Reproductive Cycle of the Edible Sea Urchin Paracentrotus lividus (Echinodermata: Echinoidae) in the Aegean Sea
Abstract
1. Introduction
2. Materials and Methods
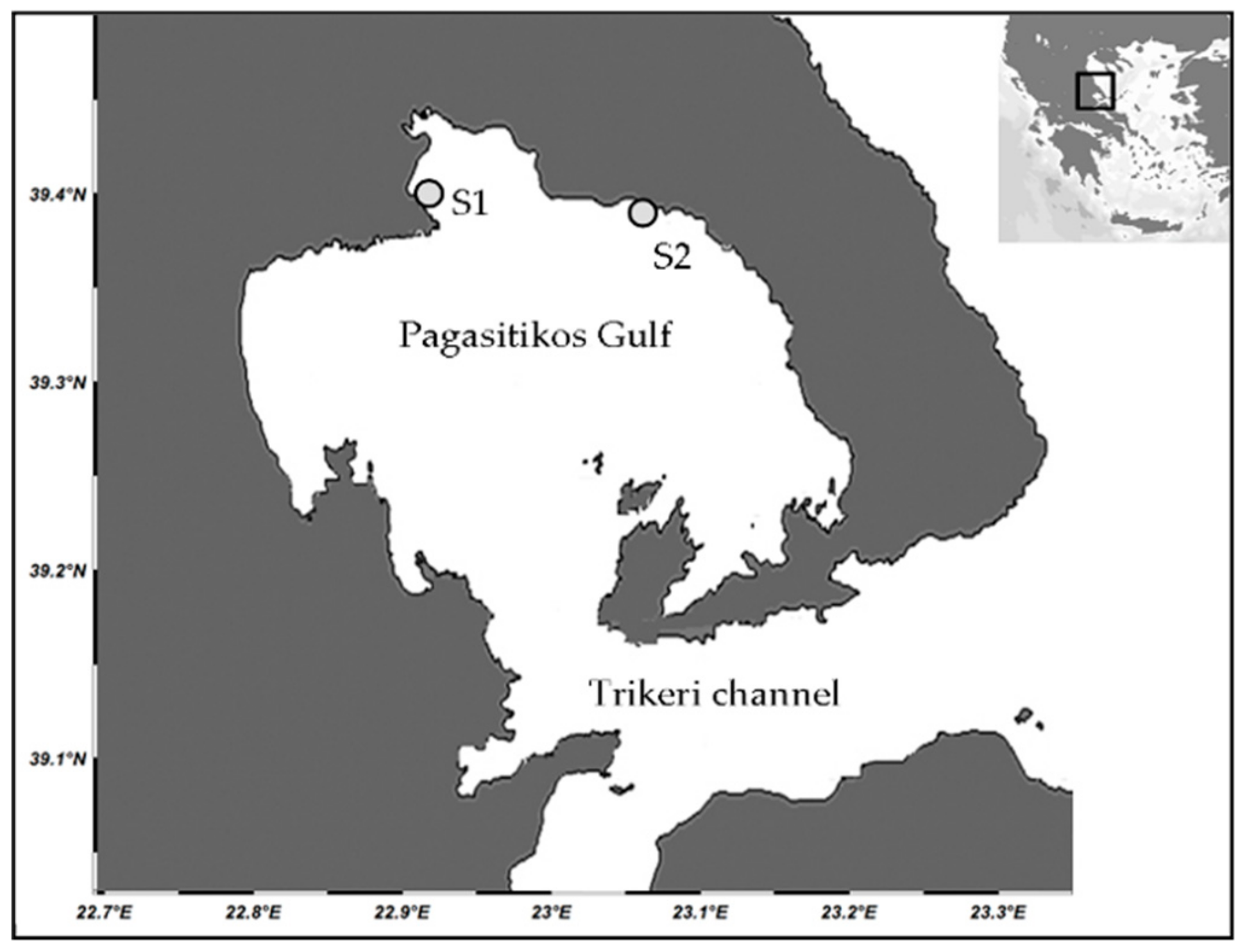
2.1. Study Area
2.2. Field Sampling
2.3. Sample Processing
2.4. Histology
2.5. Statistical Analyses
3. Results
3.1. General Biometry and Sex Ratio
3.2. Gonad-Somatic Index
3.3. Histology of Gonads
3.4. Female Fecundity
3.5. Oocyte Size
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Antoniadou, C.; Vafidis, D. Population structure and morphometric relationships of Paracentrotus lividus (Echinodermata: Echinoidae) in the south Aegean Sea. Cah. Biol. Mar. 2009, 50, 293–301. [Google Scholar]
- Andrew, N.L.; Agatsuma, Y.; Ballesteros, E.; Bazhin, A.G.; Greaser, E.P.; Barnes, D.K.A.; Botsford, L.W.; Bradbury, A.; Campbell, A.; Dixon, J.D.; et al. Status and management of the world sea urchin fisheries. Oceanogr. Mar. Biol. 2002, 40, 343–425. [Google Scholar] [CrossRef]
- Guidetti, P.; Terlizzi, A.; Boero, F. Effects of the edible sea urchin Paracentrotus lividus fishery along the Apulian rocky coast (SE Italy, Mediterranean Sea). Fish. Res. 2004, 66, 287–297. [Google Scholar] [CrossRef]
- Pais, A.; Serra, S.; Meloni, G.; Saba, S.; Ceccherelli, G. Harvesting effects on Paracentrotus lividus population structure: A case study from northwestern Sardinia, Italy, before and after the fishing season. J. Coast. Res. 2012, 28, 570–575. [Google Scholar] [CrossRef]
- Bertocci, I.; Domínguez, R.; Machado, I.; Freitas, C.; Domínguez Godino, J.; Sousa-Pinto, I.; Gonçalves, M.; Gaspar, M. Multiple effects of harvesting on populations of the purple sea urchin Paracentrotus lividus in north Portugal. Fish. Res. 2014, 150, 60–65. [Google Scholar] [CrossRef]
- Ourens, R.; Fermamdez, L.; Freire, J. Geographic, population, and seasonal patterns in the reproductive parameters of the sea urchin Paracentrotus lividus. Mar. Biol. 2011, 158, 793–804. [Google Scholar] [CrossRef]
- Perry, I.R.; Walters, C.J.; Boutillier, J.A. A framework for providing scientific advice for the management of new and developing invertebrate fisheries. Rev. Fish Biol. Fish. 1999, 9, 125–150. [Google Scholar] [CrossRef]
- De la Uz, S.; Carrasco, F.; Rodriguez, C. Temporal variability of spawning in the sea urchin Paracentrotus lividus from northern Spain. Reg. Stud. Mar. Sci. 2018, 23, 2–7. [Google Scholar] [CrossRef]
- González-Irusta, J.M.; Goni de Cerio, F.; Canteras, J.C. Reproductive cycle of the sea urchin Paracentrotus lividus in the Cantabrian Sea (northern Spain): Environmental effects. J. Mar. Biol. Assoc. UK 2009, 90, 699–709. [Google Scholar] [CrossRef]
- Yeruham, E.; Rilov, G.; Shpigel, M.; Abelson, A. Collapse of the echinoid Paracentrotus lividus populations in the eastern Mediterranean—Result of climate change? Sci. Rep. 2015, 5, 13479. [Google Scholar]
- Konstantinidis, I.; Gkafas, G.A.; Karamitros, G.; Lolas, A.; Antoniadou, C.; Vafidis, D.; Exadactylos, A. Population structure of two benthic species with different larval stages in the eastern Mediterranean Sea. J. Environ. Prot. Ecol. 2017, 18, 930–939. [Google Scholar]
- Sánchez-España, A.I.; Martinez-Pita, I.; Garcia, F.J. Gonadal growth and reproduction in the commercial sea urchin Paracentrotus lividus (Lamarck, 1816) (Echinodermata: Echinoidea) from southern Spain. Hydrobiologia 2004, 519, 61–72. [Google Scholar] [CrossRef]
- Spirlet, C.; Grosjean, P.; Jangoux, M. Reproductive cycle of the echinoid Paracentrotus lividus: An analysis by means of the maturity index. Invertebr. Reprod. Dev. 1998, 34, 69–81. [Google Scholar] [CrossRef]
- Tenuzzo, B.A.; Zaccarelli, N.; Dini, L. The reproductive cycle of the commercial sea urchin Paracentrotus lividus (Lamarck, 1816) (Echinodermata: Echinoidea) in the Ionian Sea. Ital. J. Zool. 2012, 79, 200–211. [Google Scholar] [CrossRef]
- Sellem, F.; Guillou, M. Reproductive biology of Paracentrotus lividus (Echinodermata: Echinoidea) in two contrasting habitats of northern Tunisia (south-east Mediterranean). J. Mar. Biol. Assoc. UK 2007, 87, 763–767. [Google Scholar] [CrossRef]
- Byrne, M. Annual reproductive cycles of the commercial sea urchin Paracentrotus lividus from an exposed intertidal and a sheltered subtidal habitat on the west coast of Ireland. Mar. Biol. 1990, 104, 275–289. [Google Scholar]
- Petihakis, G.; Triantafyllou, G.; Pollani, A.; Koliou, A.; Theodorou, A. Field data analysis and application of a complex water column biogeochemical model in different areas of a semi-enclosed basin: Towards the development of an ecosystem management tool. Mar. Environ. Res. 2005, 59, 493–518. [Google Scholar] [CrossRef]
- Korres, G.; Triantafyllou, G.; Petihakis, G.; Raitsos, D.E.; Hoteit, I.; Pollani, A.; Colella, S.; Tsiaras, K. A data assimilation tool for the Pagasitikos Gulf ecosystem dynamics: Methods and benefits. J. Mar. Syst. 2012, 94, 102–117. [Google Scholar] [CrossRef]
- Garrido, M.J.; Haroun, R.J.; Lessios, H.A. Annual reproductive periodicity of the sea urchin Diadema antillarum Philippi in the Canary Islands. Bull. Mar. Sci. 2000, 67, 989–996. [Google Scholar]
- Ramirez-Llodra, E. Fecundity and life history strategies in marine invertebrates. Adv. Mar. Biol. 2002, 43, 87–170. [Google Scholar] [CrossRef]
- Underwood, A.J. Experiments in Ecology. Their Logical Design and Interpretation Using Analysis of Variance, 2nd ed.; Cambridge University Press: Cambridge, UK, 1997; 524p. [Google Scholar]
- Lozano, J.; Galera, J.; López, S.; Turon, X.; Palacín, C.; Morera, G. Biological cycles and recruitment of Paracentrotus lividus (Echinodermata: Echinoidea) in two contrasting habitats. Mar. Ecol. Prog. Ser. 1995, 122, 179–191. [Google Scholar]
- Turon, X.; Giribet, G.; Lopez, S.; Palacin, C. Growth and population structure of Paracentrotus lividus (Echinodermata: Echinoidae) in two contrasting habitats. Mar. Ecol. Prog. Ser. 1995, 122, 193–204. [Google Scholar]
- Ouréns, R.; Fernandez, L.; Fernandez-Boan, M.; Naya, I.; Freire, J. Reproductive dynamics of the sea urchin Paracentrotus lividus on the Galicia coast (NW Spain): Effects of habitat and population density. Mar. Biol. 2013, 160, 2413–2423. [Google Scholar] [CrossRef]
- Guettaf, M.; San Martin, G.A.; Francour, P. Interpopulation variability of the reproductive cycle of Paracentrotus lividus (Echinodermata: Echinoidea) in the south-western Mediterranean. J. Mar. Biol. Assoc. UK 2000, 80, 899–907. [Google Scholar] [CrossRef]
- Murillo-Navarro, R.; Jiménez-Guirado, D. Relationships between algal food and gut and gonad conditions in the Mediterranean sea urchin Paracentrotus lividus (Lam.). Mediterr. Mar. Sci. 2012, 13, 227–238. [Google Scholar] [CrossRef][Green Version]
- Gianguzza, P.; Bonaviri, C.; Prato, E.; Fanelli, G.; Chiantore, M.; Privitera, D.; Luzzu, F.; Agnetta, D. Hydrodynamism and its influence on the reproductive condition of the edible sea urchin Paracentrotus lividus. Mar. Environ. Res. 2013, 85, 29–33. [Google Scholar] [CrossRef]
- Gago, J.; Range, F.; Luis, O. Growth, reproductive biology and habitat selection of the sea urchin Paracentrotus lividus in the coastal waters of Cascais, Portugal. In Echinoderm Research, 1st ed.; Feral, J.P., David, B., Eds.; Balkema: Lisse, The Netherlands, 2003; pp. 269–276. [Google Scholar]







| Sampling Month | S1 | S2 | ||||
|---|---|---|---|---|---|---|
| Females | Males | Sum | Females | Males | Sum | |
| December 2008 | 22 | 13 | 35 | 16 | 19 | 35 |
| January 2009 | 11 | 24 | 35 | 12 | 23 | 35 |
| February 2009 | 18 | 17 | 35 | 20 | 15 | 35 |
| March 2009 | 22 | 13 | 35 | 19 | 16 | 35 |
| April 2009 | 23 | 12 | 35 | 18 | 17 | 35 |
| May 2009 | 19 | 16 | 35 | 10 | 25 | 35 |
| June 2009 | 17 | 18 | 35 | 25 | 10 | 35 |
| July 2009 | 12 | 23 | 35 | 9 | 26 | 35 |
| August 2009 | 11 | 24 | 35 | 11 | 24 | 35 |
| September 2009 | 25 | 10 | 35 | 23 | 12 | 35 |
| October 2009 | 24 | 11 | 35 | 26 | 9 | 35 |
| November 2009 | 17 | 18 | 35 | 16 | 19 | 35 |
| December 2009 | 19 | 16 | 35 | 28 | 7 | 35 |
| January 2010 | 12 | 23 | 35 | 19 | 16 | 35 |
| February 2010 | 17 | 18 | 35 | 21 | 14 | 35 |
| March 2010 | 16 | 19 | 35 | 15 | 20 | 35 |
| April 2010 | 20 | 15 | 35 | 20 | 15 | 35 |
| May 2010 | 23 | 12 | 35 | 25 | 10 | 35 |
| June 2010 | 15 | 20 | 35 | 17 | 18 | 35 |
| July 2010 | 26 | 9 | 35 | 22 | 13 | 35 |
| August 2010 | 20 | 15 | 35 | 24 | 11 | 35 |
| September 2010 | 23 | 12 | 35 | 23 | 12 | 35 |
| October 2010 | 22 | 13 | 35 | 20 | 15 | 35 |
| November 2010 | 14 | 21 | 35 | 23 | 12 | 35 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vafidis, D.; Antoniadou, C.; Kyriakouli, K. Reproductive Cycle of the Edible Sea Urchin Paracentrotus lividus (Echinodermata: Echinoidae) in the Aegean Sea. Water 2019, 11, 1029. https://doi.org/10.3390/w11051029
Vafidis D, Antoniadou C, Kyriakouli K. Reproductive Cycle of the Edible Sea Urchin Paracentrotus lividus (Echinodermata: Echinoidae) in the Aegean Sea. Water. 2019; 11(5):1029. https://doi.org/10.3390/w11051029
Chicago/Turabian StyleVafidis, Dimitris, Chryssanthi Antoniadou, and Kyratso Kyriakouli. 2019. "Reproductive Cycle of the Edible Sea Urchin Paracentrotus lividus (Echinodermata: Echinoidae) in the Aegean Sea" Water 11, no. 5: 1029. https://doi.org/10.3390/w11051029
APA StyleVafidis, D., Antoniadou, C., & Kyriakouli, K. (2019). Reproductive Cycle of the Edible Sea Urchin Paracentrotus lividus (Echinodermata: Echinoidae) in the Aegean Sea. Water, 11(5), 1029. https://doi.org/10.3390/w11051029






