A Study of the Performance of Dielectric Barrier Discharge under Different Conditions for Nitrobenzene Degradation
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Materials Used
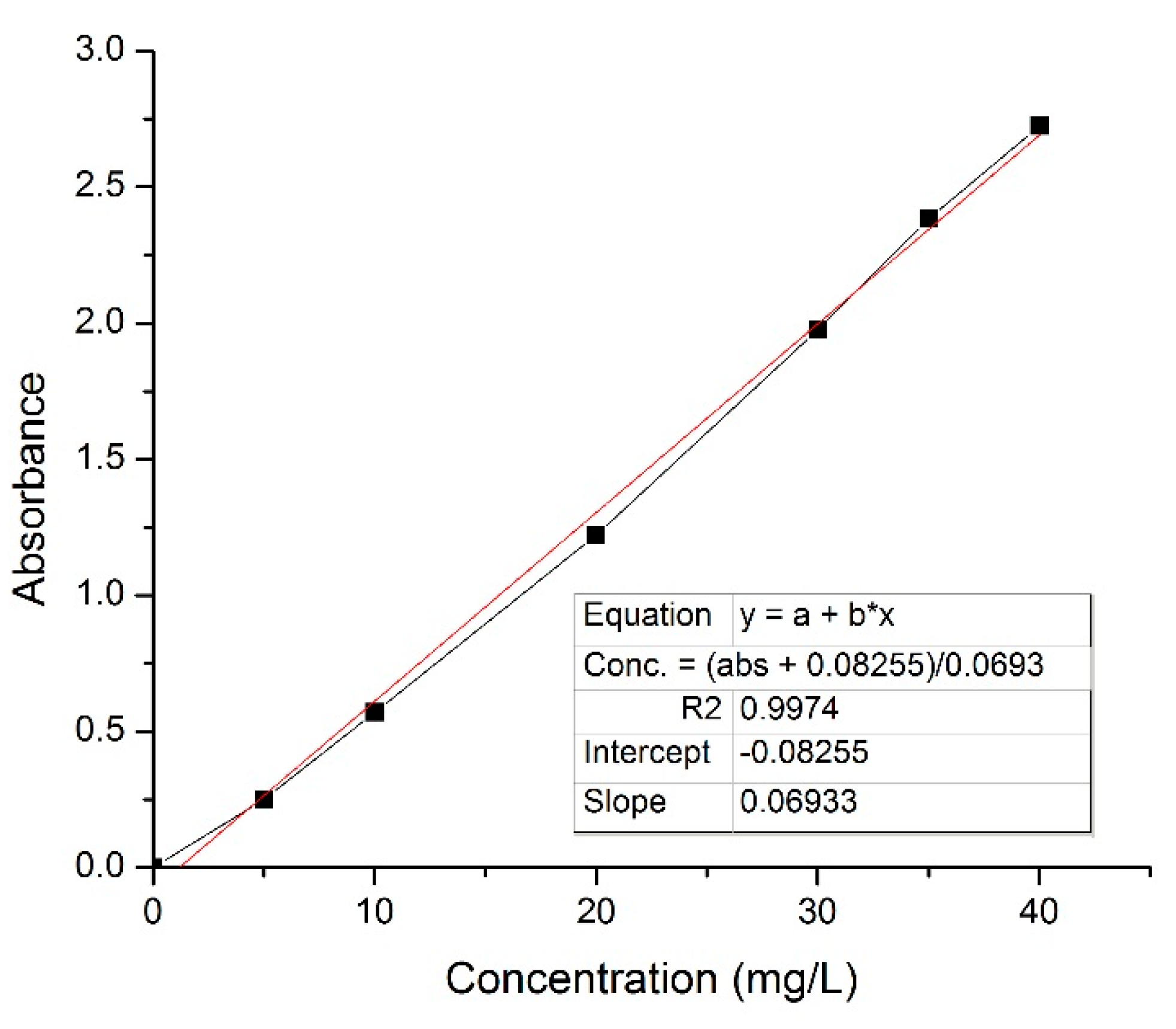
2.3. Analysis Methods
3. Results and Discussions
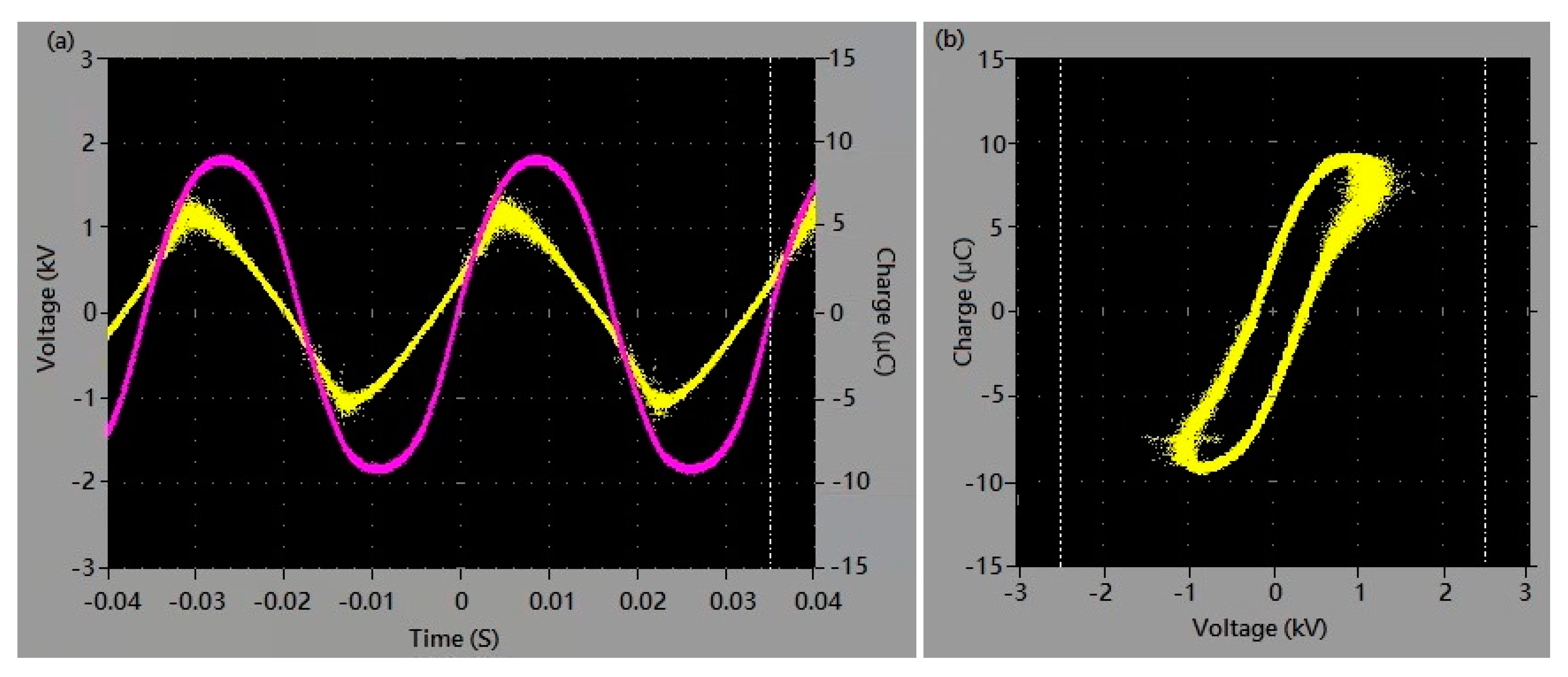
3.1. The Effect of Applied Voltage on the Production of Active Species
3.2. Effect of Varying Oxygen Flow on the Concentration of O3 in Water and Air Stream
3.3. The Effect of Different Carrier Gases on NB Degradation
3.4. The Influence of Oxygen Flow Rate on NB Degradation
3.5. The Effect of Different Inhibitors on NB Degradation
3.6. Effect of Promoters
3.7. Effect of Addition of Methanol on the Degradation of NB
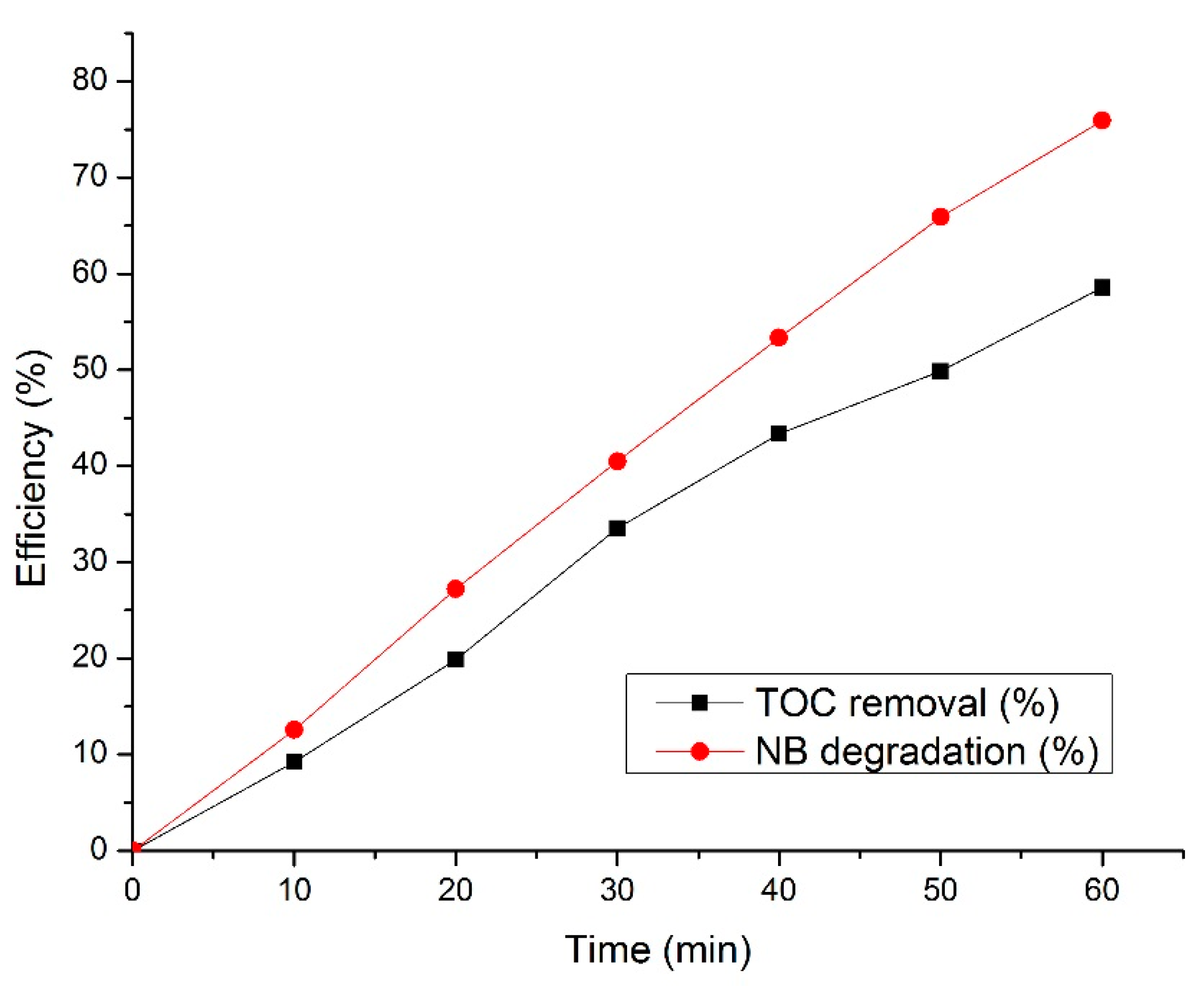
3.8. TOC Removal and NB Degradation Results
3.9. Degradation Kinetics
3.10. Energy Yield
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wen, Q.; Chen, Z.; Lian, J.; Feng, Y.; Ren, N. Removal of nitrobenzene from aqueous solution by a novel lipoid adsorption material (LAM). J. Hazard. Mater. 2012, 209, 226–232. [Google Scholar] [CrossRef]
- Wei, W.; Sun, R.; Cui, J.; Wei, Z. Removal of nitrobenzene from aqueous solution by adsorption on nanocrystalline hydroxyapatite. Desalination 2010, 263, 89–96. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, K.; Dai, C.; Zhou, X.; Si, H. An enhanced Fenton reaction catalyzed by natural heterogeneous pyrite for nitrobenzene degradation in an aqueous solution. Chem. Eng. J. 2014, 244, 438–445. [Google Scholar] [CrossRef]
- ElShafei, G.M.; Yehia, F.Z.; Dimitry, O.I.; Badawi, A.M.; Eshaq, G. Degradation of nitrobenzene at near neutral pH using Fe2+–glutamate complex as a homogeneous Fenton catalyst. Appl. Catal. B Environm. 2010, 99, 242–247. [Google Scholar] [CrossRef]
- Nawaz, M.I.; Yi, C.W.; Ni, L.X.; Zhao, H.; Wang, H.J.; Yi, R.J.; Yin, L.L.; Aleem, M.; Zaman, M. Removal of nitrobenzene from wastewater by vertical flow constructed wetland and optimizing substrate composition using Hydrus-1D: Optimizing substrate composition of vertical flow constructed wetland for removing nitrobenzene from wastewater. Int. J. Environ. Sci. Technol. 2019, 1–10. [Google Scholar] [CrossRef]
- Zhao, L.; Ma, J.; Sun, Z.-Z. Oxidation products and pathway of ceramic honeycomb-catalyzed ozonation for the degradation of nitrobenzene in aqueous solution. Appl. Catal. B Environ. 2008, 79, 244–253. [Google Scholar] [CrossRef]
- Reddy, P.M.K.; Raju, B.R.; Karuppiah, J.; Reddy, E.L.; Subrahmanyam, C. Degradation and mineralization of methylene blue by dielectric barrier discharge non-thermal plasma reactor. Chem. Eng. J. 2013, 217, 41–47. [Google Scholar] [CrossRef]
- Xu, G.-M.; Ma, Y.; Zhang, G.-J. DBD Plasma Jet in Atmospheric Pressure Argon. IEEE Trans. Plasma Sci. 2008, 36, 1352–1353. [Google Scholar]
- Zhang, Q.; Qu, G.; Wang, T.; Li, C.; Qiang, H.; Sun, Q.; Liang, D.; Hu, S. Humic acid removal from micro-polluted source water in the presence of inorganic salts in a gas-phase surface discharge plasma system. Purif. Technol. 2017, 187, 334–342. [Google Scholar] [CrossRef]
- Liu, C.; Wu, J.; Ren, L.; Tong, J.; Li, J.; Cui, N.; Brown, N.; Meenan, B. Comparative study on the effect of RF and DBD plasma treatment on PTFE surface modification. Mater. Chem. Phys. 2004, 85, 340–346. [Google Scholar] [CrossRef]
- Ren, C.-S.; Wang, K.; Nie, Q.-Y.; Wang, D.-Z.; Guo, S.-H. Surface modification of PE film by DBD plasma in air. Appl. Surf. Sci. 2008, 255, 3421–3425. [Google Scholar] [CrossRef]
- Rudolph, R.; Francke, K.-P.; Miessner, H. Concentration Dependence of VOC Decomposition by Dielectric Barrier Discharges. Plasma Chem. Plasma Process. 2002, 22, 401–412. [Google Scholar] [CrossRef]
- Karuppiah, J.; Reddy, E.L.; Reddy, P.M.K.; Ramaraju, B.; Karvembu, R.; Subrahmanyam, C. Abatement of mixture of volatile organic compounds (VOCs) in a catalytic non-thermal plasma reactor. J. Hazard. Mater. 2012, 237, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Kuai, P.-Y.; Liu, C.-J.; Huo, P.-P. Characterization of CuO-ZnO Catalyst Prepared by Decomposition of Carbonates Using Dielectric-Barrier Discharge Plasma. Catal. Lett. 2009, 129, 493–498. [Google Scholar] [CrossRef]
- Malik, M.A. Water Purification by Plasmas: Which Reactors are Most Energy Efficient? Plasma Chem. Plasma Process. 2010, 30, 21–31. [Google Scholar] [CrossRef]
- Zhang, Q.; Xue, Q.; Jiang, B.; Zheng, J.; Qiu, S.; Wu, M.; Yan, Z. Review on electrical discharge plasma technology for wastewater remediation. Chem. Eng. J. 2014, 236, 348–368. [Google Scholar]
- Mok, Y.S.; Jo, J.-O. Degradation of Organic Contaminant by Using Dielectric Barrier Discharge Reactor Immersed in Wastewater. IEEE Trans. Plasma Sci. 2006, 34, 2624–2629. [Google Scholar] [CrossRef]
- Huang, F.; Chen, L.; Wang, H.; Yan, Z. Analysis of the degradation mechanism of methylene blue by atmospheric pressure dielectric barrier discharge plasma. Chem. Eng. J. 2010, 162, 250–256. [Google Scholar] [CrossRef]
- Li, R.; Liu, Y.; Cheng, W.; Zhang, W.; Xue, G.; Ognier, S. Study on remediation of phenanthrene contaminated soil by pulsed dielectric barrier discharge plasma: The role of active species. Chem. Eng. J. 2016, 296, 132–140. [Google Scholar] [CrossRef]
- Bogaerts, A.; Neyts, E.; Gijbels, R.; Van Der Mullen, J. Gas discharge plasmas and their applications. Spectrochim. Acta Part B At. Spectrosc. 2002, 57, 609–658. [Google Scholar] [CrossRef]
- Locke, B.R.; Sato, M.; Sunka, P.; Hoffmann, M.R.; Chang, J.-S. Electrohydraulic Discharge and Nonthermal Plasma for Water Treatment. Ind. Eng. Chem. 2006, 45, 882–905. [Google Scholar] [CrossRef]
- Hong, Z.H.; Chengwu, Y.I.; Rongjie, Y.I.; Huijuan, W.A.; Lanlan, Y.I.; Muhammad, I.N.; Zhongfei, M.A. Research on the degradation mechanism of dimethyl phthalate in drinking water by strong ionization discharge. Plasma Sci. Technol. 2018, 20. [Google Scholar] [CrossRef]
- Li, Y.; Yi, R.; Yi, C.; Zhou, B.; Wang, H. Research on the degradation mechanism of pyridine in drinking water by dielectric barrier discharge. J. Environ. Sci. 2017, 53, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Brandenburg, R. Dielectric barrier discharges: Progress on plasma sources and on the understanding of regimes and single filaments. Plasma Sources Sci. Technol. 2017, 26, 053001. [Google Scholar] [CrossRef]
- Li, S.; Ma, X.; Jiang, Y.; Cao, X. Acetamiprid removal in wastewater by the low-temperature plasma using dielectric barrier discharge. Ecotoxicol. Environ. Saf. 2014, 106, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Liu, X.; Liu, Y.; Shen, X.; Lin, B.; Han, G.; Wu, Z. Adsorption properties of nitrobenzene in wastewater with silica aerogels. Sci. China Technol. Sci. 2010, 53, 2367–2371. [Google Scholar] [CrossRef]
- Xia, K.; Xie, F.; Ma, Y. Degradation of nitrobenzene in aqueous solution by dual-pulse ultrasound enhanced electrochemical process. Ultrason. Sonochemistry 2014, 21, 549–553. [Google Scholar] [CrossRef]
- Tang, S.; Lu, N.; Shang, K.; Li, J.; Wu, Y. Detection of hydroxyl radicals during regeneration of granular activated carbon in dielectric barrier discharge plasma system. J. Phys. Conf. Ser. 2013, 418, 012104. [Google Scholar] [CrossRef]
- Zhu, D.; Jiang, L.; Liu, R.-L.; Chen, P.; Lang, L.; Feng, J.-W.; Yuan, S.-J.; Zhao, D.-Y. Wire–cylinder dielectric barrier discharge induced degradation of aqueous atrazine. Chemosphere 2014, 117, 506–514. [Google Scholar] [CrossRef]
- Chen, X.; Bian, W.; Song, X.; Liu, D.; Zhang, J. Degradation of 4-chlorophenol in a dielectric barrier discharge system. Purif. Technol. 2013, 120, 102–109. [Google Scholar] [CrossRef]
- Hu, Y.; Bai, Y.; Li, X.; Chen, J. Application of dielectric barrier discharge plasma for degradation and pathways of dimethoate in aqueous solution. Purif. Technol. 2013, 120, 191–197. [Google Scholar] [CrossRef]
- Sun, M.J.; Zhang, C.; Yang, C.; Zhang, T. Degradation of Nitrobenzene by Nano-TiO2/PVDF Membrane Catalytic Ozonation; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Bai, J.; Liu, Y.; Yin, X.; Duan, H.; Ma, J. Efficient removal of nitrobenzene by Fenton-like process with Co-Fe layered double hydroxide. Appl. Surf. Sci. 2017, 416, 45–50. [Google Scholar] [CrossRef]
- Weizhou, J.; Youzhi, L.; Wenli, L.; Jing, L.; Fan, S.; Chaoran, W. Degradation of Nitrobenzene-Containing Wastewater with O3 and H2O2 by High Gravity Technology; China Petroleum Processing & Petrochemical Technology: Beijing, China, 2013. [Google Scholar]
- Feng, J.; Zheng, Z.; Sun, Y.; Luan, J.; Wang, Z.; Wang, L.; Feng, J. Degradation of diuron in aqueous solution by dielectric barrier discharge. J. Hazard. Mater. 2008, 154, 1081–1089. [Google Scholar] [CrossRef]
- Duan, H.; Liu, Y.; Yin, X.; Bai, J.; Qi, J. Degradation of nitrobenzene by Fenton-like reaction in a H2O2/schwertmannite system. Chem. Eng. J. 2016, 283, 873–879. [Google Scholar] [CrossRef]
- Iervolino, G.; Vaiano, V.; Palma, V. Enhanced Removal of Water Pollutants by Dielectric Barrier Discharge Non-Thermal Plasma Reactor; Separation and Purification Technology: Amsterdam, The Netherlands, 2019; pp. 155–162. [Google Scholar]
- Shen, J.; Chen, Z.; Li, X.; Qi, F.; Ye, M. The efficiency and mechanism of the degradation of nitrobenzene in aqueous solution by O3/H2O2. Water Sci. Technol. 2006, 6, 153–162. [Google Scholar] [CrossRef]
- Yin, W.; Wu, J.; Li, P.; Wang, X.; Zhu, N.; Wu, P.; Yang, B. Experimental study of zero-valent iron induced nitrobenzene reduction in groundwater: The effects of pH, iron dosage, oxygen and common dissolved anions. Chem. Eng. J. 2012, 184, 198–204. [Google Scholar] [CrossRef]
- Dong, Y.; He, K.; Yin, L.; Zhang, A. Catalytic Degradation of Nitrobenzene and Aniline in Presence of Ozone by Magnesia from Natural Mineral. Catal. Lett. 2007, 119, 222–227. [Google Scholar] [CrossRef]
- Zaleska, A.; Hupka, J.; Wiergowski, M.; Biziuk, M. Photocatalytic degradation of lindane, p,p′-DDT and methoxychlor in an aqueous environment. J. Photochem. Photobiol. A Chem. 2000, 135, 213–220. [Google Scholar] [CrossRef]
- Kuşvuran, E.; Samil, A.; Atanur, O.M.; Erbatur, O. Photocatalytic degradation kinetics of di- and tri-substituted phenolic compounds in aqueous solution by TiO2/UV. Appl. Catal. B Environ. 2005, 58, 211–216. [Google Scholar] [CrossRef]
- Farré, M.J.; Franch, M.I.; Malato, S.; Ayllón, J.A.; Peral, J.; Domènech, X. Degradation of some biorecalcitrant pesticides by homogeneous and heterogeneous photocatalytic ozonation. Chemosphere 2005, 58, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Magureanu, M.; Mandache, N.B.; Parvulescu, V.I. Degradation of pharmaceutical compounds in water by non-thermal plasma treatment. Water Res. 2015, 81, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, K.; Shu, C.; Lou, Z.; Sun, T.; Jia, J. Enhancement of styrene removal using a novel double-tube dielectric barrier discharge (DDBD) reactor. Chem. Eng. J. 2014, 256, 107–118. [Google Scholar] [CrossRef]
- Kostov, K.; Honda, R.Y.; Alves, L.; Kayama, M. Characteristics of dielectric barrier discharge reactor for material treatment. Braz. J. Phys. 2009, 39, 322–325. [Google Scholar] [CrossRef]
- Karuppiah, J.; Karvembu, R.; Subrahmanyam, C. The catalytic effect of MnOx and CoOx on the decomposition of nitrobenzene in a non-thermal plasma reactor. Chem. Eng. J. 2012, 180, 39–45. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, X.; Liu, Y. Efficient Degradation of Nitrobenzene Induced by Glow Discharge Plasma in Aqueous Solution. Plasma Chem. Plasma Process. 2007, 27, 504–515. [Google Scholar] [CrossRef]
- Appleton, A.T.; Lukes, P.; Finney, W.C.; Locke, B.R. A Study of the Effectiveness of Different Hybrid Pulsed Corona reactors in Degrading Aqueous Pollutants. Ph.D. Thesis, Florida State University, Tallahassee, FL, USA, 2002. [Google Scholar]
- Vanraes, P.; Willems, G.; Nikiforov, A.; Surmont, P.; Lynen, F.; Vandamme, J.; Van Durme, J.; Verheust, Y.P.; Van Hulle, S.W.; Dumoulin, A.; et al. Removal of atrazine in water by combination of activated carbon and dielectric barrier discharge. J. Hazard. Mater. 2015, 299, 647–655. [Google Scholar] [CrossRef]
- Achilleos, A.; Hapeshi, E.; Xekoukoulotakis, N.P.; Mantzavinos, D.; Fatta-Kassinos, D. Factors affecting diclofenac decomposition in water by UV-A/TiO2 photocatalysis. Chem. Eng. J. 2010, 161, 53–59. [Google Scholar] [CrossRef]
- Panorel, I.; Preis, S.; Kornev, I.; Hatakka, H.; Louhi-Kultanen, M. Oxidation of aqueous pharmaceuticals by pulsed corona discharge. Environ. Technol. 2013, 34, 923–930. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, X. Unusual catalytic effects of iron salts on phenol degradation by glow discharge plasma in aqueous solution. J. Hazard. Mater. 2009, 161, 926–932. [Google Scholar] [CrossRef]
- Grymonpré, D.R.; Finney, W.C.; Locke, B.R. Aqueous-phase pulsed streamer corona reactor using suspended activated carbon particles for phenol oxidation: Model-data comparison. Chem. Eng. Sci. 1999, 54, 3095–3105. [Google Scholar] [CrossRef]
- Du, C.M.; Yan, J.H.; Cheron, B.G. Degradation of 4-Chlorophenol using a Gas–Liquid Gliding Arc Discharge Plasma Reactor. Plasma Chem. Plasma Process. 2007, 27, 635–646. [Google Scholar] [CrossRef]







| Parameters | Values |
|---|---|
| Oxygen Concentration | 3 L/min |
| Gas pressure | ≥0.1 MPa |
| Water circulation flow rate | 1.0 m3/h |
| Voltage range | 1.0–1.8 kV |
| Average electron energy | >0 eV |
| Sample barrel volume | 40 L |
| Water temperature | 20 °C |
| Treatment System | Type of Water | Initial Conc. (mg/L) | Treatment Time | Degradation (%) | K (min−1) | R2 | References |
|---|---|---|---|---|---|---|---|
| DBD reactor | Nitrobenzene | 20 | 60 min | 75 | 0.0237 | 0.9983 | current work |
| ZVI reduction | Nitrobenzene | 25 | 8 h | 56 | 0.0165 | 0.1038 | [40] |
| Pyrite Fenton system | Nitrobenzene | 20 | 5 h | 77 | 0.0113 | 0.9644 | [3] |
| Catalytic ozonation | Nitrobenzene | 100 | 120 min | 55 | 0.0065 | - | [41] |
| UV/TiO2/O2 photocatalysis | Lindane | 40 | 90 min | 55 | 0.60 | - | [42] |
| TiO2/UV photocatalysis | 2,4-dibromophenol | 25–126 | 30 min | 53–14 | 0.025–0.0056 | - | [43] |
| Photo-Fenton/O3 | Atrazine | 35 | 5 min | 66 | 0.24 | - | [44] |
| Treatment System | Type of Water | Initial Conc. (mg/L) | Treatment Time (min) | Power (W) | Energy Yield (mg/kWh) | Degradation (%) | References |
|---|---|---|---|---|---|---|---|
| DBD reactor | Nitrobenzene | 20 | 60 | 0.538 | 1.25 | 75 | current work |
| Glow discharge plasma | Nitrobenzene | 50 | 60 | - | 0.1512 | 50 | [49] |
| PCD Reference reactor | Nitrobenzene | 10 | 60 | 66 | 0.067 | 35 | [50] |
| PCD Hybrid-series reactor | Nitrobenzene | 10 | 60 | 66 | 0.030 | 65 | [50] |
| Pyrite Fenton system | Nitrobenzene | 20 | 300 | - | - | 77 | [3] |
| Classic Fenton system | Nitrobenzene | 20 | 300 | - | - | 30 | [3] |
| DBD reactor | Atrazine | 0.03 | 20 | 27 | 2.25 | 50 | [51] |
| TiO2 photocatalysis | diclofenac | 10 | 240 | 9 | 82.6 | 85 | [52] |
| Corona with liquid shower | b-Oestradiol | 3 | 30 | 120 | 1400 | 70 | [53] |
| Contact glow discharge reactor/point-plate | phenol | 100 | 30 | 44 | 340 | 61 | [54] |
| Pulsed liquid discharge | phenol | 100 | 15 | 55 | 570 | 40 | [55] |
| Gas/liquid gliding arc discharge | 4-Chlorophenol | 40 | 76 | 230 | 1020 | 89 | [56] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nawaz, M.I.; Yi, C.; Asilevi, P.J.; Geng, T.; Aleem, M.; Zafar, A.M.; Azeem, A.; Wang, H. A Study of the Performance of Dielectric Barrier Discharge under Different Conditions for Nitrobenzene Degradation. Water 2019, 11, 842. https://doi.org/10.3390/w11040842
Nawaz MI, Yi C, Asilevi PJ, Geng T, Aleem M, Zafar AM, Azeem A, Wang H. A Study of the Performance of Dielectric Barrier Discharge under Different Conditions for Nitrobenzene Degradation. Water. 2019; 11(4):842. https://doi.org/10.3390/w11040842
Chicago/Turabian StyleNawaz, Muhammad Imran, Chengwu Yi, Prince Junior Asilevi, Tingting Geng, Muhammad Aleem, Abdul Mannan Zafar, Ahmad Azeem, and Huijuan Wang. 2019. "A Study of the Performance of Dielectric Barrier Discharge under Different Conditions for Nitrobenzene Degradation" Water 11, no. 4: 842. https://doi.org/10.3390/w11040842
APA StyleNawaz, M. I., Yi, C., Asilevi, P. J., Geng, T., Aleem, M., Zafar, A. M., Azeem, A., & Wang, H. (2019). A Study of the Performance of Dielectric Barrier Discharge under Different Conditions for Nitrobenzene Degradation. Water, 11(4), 842. https://doi.org/10.3390/w11040842





