Diethylene Glycol-Assisted Organized TiO2 Nanostructures for Photocatalytic Wastewater Treatment Ceramic Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. DEG-Assisted Synthesis of TiO2 Nanostructure
2.3. Membrane Characterization
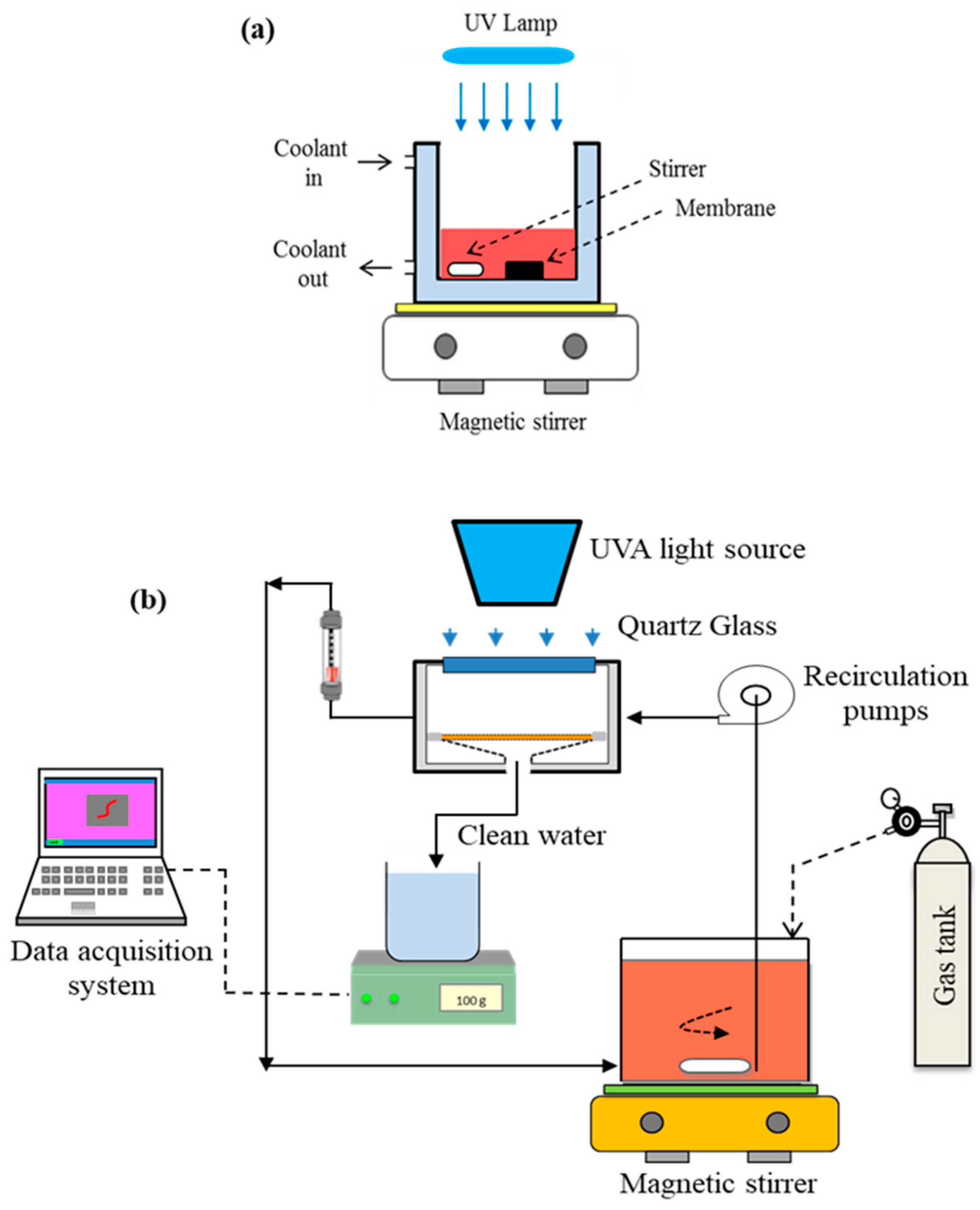
2.4. Photocatalytic Membrane Reactor
3. Results
3.1. DEG-Assistend Synthesis of TiO2 Nanostrtuctures
3.2. Intrinstic Properties of Membranes
3.3. Static Adsorption Tests, Photodegradation and Self-Cleaning
3.4. Antifouling Property by Membrane Fouling Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, L.; Zheng, G.; Yang, F. Adsorptive removal and oxidation of organic pollutants from water using a novel membrane. Chem. Eng. J. 2010, 156, 553–556. [Google Scholar] [CrossRef]
- Gao, Y.; Hu, M.; Mi, B. Membrane surface modification with TiO2–graphene oxide for enhanced photocatalytic performance. J. Membr. Sci. 2014, 455, 349–356. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, G.; Zhi, S.; Xu, K.; Zhu, L.; Li, W.; Zeng, Z.; Xue, Q. Superhydrophilicity and underwater superoleophobicity TiO2/Al2O3 composite membrane with ultra low oil adhesion for highly efficient oil-in-water emulsions separation. Appl. Surf. Sci. 2018, 458, 157–165. [Google Scholar] [CrossRef]
- Tabriz, A.; Alvi, M.A.U.R.; Niazi, M.B.K.; Batool, M.; Bhatti, M.F.; Khan, A.L.; Khan, A.U.; Jamil, T.; Ahmad, N.M. Quaternized trimethyl functionalized chitosan based antifungal membranes for drinking water treatment. Carbohydr. Polym. 2019, 207, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Davies, S.H.; Masten, S.J. Analysis of energy costs for catalytic ozone membrane filtration. Sep. Purif. Technol. 2017, 186, 182–187. [Google Scholar] [CrossRef]
- Zhu, R.; Diaz, A.J.; Shen, Y.; Qi, F.; Chang, X.; Durkin, D.P.; Sun, Y.; Solares, S.D.; Shuai, D. Mechanism of Humic Acid Fouling in a Photocatalytic Membrane System. J. Membr. Sci. 2018. [Google Scholar] [CrossRef]
- Lin, Y.-F.; Tung, K.-L.; Tzeng, Y.-S.; Chen, J.-H.; Chang, K.-S. Rapid atmospheric plasma spray coating preparation and photocatalytic activity of macroporous titania nanocrystalline membranes. J. Membr. Sci. 2012, 389, 83–90. [Google Scholar] [CrossRef]
- Ai, J.; Yang, L.; Liao, G.; Xia, H.; Xiao, F. Applications of graphene oxide blended poly(vinylidene fluoride) membranes for the treatment of organic matters and its membrane fouling investigation. Appl. Surf. Sci. 2018, 455, 502–512. [Google Scholar] [CrossRef]
- Kazemi, M.; Jahanshahi, M.; Peyravi, M. Hexavalent chromium removal by multilayer membrane assisted by photocatalytic couple nanoparticle from both permeate and retentate. J. Hazard. Mater. 2018, 344, 12–22. [Google Scholar] [CrossRef]
- Hatat-Fraile, M.; Liang, R.; Arlos, M.J.; He, R.X.; Peng, P.; Servos, M.R.; Zhou, Y.N. Concurrent photocatalytic and filtration processes using doped TiO2 coated quartz fiber membranes in a photocatalytic membrane reactor. Chem. Eng. J. 2017, 330, 531–540. [Google Scholar] [CrossRef]
- Najma, B.; Kasi, A.K.; Khan Kasi, J.; Akbar, A.; Bokhari, S.M.A.; Stroe, I.R.C. ZnO/AAO photocatalytic membranes for efficient water disinfection: Synthesis, characterization and antibacterial assay. Appl. Surf. Sci. 2018, 448, 104–114. [Google Scholar] [CrossRef]
- Ahmad, R.; Aslam, M.; Park, E.; Chang, S.; Kwon, D.; Kim, J. Submerged low-cost pyrophyllite ceramic membrane filtration combined with GAC as fluidized particles for industrial wastewater treatment. Chemosphere 2018, 206, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Kim, J.; Kim, J.; Kim, J. In-situ TiO2 formation and performance on ceramic membranes in photocatalytic membrane reactor. Membr. J. 2017, 27, 328–335. [Google Scholar] [CrossRef]
- Ferreiro, C.; Villota, N.; Lombraña, J.I.; Rivero, M.J.; Zúñiga, V.; Rituerto, J.M. Analysis of a Hybrid Suspended-Supported Photocatalytic Reactor for the Treatment of Wastewater Containing Benzothiazole and Aniline. Water 2019, 11, 337. [Google Scholar] [CrossRef]
- Ahmad, R.; Ahmad, Z.; Khan, A.U.; Mastoi, N.R.; Aslam, M.; Kim, J. Photocatalytic systems as an advanced environmental remediation: Recent developments, limitations and new avenues for applications. J. Environ. Chem. Eng. 2016, 4, 4143–4164. [Google Scholar] [CrossRef]
- Karnik, B.S.; Davies, S.H.; Baumann, M.J.; Masten, S.J. Fabrication of catalytic membranes for the treatment of drinking water using combined ozonation and ultrafiltration. Environ. Sci. Technol. 2005, 39, 7656–7661. [Google Scholar] [CrossRef]
- Aoudjit, L.; Martins, P.M.; Madjene, F.; Petrovykh, D.; Lanceros-Mendez, S. Photocatalytic reusable membranes for the effective degradation of tartrazine with a solar photoreactor. J. Hazard. Mater. 2018, 344, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Mendret, J.; Hatat-Fraile, M.; Rivallin, M.; Brosillon, S. Hydrophilic composite membranes for simultaneous separation and photocatalytic degradation of organic pollutants. Sep. Purif. Technol. 2013, 111, 9–19. [Google Scholar] [CrossRef]
- Mondal, K.; Kumar, J.; Sharma, A. TiO2-nanoparticles-impregnated photocatalytic macroporous carbon films by spin coating. Nanomater. Energy 2013, 2, 121–133. [Google Scholar] [CrossRef]
- Grčić, I.; Papić, S.; Brnardić, I. Photocatalytic activity of TiO2 thin films: Kinetic and efficiency study. Int. J. Chem. React. Eng. 2017, 16. [Google Scholar] [CrossRef]
- Sanabria Arenas, B.; Strini, A.; Schiavi, L.; Li Bassi, A.; Russo, V.; Del Curto, B.; Diamanti, M.; Pedeferri, M. Photocatalytic Activity of Nanotubular TiO2 Films Obtained by Anodic Oxidation: A Comparison in Gas and Liquid Phase. Materials 2018, 11, 488. [Google Scholar] [CrossRef] [PubMed]
- Cámara, R.; Portela, R.; Gutierrez-Martin, F.; Sánchez, B. Photocatalytic activity of TiO2 films prepared by surfactant-mediated sol–gel methods over commercial polymer substrates. Chem. Eng. J. 2016, 283, 535–543. [Google Scholar] [CrossRef]
- Goei, R.; Lim, T.-T. Asymmetric TiO2 hybrid photocatalytic ceramic membrane with porosity gradient: Effect of structure directing agent on the resulting membranes architecture and performances. Ceram. Int. 2014, 40, 6747–6757. [Google Scholar] [CrossRef]
- Pan, J.H.; Zhao, X.; Lee, W.I. Block copolymer-templated synthesis of highly organized mesoporous TiO2-based films and their photoelectrochemical applications. Chem. Eng. J. 2011, 170, 363–380. [Google Scholar] [CrossRef]
- Ahmad, R.; Kim, J.K.; Kim, J.H.; Kim, J. Well-organized, mesoporous nanocrystalline TiO2 on alumina membranes with hierarchical architecture: Antifouling and photocatalytic activities. Catal. Today 2017, 282, 2–12. [Google Scholar] [CrossRef]
- Cao, X.P.; Li, D.; Jing, W.H.; Xing, W.H.; Fan, Y.Q. Synthesis of visible-light responsive C, N and Ce co-doped TiO2 mesoporous membranes via weak alkaline sol–gel process. J. Mater. Chem. 2012, 22, 15309–15315. [Google Scholar] [CrossRef]
- Ahmad, R.; Kim, J.K.; Kim, J.H.; Kim, J. Effect of polymer template on structure and membrane fouling of TiO2/Al2O3 composite membranes for wastewater treatment. J. Ind. Eng. Chem. 2018, 57, 55–63. [Google Scholar] [CrossRef]
- Alem, A.; Sarpoolaky, H.; Keshmiri, M. Titania ultrafiltration membrane: Preparation, characterization and photocatalytic activity. J. Eur. Ceram. Soc. 2009, 29, 629–635. [Google Scholar] [CrossRef]
- Zhang, S.; Du, Y.; Jiang, H.; Liu, Y.; Chen, R. Controlled synthesis of TiO2 nanorod arrays immobilized on ceramic membranes with enhanced photocatalytic performance. Ceram. Int. 2017, 43, 7261–7270. [Google Scholar] [CrossRef]
- Zinadini, S.; Zinatizadeh, A.A.; Rahimi, M.; Vatanpour, V.; Zangeneh, H. Preparation of a novel antifouling mixed matrix PES membrane by embedding graphene oxide nanoplates. J. Membr. Sci. 2014, 453, 292–301. [Google Scholar] [CrossRef]
- Cozzoli, P.D.; Kornowski, A.; Weller, H. Low-temperature synthesis of soluble and processable organic-capped anatase TiO2 nanorods. J. Am. Chem. Soc. 2003, 125, 14539–14548. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhong, X.; Liu, S.; Li, D.; Han, M. Aminolysis route to monodisperse titania nanorods with tunable aspect ratio. Angew. Chem. Int. Ed. 2005, 44, 3466–3470. [Google Scholar] [CrossRef]
- Roh, D.K.; Chi, W.S.; Ahn, S.H.; Jeon, H.; Kim, J.H. One-step Synthesis of Vertically Aligned Anatase Thornbush-like TiO2 Nanowire Arrays on Transparent Conducting Oxides for Solid-State Dye-Sensitized Solar Cells. ChemSusChem 2013, 6, 1384–1391. [Google Scholar] [CrossRef]
- Roh, D.K.; Chi, W.S.; Jeon, H.; Kim, S.J.; Kim, J.H. High efficiency solid-state dye-sensitized solar cells assembled with hierarchical anatase pine tree-like TiO2 nanotubes. Adv. Funct. Mater. 2014, 24, 379–386. [Google Scholar] [CrossRef]
- Niu, F.; Zhang, L.-S.; Chen, C.-Q.; Li, W.; Li, L.; Song, W.-G.; Jiang, L. Hydrophilic TiO2 porous spheres anchored on hydrophobic polypropylene membrane for wettability induced high photodegrading activities. Nanoscale 2010, 2, 1480–1484. [Google Scholar] [CrossRef]
- Qin, A.; Li, X.; Zhao, X.; Liu, D.; He, C. Engineering a highly hydrophilic PVDF membrane via binding TiO2 nanoparticles and a PVA layer onto a membrane surface. ACS Appl. Mater. Interfaces 2015, 7, 8427–8436. [Google Scholar] [CrossRef]
- Ozawa, K.; Yamamoto, S.; Yukawa, R.; Liu, R.-Y.; Terashima, N.; Natsui, Y.; Kato, H.; Mase, K.; Matsuda, I. Correlation between Photocatalytic Activity and Carrier Lifetime: Acetic Acid on Single-Crystal Surfaces of Anatase and Rutile TiO2. J. Phys. Chem. C 2018, 122, 9562–9569. [Google Scholar] [CrossRef]
- Pigeot-Rémy, S.; Dufour, F.; Herissan, A.; Ruaux, V.; Maugé, F.; Hazime, R.; Foronato, C.; Guillard, C.; Chaneac, C.; Durupthy, O. Bipyramidal anatase TiO2 nanoparticles, a highly efficient photocatalyst? Towards a better understanding of the reactivity. Appl. Catal. B Environ. 2017, 203, 324–334. [Google Scholar] [CrossRef]
- Ahmad, R.; Kim, J.K.; Kim, J.H.; Kim, J. Nanostructured ceramic photocatalytic membrane modified with a polymer template for textile wastewater treatment. Appl. Sci. 2017, 7, 1284. [Google Scholar] [CrossRef]
- Zhao, K.; Feng, L.; Lin, H.; Fu, Y.; Lin, B.; Cui, W.; Li, S.; Wei, J. Adsorption and photocatalytic degradation of methyl orange imprinted composite membranes using TiO2/calcium alginate hydrogel as matrix. Catal. Today 2014, 236, 127–134. [Google Scholar] [CrossRef]
- Mohtor, N.H.; Othman, M.H.D.; Bakar, S.A.; Kurniawan, T.A.; Dzinun, H.; Norddin, M.N.A.M.; Rajis, Z. Synthesis of nanostructured titanium dioxide layer onto kaolin hollow fibre membrane via hydrothermal method for decolourisation of reactive black 5. Chemosphere 2018, 208, 595–605. [Google Scholar] [CrossRef]
- Tao, J.; Gong, Z.; Yao, G.; Cheng, Y.; Zhang, M.; Lv, J.; Shi, S.; He, G.; Chen, X.; Sun, Z. Hydrothermal growth of nanorod arrays and in situ conversion to nanotube arrays for highly efficient Ag-sensitized photocatalyst. J. Alloys Compd. 2016, 689, 451–459. [Google Scholar] [CrossRef]
- You, S.-J.; Semblante, G.U.; Lu, S.-C.; Damodar, R.A.; Wei, T.-C. Evaluation of the antifouling and photocatalytic properties of poly (vinylidene fluoride) plasma-grafted poly (acrylic acid) membrane with self-assembled TiO2. J. Hazard. Mater. 2012, 237, 10–19. [Google Scholar] [CrossRef]
- Xu, H.; Ding, M.; Chen, W.; Li, Y.; Wang, K. Nitrogen–doped GO/TiO2 nanocomposite ultrafiltration membranes for improved photocatalytic performance. Sep. Purif. Technol. 2018, 195, 70–82. [Google Scholar] [CrossRef]
- Zhang, E.; Wang, L.; Zhang, B.; Xie, Y.; Sun, C.; Jiang, C.; Zhang, Y.; Wang, G. Modification of polyvinylidene fluoride membrane with different shaped α-Fe2O3 nanocrystals for enhanced photocatalytic oxidation performance. Mater. Chem. Phys. 2018, 214, 41–47. [Google Scholar] [CrossRef]
- Horovitz, I.; Avisar, D.; Baker, M.A.; Grilli, R.; Lozzi, L.; Di Camillo, D.; Mamane, H. Carbamazepine degradation using a N-doped TiO2 coated photocatalytic membrane reactor: Influence of physical parameters. J. Hazard. Mater. 2016, 310, 98–107. [Google Scholar] [CrossRef]
- Lee, H.Y.; Park, Y.H.; Ko, K.H. Correlation between Surface Morphology and Hydrophilic/Hydrophobic Conversion of MOCVD−TiO2 Films. Langmuir 2000, 16, 7289–7293. [Google Scholar] [CrossRef]
- Moustakas, N.; Katsaros, F.; Kontos, A.; Romanos, G.E.; Dionysiou, D.; Falaras, P. Visible light active TiO2 photocatalytic filtration membranes with improved permeability and low energy consumption. Catal. Today 2014, 224, 56–69. [Google Scholar] [CrossRef]











| Membrane | Porosity (%) | Thickness (cm) | Permeability (L m−2 h−1 bar−1) |
|---|---|---|---|
| T1 | 49 | 0.219 | 33.4 |
| T2 | 49 | 0.221 | 37.7 |
| T3 | 48 | 0.225 | 8.3 |
| Bare | 52 | 0.208 | 44.3 |
| Photocatalyst | Degradation/Color Removal Efficiency (%) | Dye/Concentration (ppm) | UV/Solar Lamp Wattage (W) | Degradation Time (min) | Reference |
|---|---|---|---|---|---|
| Immobilized TNR | 52 | Reactive Black 5/(10) | 36 | 360 | [41] |
| Immobilized TNR | 82 | Methyl Orange/(15) | 36 | 100 | [42] |
| Immobilized TiO2 NPs | 42 | Reactive Black 5/(40) | 15 | 120 | [43] |
| Immobilized nitrogen-doped graphene/TiO2 | 80.6 | Methylene Blue/(50) | 125 | 120 | [44] |
| Immobilized α-Fe2O3 NR in presence of 75ml/L H2O2 | 88 | Congo Red/(20) | 150 | 120 | [45] |
| Immobilized TNR | 93.5 | Congo Red/(5) | 300 | 180 | This study |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, R.; Kim, J.K.; Kim, J.H.; Kim, J. Diethylene Glycol-Assisted Organized TiO2 Nanostructures for Photocatalytic Wastewater Treatment Ceramic Membranes. Water 2019, 11, 750. https://doi.org/10.3390/w11040750
Ahmad R, Kim JK, Kim JH, Kim J. Diethylene Glycol-Assisted Organized TiO2 Nanostructures for Photocatalytic Wastewater Treatment Ceramic Membranes. Water. 2019; 11(4):750. https://doi.org/10.3390/w11040750
Chicago/Turabian StyleAhmad, Rizwan, Jin Kyu Kim, Jong Hak Kim, and Jeonghwan Kim. 2019. "Diethylene Glycol-Assisted Organized TiO2 Nanostructures for Photocatalytic Wastewater Treatment Ceramic Membranes" Water 11, no. 4: 750. https://doi.org/10.3390/w11040750
APA StyleAhmad, R., Kim, J. K., Kim, J. H., & Kim, J. (2019). Diethylene Glycol-Assisted Organized TiO2 Nanostructures for Photocatalytic Wastewater Treatment Ceramic Membranes. Water, 11(4), 750. https://doi.org/10.3390/w11040750







