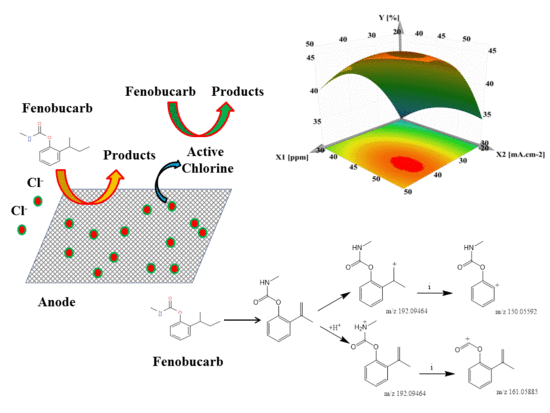
Response Surface Analysis of Fenobucarb Removal by Electrochemically Generated Chlorine
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
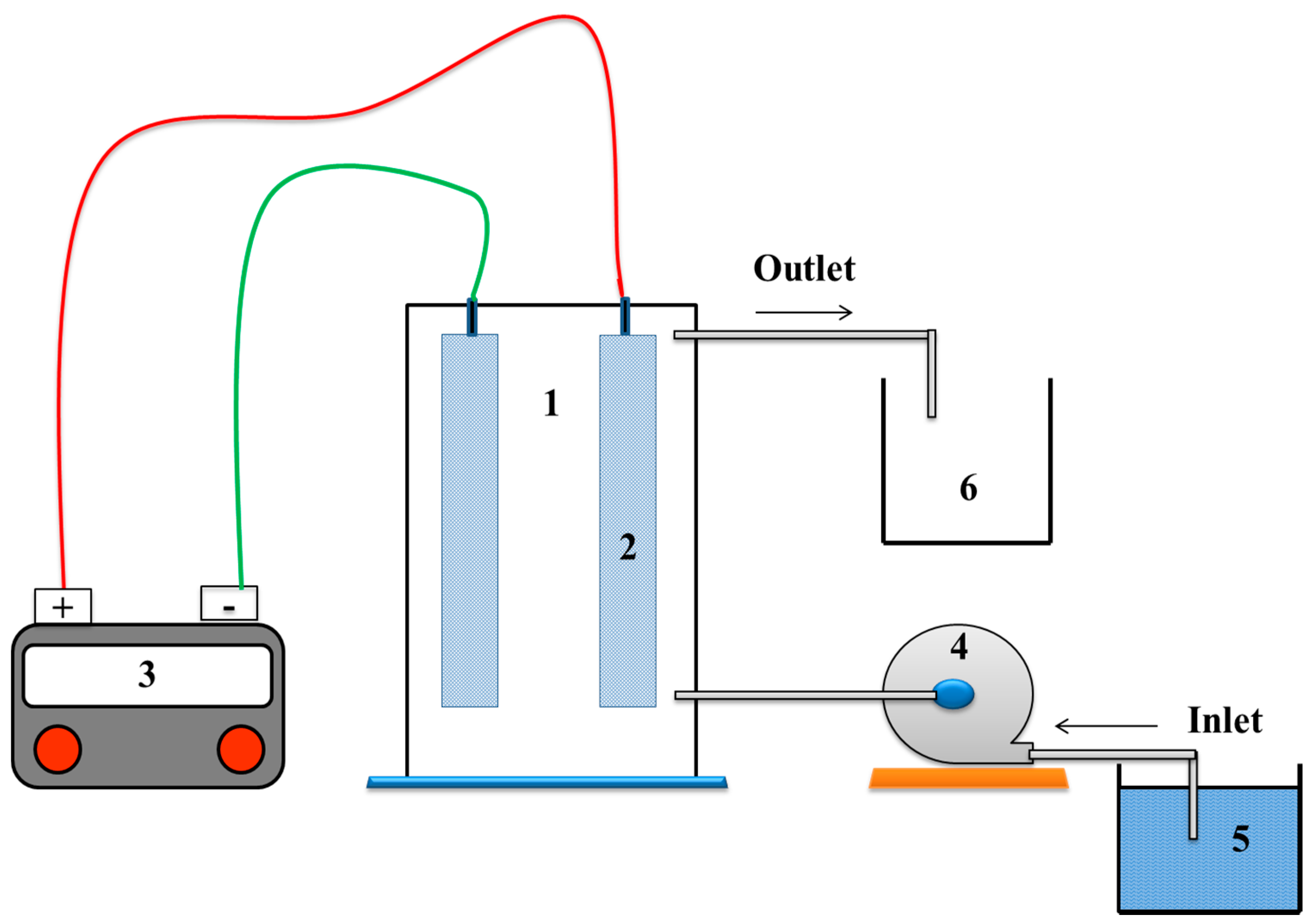
2.2. Electrochemical Equipment and Procedure
2.3. Analysis of the Sample Solutions
2.4. Response Surface Methodology (RSM)
3. Results and Discussion
3.1. The Formation of AC and the Mass Balance during the NaCl Electrolysis Process
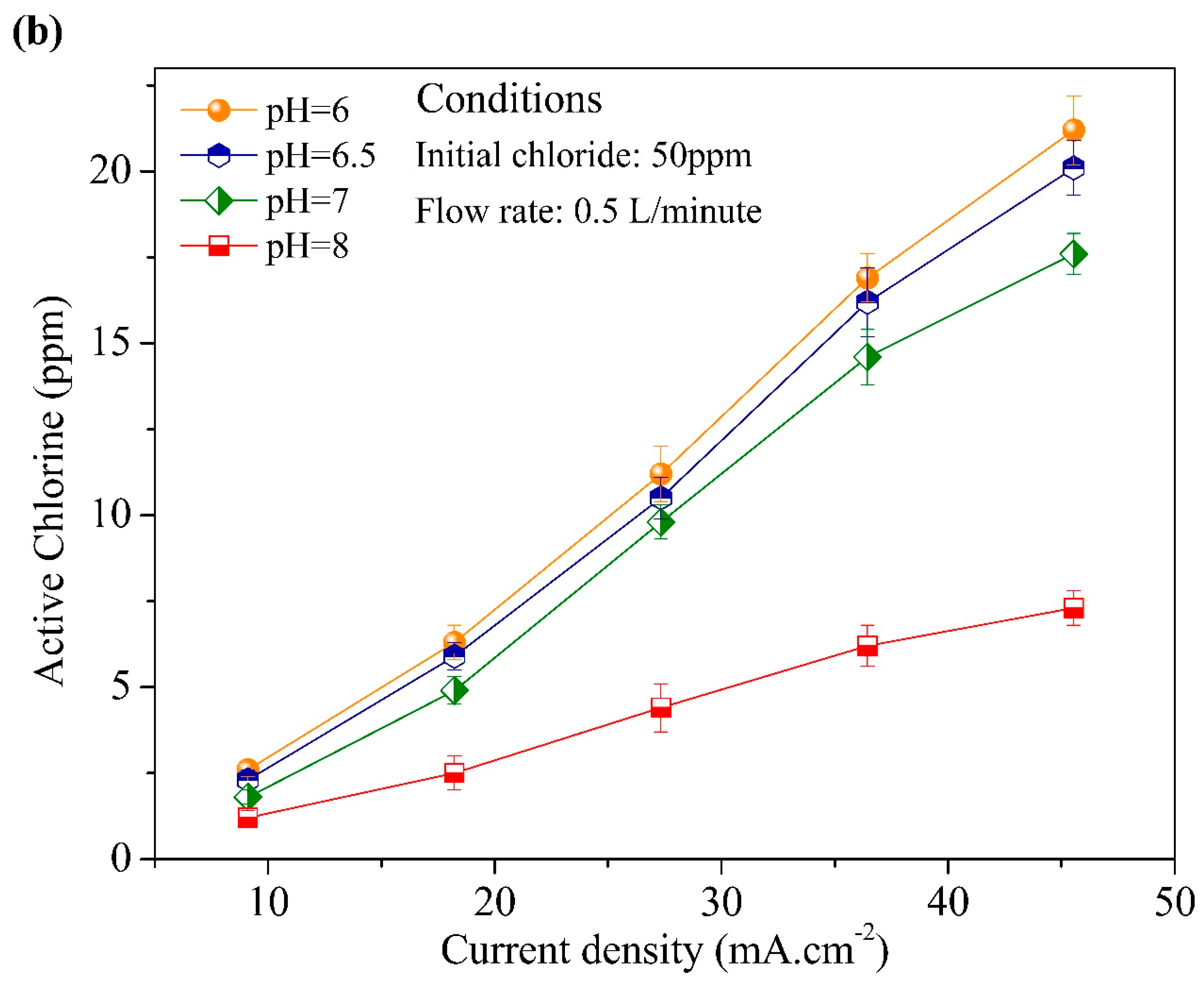
3.2. Effect of Initial Chloride Concentration, pH, Flow Rate, and Current Density
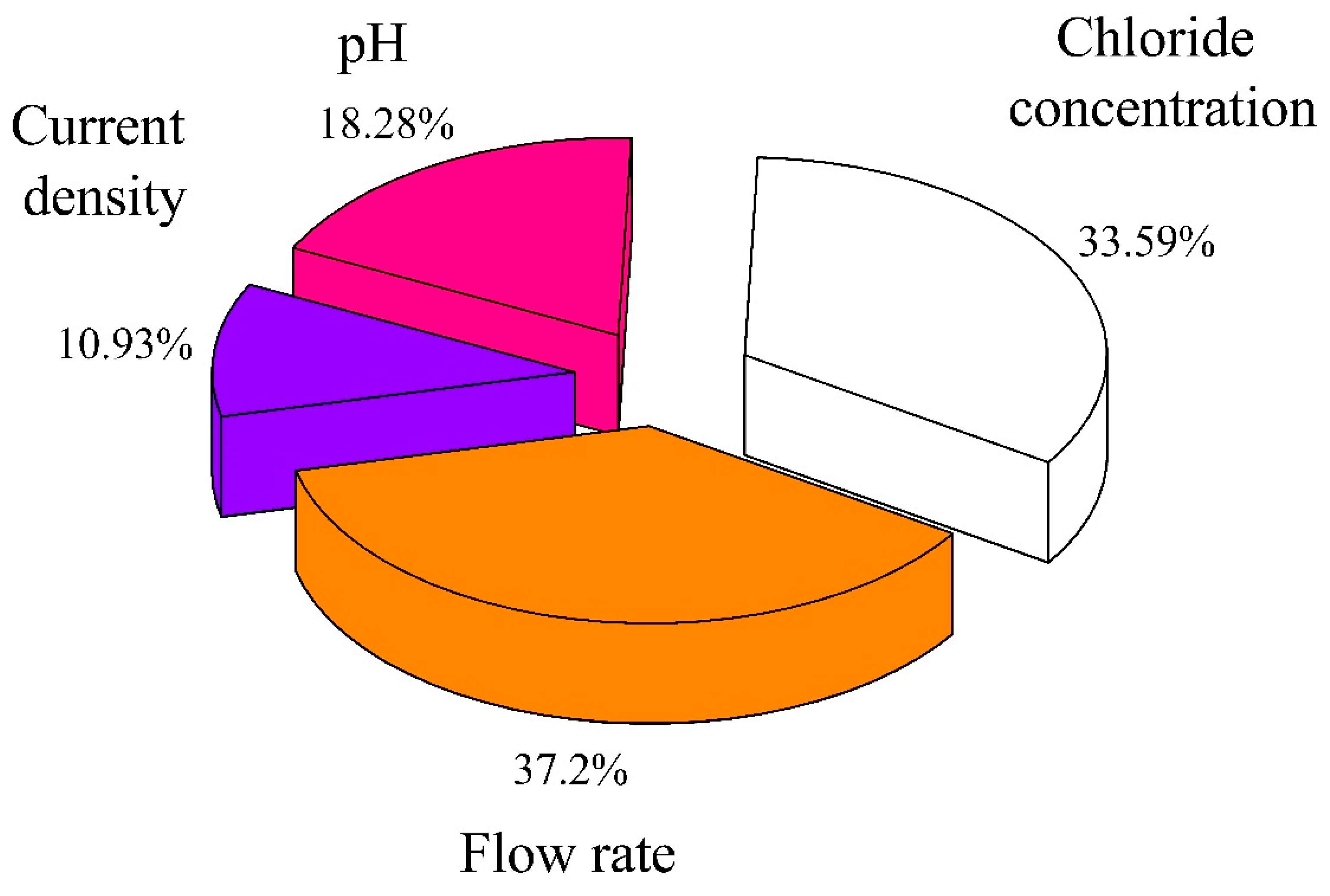
3.3. Main Influence on the Formation of Active Chlorine during the Chloride Electrolysis Process
3.4. Application to Removal of Fenobucarb from Surface Water
3.5. By-Products of Fenobucarb’s Degradation
4. Conclusions
- Factors influencing active chlorine formation have been shown, with four main factors: Chloride content, electric current, flow rate (retention time of Cl- ion), and initial pH.
- The contribution percentage of the four main factors were indicated by RSM, in which the factors were arranged in the direction of diminishing influence: Flow rate > chloride concentration > pH > current density.
- RSM was used to assess the interaction of four independent variables in the process of eliminating fenobucarb. The results showed that the ability to remove fenobucarb was greatest under the experimental conditions of chloride concentration 40.05 mg/L; current density 54.65 mA·cm−2; flow rate 0.177 L/min (retention time 94.4 s); and fenobucarb 1.0 mg/L.
- Seven metabolites were detected by LC–MS/MS in combination with Compound Discoverer 2.1. The decomposition of fenobucarb occurs via four pathways: Hydrolysis of fenobucarb, oxidation by Cl●, ●OH free radicals, and the direct oxidation of fenobucarb at the electrode surface.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vasudevan, S.; Oturan, M.A. Electrochemistry: As cause and cure in water pollution—An overview. Environ. Chem. Lett. 2013, 12, 97–108. [Google Scholar] [CrossRef]
- Brillas, E.; Martínez-Huitle, C.A. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods. An updated review. Appl. Catal. B Environ. 2015, 166–167, 603–643. [Google Scholar] [CrossRef]
- Bergmann, M.E.H.; Koparal, A.S.; Iourtchouk, T. Electrochemical Advanced Oxidation Processes, Formation of Halogenate and Perhalogenate Species: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 348–390. [Google Scholar] [CrossRef]
- Vos, J.G.; Koper, M.T.M. Measurement of competition between oxygen evolution and chlorine evolution using rotating ring-disk electrode voltammetry. J. Electroanal. Chem. 2018, 819, 260–268. [Google Scholar] [CrossRef]
- Pinto, C.F.; Antonelli, R.; de Araujo, K.S.; Fornazari, A.L.T.; Fernandes, D.M.; Granato, A.C.; Azevedo, E.B.; Malpass, G.R.P. Experimental-design-guided approach for the removal of atrazine by sono-electrochemical-UV-chlorine techniques. Environ. Technol. 2017, 40, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters. Appl. Catal. B Environ. 2017, 202, 217–261. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, C.; Yoon, J. The effect of electrode material on the generation of oxidants and microbial inactivation in the electrochemical disinfection processes. Water Res. 2009, 43, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Ming, R.; Zhu, Y.; Deng, L.; Zhang, A.; Wang, J.; Han, Y.; Chai, B.; Ren, Z. Effect of electrode material and electrolysis process on the preparation of electrolyzed oxidizing water. New J. Chem. 2018, 42, 12143–12151. [Google Scholar] [CrossRef]
- Song, X.; Zhao, H.; Fang, K.; Lou, Y.; Liu, Z.; Liu, C.; Ren, Z.; Zhou, X.; Fang, H.; Zhu, Y. Effect of platinum electrode materials and electrolysis processes on the preparation of acidic electrolyzed oxidizing water and slightly acidic electrolyzed water. RSC Adv. 2019, 9, 3113–3119. [Google Scholar] [CrossRef]
- Canizares, P.; Martınez, F.; Dıaz, M.; Garcıa-Gómez, J.; Rodrigo, M.A. Electrochemical oxidation of aqueous phenol wastes using active and nonactive electrodes. Electrochem. Soc. 2002, 149, 118–124. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, H.; Li, Y.; Zhang, H.; Zhang, G.; Lu, Z.; Sun, X.; Jiang, L. Superaerophobic RuO2 -Based Nanostructured Electrode for High-Performance Chlorine Evolution Reaction. Small 2017, 13, 1602240. [Google Scholar] [CrossRef]
- Patel, P.S.; Bandre, N.; Saraf, A.; Ruparelia, J.P. Electro-catalytic Materials (Electrode Materials) in Electrochemical Wastewater Treatment. Procedia Eng. 2013, 51, 430–435. [Google Scholar] [CrossRef]
- Luu, T.L.; Kim, C.; Kim, S.; Kim, J.; Yoon, J. Fabricating macroporous RuO2-TiO2 electrodes using polystyrene templates for high chlorine evolution efficiencies. Desalin. Water Treat. 2017, 77, 94–104. [Google Scholar] [CrossRef]
- Urbansky, E.T.; Schock, M.R. Issues in managing the risks associated with perchlorate in drinking water. J. Environ. Manag. 1999, 56, 79–95. [Google Scholar] [CrossRef]
- Srinivasan, R.; Sorial, G.A. Treatment of perchlorate in drinking water: A critical review. Sep. Purif. Technol. 2009, 69, 7–21. [Google Scholar] [CrossRef]
- Siddiqui, M.S. Chlorine-ozone interactions: Formation of chlorate. Water Res. 1996, 30, 2160–2170. [Google Scholar] [CrossRef]
- Richardson, S.D.; Plewa, M.J.; Wagner, E.D.; Schoeny, R.; DeMarini, D.M. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection byproducts in drinking water: A review and roadmap for research. Mutat. Res. 2007, 636, 178–242. [Google Scholar] [CrossRef]
- Yoon, Y.; Cho, E.; Jung, Y.; Kwon, M.; Yoon, J.; Kang, J.W. Evaluation of the formation of oxidants and by-products using Pt/Ti, RuO2/Ti, and IrO2/Ti electrodes in the electrochemical process. Environ. Technol. 2015, 36, 317–326. [Google Scholar] [CrossRef]
- Michalski, B.M. The occurrence of Chlorite, Chlorate, and Bromate in Disinfected Swimming Pool Water. Pol. J. Environ. 2007, 16, 237–241. [Google Scholar]
- Khuri, A.I.; Mukhopadhyay, S. Response surface methodology. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 128–149. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Hsu, G.W.; Lu, Y.F.; Hsu, S.Y. Effects of electrolysis time and electric potential on chlorine generation of electrolyzed deep ocean water. J. Food Drug Anal. 2017, 25, 759–765. [Google Scholar] [CrossRef]
- Ciblak, A.; Mao, X.; Padilla, I.; Vesper, D.; Alshawabkeh, I.; Alshawabkeh, A.N. Electrode effects on temporal changes in electrolyte pH and redox potential for water treatment. J. Environ. Sci. Health Part A 2012, 47, 718–726. [Google Scholar] [CrossRef]
- Jung, Y.J.; Baek, K.W.; Oh, B.S.; Kang, J.W. An investigation of the formation of chlorate and perchlorate during electrolysis using Pt/Ti electrodes: The effects of pH and reactive oxygen species and the results of kinetic studies. Water Res. 2010, 44, 5345–5355. [Google Scholar] [CrossRef]
- Greenland, S.; Senn, S.J.; Rothman, K.J.; Carlin, J.B.; Poole, C.; Goodman, S.N.; Altman, D.G. Statistical tests, P values, confidence intervals, and power: A guide to misinterpretations. Eur. J. Epidemiol. 2016, 31, 337–350. [Google Scholar] [CrossRef]
- Wu, W.; Huang, Z.-H.; Lim, T.-T. Recent development of mixed metal oxide anodes for electrochemical oxidation of organic pollutants in water. Appl. Catal. A Gen. 2014, 480, 58–78. [Google Scholar] [CrossRef]
- Wang, L.; Wu, B.; Li, P.; Zhang, B.; Balasubramanian, N.; Zhao, Y. Kinetics for electro-oxidation of organic pollutants by using a packed-bed electrode reactor (PBER). Chem. Eng. J. 2016, 284, 240–246. [Google Scholar] [CrossRef]
- Song, H.-L.; Zhu, Y.; Li, J. Electron transfer mechanisms, characteristics and applications of biological cathode microbial fuel cells—A mini review. Arab. J. Chem. 2015, in press. [Google Scholar] [CrossRef]
- Wang, Q.; Lemley, A.T. Competitive Degradation and Detoxification of Carbamate Insecticides by Membrane Anodic Fenton Treatment. J. Agric. Food Chem. 2003, 51, 5382–5390. [Google Scholar] [CrossRef]
- Roberts, T.R.; Hutson, D.H. Phenyl carbamates. In Metabolic Pathways of Agrochemicals: Part 2: Insecticides and Fungicides; Roberts, T.R., Hutson, D.H., Eds.; The Royal Society of Chemistry: Cambridge, UK, 1999; pp. 1283–1288. [Google Scholar]






| Flow Rate (L/min) | Retention Time (s) | Current (A) | Current Density (mA cm−2) |
|---|---|---|---|
| 0.1 | 167 | 0.2 | 3.64 |
| 0.2 | 83 | 0.5 | 9.11 |
| 0.3 | 56 | 1.0 | 18.22 |
| 0.4 | 42 | 1.5 | 27.33 |
| 0.5 | 33 | 2.0 | 36.44 |
| 0.6 | 28 | 2.5 | 45.55 |
| 0.8 | 21 | 3.0 | 54.66 |
| 1.0 | 17 | 3.5 | 63.77 |
| Symbol | Variable | Coded Variable and Independent Variables | ||||
|---|---|---|---|---|---|---|
| −α | −1 | 0 | +1 | +α | ||
| X1 | Chloride concentration (mg/L) | 10 | 30 | 50 | 70 | 90 |
| X2 | Flow rate (L/min) | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 |
| X3 | Current density (mA cm−2) | 9.11 | 18.22 | 27.33 | 36.44 | 45.55 |
| X4 | pH | 5 | 6 | 7 | 8 | 9 |
| Symbol | Variable | Coded Variable and Independent Variables | ||||
|---|---|---|---|---|---|---|
| −α | −1 | 0 | +1 | +α | ||
| X’1 | Chloride concentration (mg/L) | 5 | 20 | 35 | 50 | 65 |
| X’2 | Current density (mA cm−2) | 27.33 | 33.64 | 45.55 | 54.66 | 63.77 |
| X’3 | Flow rate (L/min) | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 |
| X’4 | Fenobucarb concentration (mg/L) | 0.5 | 1.0 | 1.5 | 2.0 | 2.5 |
| Y2 | Coeff. SC | Std. Err. | P | Conf. int (±) |
|---|---|---|---|---|
| b’0 | 45.4714 | 1.9002 | 5.92352 × 10−14 | 4.0282 |
| b’1 | 4.6788 | 1.0262 | 0.000321708 | 2.1755 |
| b’2 | 2.2779 | 1.0262 | 0.0412374 | 2.1755 |
| b’3 | −9.0738 | 1.0262 | 1.47924 × 10−7 | 2.1755 |
| b’4 | −2.4796 | 1.0262 | 0.0279997 | 2.1755 |
| b’11 | −5.6294 | 0.9401 | 1.89544 × 10−5 | 1.9930 |
| b’22 | −2.2832 | 0.9401 | 0.027321 | 1.9930 |
| b’33 | −4.3269 | 0.9401 | 0.000294319 | 1.9930 |
| b’44 | −0.7832 | 0.9401 | 0.41709 | 1.9930 |
| b’12 | −0.1544 | 1.2569 | 0.903778 | 2.6644 |
| b’13 | −0.4981 | 1.2569 | 0.697102 | 2.6644 |
| b’14 | 0.0494 | 1.2569 | 0.96915 | 2.6644 |
| b’23 | −1.5206 | 1.2569 | 0.243906 | 2.6644 |
| b’24 | −0.8681 | 1.2569 | 0.499648 | 2.6644 |
| b’34 | 1.5006 | 1.2569 | 0.249901 | 2.6644 |
| N = 31 | Q2 = 0.570 | Cond. no. = 4.686 | ||
| DF = 16 | R2 = 0.913 | RSD = 5.027 | ||
| R2 adj = 0.836 | ||||
| Y2 | DF | SS | MS (variance) | F | p | SD |
|---|---|---|---|---|---|---|
| Total | 31 | 43,453.9 | 1401.7 | |||
| Constant | 1 | 38,824.6 | 38824.6 | |||
| Total corrected | 30 | 4629.3 | 154.3 | 12.4222 | ||
| Regression | 7 | 4117.9 | 588.3 | 26.4564 | 0.000 | 24.2543 |
| Residual | 23 | 511.4 | 22.2 | 4.71546 | ||
| Lack of Fit | 17 | 434.5 | 25.6 | 1.99339 | 0.201 | 5.05551 |
| Pure error | 6 | 76.9 | 12.8 | 3.58071 | ||
| N = 31 | Q2 = 0.726 | Cond. no. = 3.783 | ||||
| DF = 23 | R2 = 0.890 | RSD = 4.715 | ||||
| R2 adj = 0.856 | ||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, G.T.; Ta, N.T.; Pham, T.Q.; Dao, Y.H. Response Surface Analysis of Fenobucarb Removal by Electrochemically Generated Chlorine. Water 2019, 11, 899. https://doi.org/10.3390/w11050899
Le GT, Ta NT, Pham TQ, Dao YH. Response Surface Analysis of Fenobucarb Removal by Electrochemically Generated Chlorine. Water. 2019; 11(5):899. https://doi.org/10.3390/w11050899
Chicago/Turabian StyleLe, Giang Truong, Nguyen Thuy Ta, Trung Quoc Pham, and Yen Hai Dao. 2019. "Response Surface Analysis of Fenobucarb Removal by Electrochemically Generated Chlorine" Water 11, no. 5: 899. https://doi.org/10.3390/w11050899
APA StyleLe, G. T., Ta, N. T., Pham, T. Q., & Dao, Y. H. (2019). Response Surface Analysis of Fenobucarb Removal by Electrochemically Generated Chlorine. Water, 11(5), 899. https://doi.org/10.3390/w11050899