell, W.H.; Asbury, C.E. Exports of carbon, nitrogen, and major ions from three tropical montane watersheds. Limnol. Oceanogr. 1994, 39, 111–125. [Google Scholar] [CrossRef]
- Williams, M.R.; Melack, J.M. Solute export from forested and partially deforested catchments in the central amazon. Biogeochemistry 1997, 38, 67–102. [Google Scholar] [CrossRef]
- Newbold, J.D.; Sweeney, B.W.; Jackson, J.K.; Kaplan, L.A. Concentrations and export of solutes from six mountain streams in northwestern Costa Rica. J. North Am. Benthol. Soc. 1995, 14, 21–37. [Google Scholar] [CrossRef]
- Gücker, B.; Silva, R.C.S.; Graeber, D.; Monteiro, J.A.F.; Brookshire, E.N.J.; Chaves, R.C.; Boëchat, I.G. Dissolved nutrient exports from natural and human-impacted Neotropical catchments. Glob. Ecol. Biogeogr. 2016, 25, 378–390. [Google Scholar] [CrossRef]
- Young, W.J.; Marston, F.M.; Davis, J.R. Nutrient exports and land use in Australian catchments. J. Environ. Manag. 1996, 47, 165–183. [Google Scholar] [CrossRef]
- Borbor-Cordova, M.J.; Boyer, E.W.; McDowell, W.H.; Hall, C.A. Nitrogen and phosphorus budgets for a tropical watershed impacted by agricultural land use: Guayas, Ecuador. Biogeochemistry 2006, 79, 135–161. [Google Scholar] [CrossRef]
- Junk, W.J.; Bayley, P.B.; Sparks, R.E. The flood pulse concept in river-floodplain systems. Can. Spec. Publ. Fish Aquat. Sci. 1989, 106, 110–127. [Google Scholar]
- Douglas, M.M.; Bunn, S.E.; Davies, P.M. River and wetland food webs in Australia’s wet-dry tropics: General principles and implications for management. Mar. Freshw. Res. 2005, 56, 329–342. [Google Scholar] [CrossRef]
- Williams, M.R.; Fisher, T.R.; Melack, J.M. Solute dynamics in soil water and groundwater in a central Amazon catchment undergoing deforestation. Biogeochemistry 1997, 38, 303–335. [Google Scholar] [CrossRef]
- Wollheim, W.M.; Vörösmarty, C.J.; Peterson, B.J.; Seitzinger, S.P.; Hopkinson, C.S. Relationship between river size and nutrient removal. Geophys. Res. Lett. 2006, 33, L06410. [Google Scholar] [CrossRef]
- Naiman, R.J.; Decamps, H. The ecology of interfaces: Riparian zones. Annu. Rev. Ecol. Syst. 1997, 28, 621–658. [Google Scholar] [CrossRef]
- Buck, D.G. Linking Land Use-Land Cover Change and Ecosystem Function in Tropical Lowland Watersheds of Belize, Central America. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 2012. [Google Scholar]
- Pringle, C.M.; Triska, F.J. Effects of geothermal groundwater on nutrient dynamics of a lowland Costa Rican stream. Ecology 1991, 72, 951–965. [Google Scholar] [CrossRef]
- Wilk, R.R. Household Ecology: Economic Change and Domestic Life among the Kekchi Maya in Belize; Northern Illinois University Press: Dekalb, IL, USA, 1997. [Google Scholar]
- Wainwright, J. Decolonizing Development: Colonial Power and the Maya; Blackwell Publishing: Hoboken, NJ, USA, 2008. [Google Scholar]
- Grandia, L. Enclosed: Conservation, Cattle and Commerce among the Qeqchi Maya Lowlanders; University of Washington Press: Seattle, WA, USA, 2012. [Google Scholar]
- Downey, S.S. Can properties of labor-exchange networks explain the reslience of swidden agriculture? Ecol. Soc. 2010, 15, 15. [Google Scholar] [CrossRef]
- Cortez, C.J. Intensive Smallholding Farming in the Belizean Maya Milpa and Matahambre Agro-Ecosystems. Ph.D. Thesis, University of California, Davis, CA, USA, 2016. [Google Scholar]
- Chomitz, K.M.; Gray, D.A. Roads, Lands, Markets and Deforestation: A Spatial Model of Land Use in Belize; The World Bank: Washington, DC, USA, 1995. [Google Scholar]
- Zarger, R.K. Mosaics of Maya Livelihoods: Readjusting to Global and Local Food Crises. Napa Bull. 2009, 32, 130–151. [Google Scholar] [CrossRef]
- Curtis, P.G.; Slay, C.M.; Harris, N.L.; Tyukavina, A.; Hansen, M.C. Classifying drivers of global forest loss. Science 2018, 361, 1108–1111. [Google Scholar] [CrossRef]
- Emch, M. The human ecology of Mayan Cacao farming in Belize. Hum. Ecol. 2003, 31, 111–132. [Google Scholar] [CrossRef]
- Downey, S.S. Q’eqchi’ Maya Swidden Agriculture, Settlement History, and Colonial Enterprise in Modern Belize. Ethnohistory 2015, 62, 751–779. [Google Scholar] [CrossRef]
- Steinberg, M.K. Political Ecology and Cultural Change: Impacts on Swidden-fallow Agroforestry Practices among the Mopan Maya in Southern Belize. Prof. Geogr. 1998, 50, 407–417. [Google Scholar] [CrossRef]
- Sánchez, P.A. Nitrogen in shifting cultivation systems of Latin America. In Nitrogen Cycling in Ecosystems of Latin America and the Caribbean; Robertson, G.P., Herrera, R., Rosswall, T., Eds.; Developments in Plant and Soil Sciences; Springer: Dordrecht, The Netherlands, 1982; pp. 91–103. ISBN 978-94-009-7639-9. [Google Scholar]
- Eaton, J.M.; Lawrence, D. Loss of carbon sequestration potential after several decades of shifting cultivation in the Southern Yucatán. For. Ecol. Manag. 2009, 258, 949–958. [Google Scholar] [CrossRef]
- Ewel, J.; Berish, C.; Brown, B.; Price, N.; Raich, J. Slash and Burn Impacts on a Costa Rican Wet Forest Site. Ecology 1981, 62, 816–829. [Google Scholar] [CrossRef]
- Giardina, C.P.; Sanford, R.L.; Døckersmith, I.C.; Jaramillo, V.J. The effects of slash burning on ecosystem nutrients during the land preparation phase of shifting cultivation. Plant Soil 2000, 220, 247–260. [Google Scholar] [CrossRef]
- Sommer, R.; Vlek, P.L.G.; Deane De Abreu Sá, T.; Vielhauer, K.; De Fátima Rodrigues Coelho, R.; Fölster, H. Nutrient balance of shifting cultivation by burning or mulching in the Eastern Amazon—Evidence for subsoil nutrient accumulation. Nutr. Cycl. Agroecosyst. 2004, 68, 257–271. [Google Scholar] [CrossRef]
- Lambert, J.D.H.; Arnason, J.T. Nutrient dynamics in milpa agriculture and the role of weeds in initial stages of secondary succession in Belize, C.A. Plant Soil 1986, 93, 303–322. [Google Scholar] [CrossRef]
- Malmer, A.; Grip, H. Converting tropical rainforest to forest plantation in sabah, malaysia. Part II. Effects on nutrient dynamics and net losses in streamwater. Hydrol. Process. 1994, 8, 195–209. [Google Scholar] [CrossRef]
- Ferreira Marmontel, C.V.; Lucas-Borja, M.E.; Rodrigues, V.A.; Zema, D.A. Effects of land use and sampling distance on water quality in tropical headwater springs (Pimenta creek, São Paulo State, Brazil). Sci. Total Environ. 2018, 622–623, 690–701. [Google Scholar] [CrossRef]
- Jacobs, S.R.; Breuer, L.; Butterbach-Bahl, K.; Pelster, D.E.; Rufino, M.C. Land use affects total dissolved nitrogen and nitrate concentrations in tropical montane streams in Kenya. Sci. Total Environ. 2017, 603–604, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Bücker, A.; Crespo, P.; Frede, H.-G.; Breuer, L. Solute behaviour and export rates in neotropical montane catchments under different land-uses. J. Trop. Ecol. 2011, 27, 305–317. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, A.W.; Bramley, R.G.V.; Johnson, A.K.L. Export of nutrients and suspended sediment during a cyclone-mediated flood event in the Herbert River catchment, Australia. Mar. Freshw. Res. 1997, 48, 79. [Google Scholar] [CrossRef]
- Taniwaki, R.; Piggott, J.; Ferraz, S.; Matthaei, C. Climate change and multiple stressors in small tropical streams. Hydrobiologia 2017, 793, 41–53. [Google Scholar] [CrossRef]






| Crique Sarco (CRS05) | Yax Cal (YXL05) | Sunday Wood (SWD04) | Conejo (CON04) | |
|---|---|---|---|---|
| Area (ha) | 1084 | 469 | 3204 | 1384 |
| Elevation (m) | 53 | 26 | 41 | 28 |
| Slope (%) | 6.6 | 2.9 | 5.4 | 2.9 |
| Geology (%) | ||||
| Alluvial | 0 | 0 | 60 | 27 |
| Limestone | 23 | 0 | 15 | 0 |
| Clastic Sedimentary | 77 | 100 | 25 | 73 |
| Soils (%) | ||||
| Cambisol | 79 | 95 | 30 | 52 |
| Fluvisol | 0 | 5 | 61 | 48 |
| Leptosol | 11 | 0 | 9 | 0 |
| Leptosol–vertisol | 10 | 0 | 0 | 0 |
| Land Cover (%)^ Semideciduous/evergreen forest | 23 | 23 | 40 | 36 |
| Lowland swamp forest | 0 | 0 | 0 | 5 |
| Waterbody | 2 | 0 | 0 | 0 |
| Land Use (%) | ||||
| Historic agricultural lands^ | 75 | 77 | 60 | 59 |
| Actively cultivated lands+ | 1.8 | 6.4 | 6.9 | 8.7 |
| Dry Season Discharge (m3 s−1) | Wet Season Discharge (m3 s−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| Sampling Site | Median (N) | 25% | 75% | Median (N) | 25% | 75% | U Statistic | p-Value |
| CRS01 | 0.047 (9) | 0.017 | 0.248 | 0.353 (11) | 0.088 | 0.642 | 15.0 | 0.037* |
| CRS02 | 0.060 (9) | 0.000 | 0.263 | 0.446 (10) | 0.167 | 0.970 | 10.0 | 0.017* |
| CRS03 | 0.123 (9) | 0.000 | 0.234 | 0.680 (10) | 0.257 | 1.541 | 10.0 | 0.017* |
| CRS04 | 0.181 (10) | 0.047 | 0.257 | 0.383 (10) | 0.171 | 0.859 | 14.0 | 0.045* |
| CRS05 | 0.288 (9) | 0.116 | 0.539 | 0.365 (9) | 0.142 | 0.926 | 23.5 | 0.613 |
| YXL01 | 0.095 (10) | 0.036 | 0.199 | 0.210 (11) | 0.155 | 0.291 | 15.0 | 0.079 |
| YXL02 | 0.037 (10) | 0.019 | 0.088 | 0.148 (11) | 0.056 | 0.246 | 16.0 | 0.046* |
| YXL03 | 0.053 (10) | 0.033 | 0.113 | 0.236 (11) | 0.089 | 0.403 | 8.0 | 0.007* |
| YXL04 | 0.088 (8) | 0.035 | 0.133 | 0.382 (11) | 0.137 | 0.716 | 8.0 | 0.007* |
| YXL05 | 0.000 (9) | 0.000 | 0.000 | 4.2 × 10−5 (8) | 0.000 | 0.222 | 17.0 | 0.232 |
| SWD01 | 0.000 (9) | 0.000 | 0.000 | 0.031 (9) | 0.000 | 0.080 | 13.5 | 0.029* |
| SWD02 | 0.000 (9) | 0.000 | 0.000 | 0.135 (9) | 0.010 | 0.264 | 6.0 | 0.013* |
| SWD03 | 0.019 (10) | 0.011 | 0.083 | 0.250 (9) | 0.100 | 0.299 | 7.0 | 0.006* |
| SWD04 | 0.031 (9) | 0.006 | 0.043 | 0.293 (8) | 0.169 | 0.316 | 2.0 | 0.002* |
| CON01 | 0.000 (9) | 0.000 | 0.006 | 0.059 (10) | 0.017 | 0.155 | 9.5 | 0.005* |
| CON02 | 0.009 (9) | 0.000 | 0.025 | 0.124 (9) | 0.057 | 0.280 | 7.0 | 0.006* |
| CON03 | 0.000 (9) | 0.000 | 0.000 | 0.260 (9) | 0.129 | 0.406 | 5.0 | 0.002* |
| CON04 | 0.000 (9) | 0.000 | 0.000 | 0.380 (9) | 0.076 | 0.844 | 4.0 | 0.001* |
| Antecedent Rainfall | ||||
|---|---|---|---|---|
| Catchment | 1-day | 7-day | 14-day | 28-day |
| CRS * | 0.73 | 0.75 | 0.77 | 0.74 |
| YXL * | 0.58 | 0.65 | 0.73 | 0.70 |
| SWD ^ | 0.55 | 0.66 | 0.65 | 0.62 |
| CON ^ | 0.52 | 0.82 | 0.76 | 0.72 |
| DIN (mg/L) | TSN (mg/L) | TN (mg/L) | ||||
|---|---|---|---|---|---|---|
| Dry | Wet | Dry | wet | Dry | Wet | |
| Crique Sarco | ||||||
| CRS01 | 0.276 ± 0.442 (10) | 0.142 ± 0.269 (13) | 0.391 ± 0.401 (10) | 0.207 ± 0.267 (13) | 0.586 ± 0.298 (6) | 0.097 ± 0.063 (3) |
| CRS02 | 0.291 ± 0.443 (11) | 0.159 ± 0.342 (11) | 0.351 ± 0.416 (11) | 0.219 ± 0.330 (11) | 0.496 ± 0.377 (6) | 0.106 ± 0.065 (2) |
| CRS03 | 0.292 ± 0.452 (11) | 0.178 ± 0.360 (11) | 0.355 ± 0.421 (11) | 0.218 ± 0.350 (11) | 0.513 ± 0.364 (6) | 0.108 ± 0.079 (3) |
| CRS04 | 0.270 ± 0.407 (12) | 0.169 ± 0.355 (11) | 0.406 ± 0.386 (11) | 0.204 ± 0.346 (11) | 0.517 ± 0.323 (7) | 0.093 ± 0.051 (3) |
| CRS05 | 0.329 ± 0.434 (11) | 0.218 ± 0.457 (9) | 0.507 ± 0.374 (11) | 0.275 ± 0.437 (9) | 0.602 ± 0.275 (6) | 0.124 ± 0.038 (2) |
| Yax Cal | ||||||
| YXL01 | 0.154 ± 0.259 (12) | 0.214 ± 0.511 (12) | 0.179 ± 0.252 (12) | 0.225 ± 0.508 (12) | 0.378 ± 0.401 (7) | 0.058 ± 0.026 (3) |
| YXL02 | 0.227 ± 0.374 (12) | 0.204 ± 0.309 (12) | 0.255 ± 0.360 (12) | 0.227 ± 0.305 (12) | 0.405 ± 0.408 (7) | 0.040 ± 0.027 (3) |
| YXL03 | 0.216 ± 0.428 (12) | 0.205 ± 0.293 (12) | 0.314 ± 0.408 (12) | 0.220 ± 0.296 (12) | 0.446 ± 0.371 (7) | 0.047 ± 0.040 (3) |
| YXL04 | 0.278 ± 0.483 (12) | 0.191 ± 0.291 (12) | 0.334 ± 0.459 (12) | 0.222 ± 0.288 (12) | 0.432 ± 0.401 (7) | 0.047 ± 0.023 (3) |
| YXL05 | 0.272 ± 0.399 (12) | 0.170 ± 0.315 (10) | 0.357 ± 0.366 (12) | 0.201 ± 0.306 (10) | 0.491 ± 0.342 (7) | 0.082 ± 0.055 (3) |
| Sunday Wood | ||||||
| SWD01 | 0.120 ± 0.204 (11) | 0.161 ± 0.401 (10) | 0.143 ± 0.187 (11) | 0.183 ± 0.394 (10) | 0.424 ± 0.405 (6) | 0.027 ± 0.009 (3) |
| SWD02 | 0.118 ± 0.190 (10) | 0.163 ± 0.369 (11) | 0.240 ± 0.230 (10) | 0.177 ± 0.364 (11) | 0.431 ± 0.395 (6) | 0.064 ± 0.052 (3) |
| SWD03 | 0.121 ± 0.198 (11) | 0.219 ± 0.480 (11) | 0.308 ± 0.163 (10) | 0.246 ± 0.472 (11) | 0.522 ± 0.300 (7) | 0.057 ± 0.037 (3) |
| SWD04 | 0.143 ± 0.219 (11) | 0.286 ± 0.630 (9) | 0.213 ± 0.192 (11) | 0.306 ± 0.623 (9) | 0.494 ± 0.341 (6) | 0.063 ± 0.039 (2) |
| Conejo | ||||||
| CON01 | 0.178 ± 0.302 (11) | 0.381 ± 0.723 (11) | 0.353 ± 0.477 (11) | 0.402 ± 0.714 (11) | 0.556 ± 0.369 (6) | 0.071 ± 0.033 (3) |
| CON02 | 0.123 ± 0.191 (11) | 0.932 ± 0.764 (10) | 0.216 ± 0.207 (10) | 0.432 ± 0.760 (10) | 0.435 ± 0.348 (5) | 0.087 ± 0.050 (3) |
| CON03 | 0.117 ± 0.171 (11) | 0.313 ± 0.465 (10) | 0.226 ± 0.181 (11) | 0.348 ± 0.482 (10) | 0.556 ± 0.328 (6) | 0.080 ± 0.020 (3) |
| CON04 | 0.128 ± 0.176 (11) | 0.303 ± 0.450 (10) | 0.280 ± 0.167 (11) | 0.338 ± 0453 (10) | 0.636 ± 0.258 (6) | 0.086 ± 0.028 (3) |
| SRP (mg/L) | TSP (mg/L) | TP (mg/L) | ||||
|---|---|---|---|---|---|---|
| Dry | Wet | Dry | Wet | Dry | Wet | |
| Crique Sarco | ||||||
| CRS01 | 0.001 ± 0.002 (9) | 0.001 ± 0.001 (13) | 0.004 ± 0.005 (10) | 0.003 ± 0.003 (13) | 0.009 ± 0.004 (6) | 0.006 ± 0.003 (3) |
| CRS02 | 0.002 ± 0.002 (10) | 0.002 ± 0.003 (11) | 0.004 ± 0.003 (11) | 0.005 ± 0.003 (11) | 0.009 ± 0.003 (6) | 0.010 ± 0.003 (2) |
| CRS03 | 0.001 ± 0.001 (10) | 0.001 ± 0.001 (11) | 0.003 ± 0.002 (11) | 0.005 ± 0.005 (11) | 0.007 ± 0.003 (3) | 0.007 ± 0.003 (3) |
| CRS04 | 0.004 ± 0.004 (10) | 0.005 ± 0.008 (11) | 0.006 ± 0.004 (11) | 0.009 ± 0.007 (11) | 0.009 ± 0.004 (7) | 0.015 ± 0.012 (3) |
| CRS05 | 0.005 ± 0.006 (10) | 0.002 ± 0.001 (9) | 0.010 ± 0.010 (11) | 0.006 ± 0.003 (9) | 0.023 ± 0.017 (6) | 0.008 ± 0.001 (2) |
| Yax Cal | ||||||
| YXL01 | 0.019 ± 0.008 (12) | 0.006 ± 0.006 (12) | 0.022 ± 0.008 (12) | 0.010 ± 0.007 (12) | 0.029 ± 0.010 (7) | 0.011 ± 0.010 (3) |
| YXL02 | 0.004 ± 0.002 (11) | 0.004 ± 0.005 (12) | 0.006 ± 0.003 (12) | 0.006 ± 0.004 (12) | 0.011 ± 0.004 (7) | 0.010 ± 0.008 (3) |
| YXL03 | 0.002 ± 0.002 (11) | 0.001 ± 0.001 (12) | 0.006 ± 0.005 (12) | 0.004 ± 0.002 (12) | 0.011 ± 0.005 (7) | 0.004 ± 0.002 (3) |
| YXL04 | 0.001 ± 0.001 (11) | 0.001 ± 0.001 (12) | 0.004 ± 0.003 (12) | 0.004 ± 0.001 (12) | 0.008 ± 0.003 (7) | 0.005 ± 0.002 (3) |
| YXL05 | 0.005 ± 0.014 (11) | 0.005 ± 0.014 (10) | 0.007 ± 0.013 (12) | 0.011 ± 0.015 (10) | 0.011 ± 0.017 (7) | 0.018 ± 0.023 (3) |
| Sunday Wood | ||||||
| SWD01 | 0.002 ± 0.003 (10) | 0.002 ± 0.002 (10) | 0.004 ± 0.005 (10) | 0.005 ± 0.003 (10) | 0.009 ± 0.006 (6) | 0.007 ± 0.003 (3) |
| SWD02 | 0.011 ± 0.022 (9) | 0.002 ± 0.001 (10) | 0.012 ± 0.025 (10) | 0.003 ± 0.002 (11) | 0.009 ± 0.012 (6) | 0.006 ± 0.002 (3) |
| SWD03 | 0.007 ± 0.008 (9) | 0.002 ± 0.002 (11) | 0.014 ± 0.018 (10) | 0.007 ± 0.004 (11) | 0.020 ± 0.020 (7) | 0.008 ± 0.003 (3) |
| SWD04 | 0.001 ± 0.001 (10) | 0.002 ± 0.002 (9) | 0.005 ± 0.002 (11) | 0.005 ± 0.003 (9) | 0.010 ± 0.005 (6) | 0.009 ± 0.005 (2) |
| Conejo | ||||||
| CON01 | 0.010 ± 0.019 (10) | 0.004 ± 0.009 (11) | 0.012 ± 0.020 (11) | 0.005 ± 0.008 (11) | 0.017 ± 0.021 (6) | 0.005 ± 0.001 (3) |
| CON02 | 0.001 ± 0.002 (9) | 0.002 ± 0.001 (10) | 0.004 ± 0.003 (10) | 0.014 ± 0.033 (10) | 0.006 ± 0.001 (5) | 0.039 ± 0.059 (3) |
| CON03 | 0.010 ± 0.020 (10) | 0.003 ± 0.002 (10) | 0.013 ± 0.018 (11) | 0.006 (0.003) (10) | 0.028 ± 0.010 (6) | 0.007 ± 0.002 (3) |
| CON04 | 0.002 ± 0.003 (10) | 0.002 ± 0.002 (10) | 0.005 ± 0.004 (11) | 0.004 ± 0.001 (10) | 0.017 ± 0.009 (6) | 0.007 ± 0.001 (3) |
| Yield | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Stream/River | Size (ha) | Land Cover | DIN | TSN | TN | SRP | TSP | TP | Location/Reference |
| kg ha−1 year−1 | |||||||||
| Crique Sarco Creek | 1084 | 2% agriculture | 1.04 | 2.25 | 0.03 | 0.09 | southern Belize/this study | ||
| Yax Cal Creek | 469 | 6% agriculture | 0.28 | 0.53 | 0.03 | 0.04 | southern Belize/this study | ||
| Sundaywood Creek | 3204 | 7% agr | 0.12 | 0.18 | 0.00 | 0.01 | southern Belize/this study | ||
| Conejo Creek | 1384 | 9% agr | 2.04 | 2.48 | 0.02 | 0.03 | southern Belize/this study | ||
| Braco do Mota | 23.4 | 80% agr | 3.64 | 6.44 | 9.14 | 0.08 | 0.33 | 0.48 | central Amazon (A) |
| Igarape de Mota | 18 | >95% forest | 2.67 | 3.61 | 4.31 | 0.02 | 0.05 | 0.08 | central Amazon (A) |
| Tempisquito | 319 | >95% forest | 6.10 | 0.57 | Costa Rica (B) | ||||
| Tempisquito Sur | 311 | >95% forest | 4.90 | 0.33 | Costa Rica (B) | ||||
| Kathia | 264 | >95% forest | 5.60 | 0.34 | Costa Rica (B) | ||||
| Marilin | 36 | >95% forest | 4 | 0.46 | Costa Rica (B) | ||||
| El Jobo | 55 | >95% forest | 4.3 | 0.34 | Costa Rica (B) | ||||
| Zompopa | 37 | >95% forest | 6 | 0.43 | Costa Rica (B) | ||||
| Icacos | 326 | >95% forest | 3.2 | 8.01 | 9.8 | 0.07 | Puerto Rico (C) | ||
| Sonadora | 262 | >95% forest | 1.69 | 5.43 | 5.9 | 0.05 | Puerto Rico (C) | ||
| Toronja | 16.2 | >95% forest | 1.16 | 3.96 | 4.4 | 0.03 | Puerto Rico (C) | ||
| Rio das Mortes | Cerrado | 3.9 | 0.54 | Brazil (D) | |||||
| multiple basins | various | 0.17 | 0.04 | SE Brazil (D) | |||||
| multiple basins | 104–106 | forested | 2 | 1.2 | Queensland, Australia (E) | ||||
| Guayas | 3.2 × 106 | 29% forest; 52% Agriculture | 10.1 | 2.4 | Ecuador (F) | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buck, D.G.; Esselman, P.C.; Jiang, S.; Wainwright, J.D.; Brenner, M.; Cohen, M.J. Seasonal Fluxes of Dissolved Nutrients in Streams of Catchments Dominated by Swidden Agriculture in the Maya Forest of Belize, Central America. Water 2019, 11, 664. https://doi.org/10.3390/w11040664
Buck DG, Esselman PC, Jiang S, Wainwright JD, Brenner M, Cohen MJ. Seasonal Fluxes of Dissolved Nutrients in Streams of Catchments Dominated by Swidden Agriculture in the Maya Forest of Belize, Central America. Water. 2019; 11(4):664. https://doi.org/10.3390/w11040664
Chicago/Turabian StyleBuck, David G., Peter C. Esselman, Shiguo Jiang, Joel D. Wainwright, Mark Brenner, and Matthew J. Cohen. 2019. "Seasonal Fluxes of Dissolved Nutrients in Streams of Catchments Dominated by Swidden Agriculture in the Maya Forest of Belize, Central America" Water 11, no. 4: 664. https://doi.org/10.3390/w11040664
APA StyleBuck, D. G., Esselman, P. C., Jiang, S., Wainwright, J. D., Brenner, M., & Cohen, M. J. (2019). Seasonal Fluxes of Dissolved Nutrients in Streams of Catchments Dominated by Swidden Agriculture in the Maya Forest of Belize, Central America. Water, 11(4), 664. https://doi.org/10.3390/w11040664







