Satellite Estimation of Chlorophyll-a Using Moderate Resolution Imaging Spectroradiometer (MODIS) Sensor in Shallow Coastal Water Bodies: Validation and Improvement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. In Situ Data
2.3. Satellite Data
2.4. Extraction of In Situ-Satellite Matchup Pairs
2.5. Validation of the Ocean Color 3M (OC3M) Algorithm
2.6. The Green-Red Ocean Color 4 (GROC4) Algorithm
2.7. Validation of the GROC4 Algorithm
3. Results
3.1. Performance of the OC3M Algorithm in Sargasso Sea and Chesapeake Bay
3.2. The GROC4 Algorithm
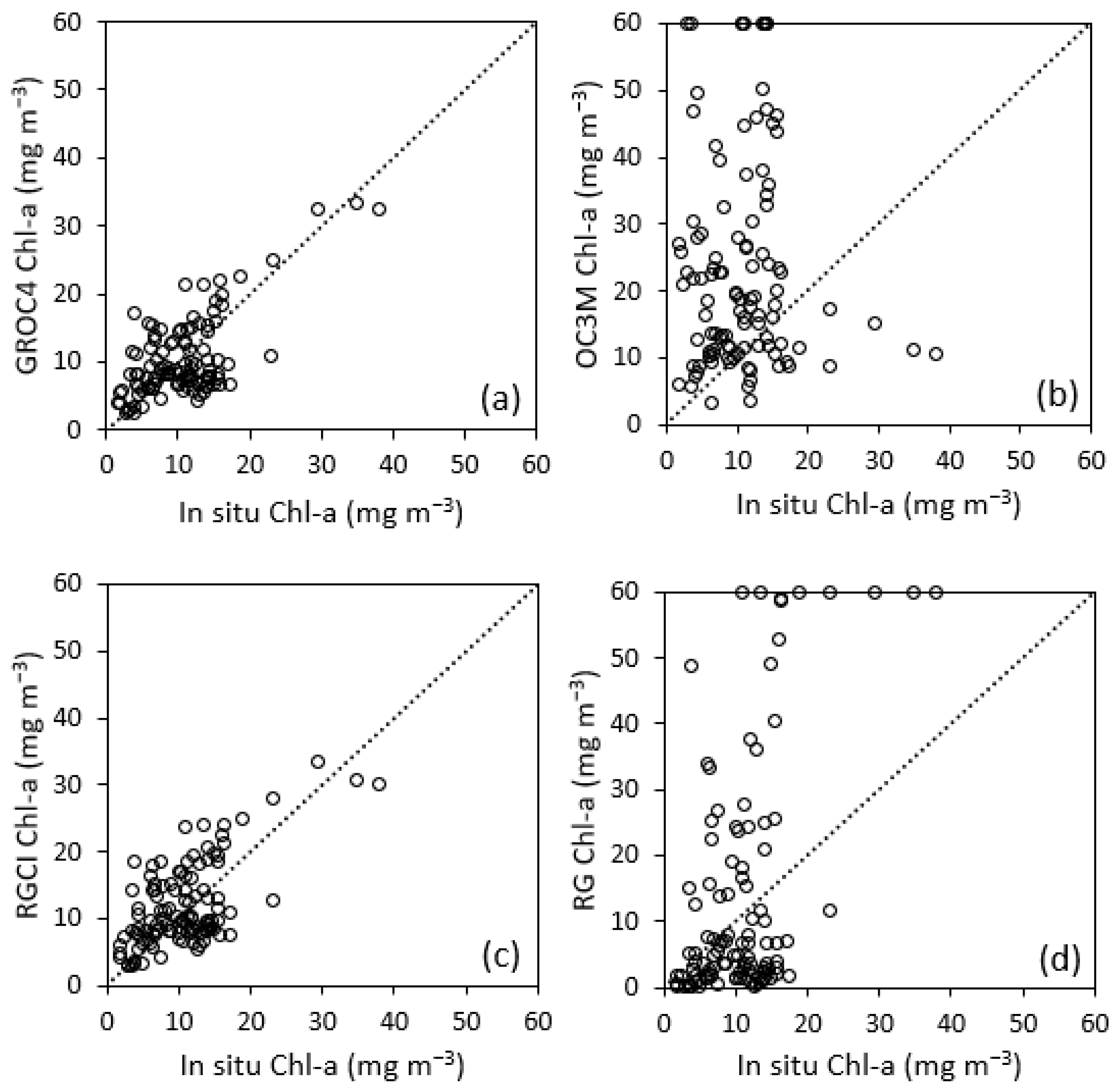
3.3. Validation and Comparison Analysis of the GROC4 Algorithm
3.4. Seasonal Performance of the GROC4 Algorithm
3.5. Chl-a Map of Chesapeake Bay
4. Discussion
4.1. OC3M Algorithm Performance
4.2. Performance Evaluation of the GROC4 Algorithm in Chesapeake Bay
4.3. Seasonality of Chl-a
4.4. Spatial Map of Chl-a
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Dolah, F.M. Marine algal toxins: Origins, health effects, and their increased occurrence. Environ. Health Perspect. 2000, 108 (Suppl. 1), 133–141. [Google Scholar] [CrossRef] [PubMed]
- Pierce, R.H.; Henry, M. Harmful algal toxins of the Florida red tide (Karenia brevis): Natural chemical stressors in South Florida coastal ecosystems. Ecotoxicology 2008, 17, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Pinckney, J.L.; Paerl, H.W.; Tester, P.; Richardson, T.L. The role of nutrient loading and eutrophication in estuarine ecology. Environ. Health Perspect. 2001, 109 (Suppl. 5), 699–706. [Google Scholar] [PubMed]
- Smith, V.H. Eutrophication of freshwater and coastal marine ecosystems a global problem. Environ. Sci. Pollut. Res. 2003, 10, 126–139. [Google Scholar] [CrossRef]
- Moore, S.K.; Trainer, V.L.; Mantua, N.J.; Parker, M.S.; Laws, E.A.; Backer, L.C.; Fleming, L.E. Impacts of climate variability and future climate change on harmful algal blooms and human health. Environ. Health 2008, 7 (Suppl. 2), S4. [Google Scholar] [CrossRef]
- Mélin, F.; Hoepffner, N. Monitoring Phytoplankton Productivity from Satellite—An Aid to Marine Resources Management. In Handbook of Satellite Remote Sensing Image Interpretation: Applications for Marine Living Resources Conservation and Management; Morales, J., Stuart, V., Platt, T., Sathyendranath, S., Eds.; EU PRESPO and IOCCG, 2011; pp. 79–93. Available online: https://www.ioccg.org/handbook/casestudy6_melin_hoepffner.pdf (accessed on 6 August 2019).
- Cullen, J.J. The deep chlorophyll maximum: Comparing vertical profiles of chlorophyll. Can. J. Fish. Aquat. Sci. 1982, 39, 791–803. [Google Scholar] [CrossRef]
- Dore, J.E.; Letelier, R.M.; Church, M.J.; Lukas, R.; Karl, D.M. Summer phytoplankton blooms in the oligotrophic North Pacific Subtropical Gyre: Historical perspective and recent observations. Prog. Oceanogr. 2008, 76, 2–38. [Google Scholar] [CrossRef]
- Joint, I.; Groom, S.B. Estimation of phytoplankton production from space: Current status and future potential of satellite remote sensing. J. Exp. Mar. Biol. Ecol. 2000, 250, 233–255. [Google Scholar] [CrossRef]
- O’Reilly, J.E.; Maritorena, S.; Mitchell, B.G.; Siegel, D.A.; Carder, K.L.; Garver, S.A.; Kahru, M.; McClain, C. Ocean color chlorophyll algorithms for SeaWiFS. J. Geophys. Res. Ocean. 1998, 103, 24937–24953. [Google Scholar] [Green Version]
- Blondeau-Patissier, D.; Gower, J.F.R.; Dekker, A.G.; Phinn, S.R.; Brando, V.E. A review of ocean color remote sensing methods and statistical techniques for the detection, mapping and analysis of phytoplankton blooms in coastal and open oceans. Prog. Oceanogr. 2014, 123, 123–144. [Google Scholar] [CrossRef] [Green Version]
- Darecki, M.; Stramski, D. An evaluation of MODIS and SeaWiFS bio-optical algorithms in the Baltic Sea. Remote Sens. Environ. 2004, 89, 326–350. [Google Scholar] [CrossRef]
- Morel, A.; Prieur, L. Analysis of variations in ocean color 1. Limnol. Oceanogr. 1977, 22, 709–722. [Google Scholar] [CrossRef]
- Schalles, J.F. Optical remote sensing techniques to estimate phytoplankton chlorophyll a concentrations in coastal. In Remote Sensing of Aquatic Coastal Ecosystem Processes; Springer: Dordrecht, The Netherlands, 2006; pp. 27–79. [Google Scholar]
- Carder, K.L.; Cannizzaro, J.P.; Lee, Z. Ocean color algorithms in optically shallow waters: Limitations and improvements. In Remote Sensing of the Coastal Oceanic Environment; International Society for Optics and Photonics: Bellingham, WA, USA, 2005. [Google Scholar]
- Moses, W.J.; Gitelson, A.A.; Berdnikov, S.; Povazhnyy, V. Estimation of chlorophyll-a concentration in case II waters using MODIS and MERIS data—Successes and challenges. Environ. Res. Lett. 2009, 4, 045005. [Google Scholar] [CrossRef]
- Richardson, L.L.; LeDrew, E.F. Remote Sensing of Aquatic Coastal Ecosystem Processes; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Gilerson, A.A.; Gitelson, A.A.; Zhou, J.; Gurlin, D.; Moses, W.; Ioannou, I.; Ahmed, S.A. Algorithms for remote estimation of chlorophyll-a in coastal and inland waters using red and near infrared bands. Opt. Express 2010, 18, 24109–24125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, C.; Hu, C.; Cannizzaro, J.; Duan, H. Long-term distribution patterns of remotely sensed water quality parameters in Chesapeake Bay. Estuar. Coast. Shelf Sci. 2013, 128, 93–103. [Google Scholar] [CrossRef]
- Tzortziou, M.; Subramaniam, A.; Herman, J.R.; Gallegos, C.L.; Neale, P.J.; Harding, L.W., Jr. Remote sensing reflectance and inherent optical properties in the mid Chesapeake Bay. Estuar. Coast. Shelf Sci. 2007, 72, 16–32. [Google Scholar] [CrossRef]
- Le, C.; Hu, C.; English, D.; Cannizzaro, J.; Chen, Z.; Feng, L.; Boler, R.; Kovach, C. Towards a long-term chlorophyll-a data record in a turbid estuary using MODIS observations. Prog. Oceanogr. 2013, 109, 90–103. [Google Scholar] [CrossRef]
- Gons, H.J.; Rijkeboer, M.; Ruddick, K.G. A chlorophyll-retrieval algorithm for satellite imagery (Medium Resolution Imaging Spectrometer) of inland and coastal waters. J. Plankton Res. 2002, 24, 947–951. [Google Scholar] [CrossRef] [Green Version]
- Gitelson, A.A.; Schalles, J.F.; Hladik, C.M. Remote chlorophyll-a retrieval in turbid, productive estuaries: Chesapeake Bay case study. Remote Sens. Environ. 2007, 109, 464–472. [Google Scholar] [CrossRef]
- Le, C.; Hu, C.; Cannizzaro, J.; English, D.; Muller-Karger, F.; Lee, Z. Evaluation of chlorophyll-a remote sensing algorithms for an optically complex estuary. Remote Sens. Environ. 2013, 129, 75–89. [Google Scholar] [CrossRef]
- Blakey, T.; Melesse, A.; Sukop, M.C.; Tachiev, G.; Whitman, D.; Miralles-Wilhelm, F. Developing Benthic Class Specific, Chlorophyll-a Retrieving Algorithms for Optically-Shallow Water Using SeaWiFS. Sensors 2016, 16, 1749. [Google Scholar] [CrossRef] [PubMed]
- Powledge, F. Chesapeake bay restoration: A model of what? BioScience 2005, 55, 1032–1038. [Google Scholar] [CrossRef]
- Ator, S.W.; Denver, J.M. Understanding the Nutrients in the Chesapeake Bay Watershed and Implications for Management and Restoration: The Eastern Shore; US Geological Survey: Reston, VA, USA, 2015.
- Ator, S.W.; Brakebill, J.W.; Blomquist, J.D. Sources, Fate, and Transport of Nitrogen and Phosphorus in the Chesapeake Bay Watershed: An Empirical Model; US Geological Survey: Reston, VA, USA, 2011; Volume 5167.
- Ha, N.; Koike, K.; Nhuan, M. Improved accuracy of chlorophyll-a concentration estimates from MODIS imagery using a two-band ratio algorithm and geostatistics: As applied to the monitoring of eutrophication processes over Tien Yen Bay (Northern Vietnam). Remote Sens. 2013, 6, 421–442. [Google Scholar] [CrossRef]
- Son, S.; Wang, M. Water properties in Chesapeake Bay from MODIS-Aqua measurements. Remote Sens. Environ. 2012, 123, 163–174. [Google Scholar] [CrossRef] [Green Version]
- Ryberg, K.R.; Blomquist, J.D.; Sprague, L.A.; Sekellick, A.J.; Keisman, J. Modeling drivers of phosphorus loads in Chesapeake Bay tributaries and inferences about long-term change. Sci. Total Environ. 2018, 616, 1423–1430. [Google Scholar] [CrossRef]
- Freestone, D.; Bulger, F. The Sargasso Sea commission: An innovative approach to the conservation of areas beyond national jurisdiction. Ocean Yearb. 2016, 30, 80–90. [Google Scholar]
- Morel, A.; Claustre, H.; Gentili, B. The most oligotrophic subtropical zones of the global ocean: Similarities and differences in terms of chlorophyll and yellow substance. Biogeosciences 2010, 7, 3139–3151. [Google Scholar] [CrossRef]
- Mackey, K.R.M.; Buck, K.N.; Casey, J.R.; Cid, A.; Lomas, M.W.; Sohrin, Y.; Paytan, A. Phytoplankton responses to atmospheric metal deposition in the coastal and open-ocean Sargasso Sea. Front. Microbiol. 2012, 3, 359. [Google Scholar] [CrossRef] [Green Version]
- Chesapeake Bay Program. Chesapeake Bay Program Water Quality Database 1984–Present. Available online: http://www.chesapeakebay.net/data/downloads/cbp_water_quality_database_1984_present (accessed on 20 December 2018).
- National Centers for Environmental Information. Available online: https://www.nodc.noaa.gov/archive/arc0030/0067471/1.1/data/ (accessed on 15 April 2018).
- NASA Goddard Space Flight Center, Ocean Ecology Laboratory, Ocean Biology Processing Group. Moderate-Resolution Imaging Spectroradiometer (MODIS) Aqua Ocean Color Data. 2018. Available online: https://oceancolor.gsfc.nasa.gov/ (accessed on 6 August 2019).
- Bailey, S.W.; Werdell, P.J. A multi-sensor approach for the on-orbit validation of ocean color satellite data products. Remote Sens. Environ. 2006, 102, 12–23. [Google Scholar] [CrossRef]
- Hattab, T.; Jamet, C.; Sammari, C.; Lahbib, S. Validation of chlorophyll-α concentration maps from Aqua MODIS over the Gulf of Gabes (Tunisia): Comparison between MedOC3 and OC3M bio-optical algorithms. Int. J. Remote Sens. 2013, 34, 7163–7177. [Google Scholar] [CrossRef]
- Minu, P.; Lotliker, A.A.; Shaju, S.S.; Ashraf, P.M.; Kumar, T.S.; Meenakumari, B. Performance of operational satellite bio-optical algorithms in different water types in the southeastern Arabian Sea. Oceanologia 2016, 58, 317–326. [Google Scholar] [CrossRef] [Green Version]
- Zheng, G.; DiGiacomo, P.M. Remote sensing of chlorophyll-a in coastal waters based on the light absorption coefficient of phytoplankton. Remote Sens. Environ. 2017, 201, 331–341. [Google Scholar] [CrossRef]
- Ha, N.T.T.; Thao, N.T.P.; Koike, K.; Nhuan, M.T. Selecting the Best Band Ratio to Estimate Chlorophyll-a Concentration in a Tropical Freshwater Lake Using Sentinel 2A Images from a Case Study of Lake Ba Be (Northern Vietnam). ISPRS Int. J. Geo-Inf. 2017, 6, 290. [Google Scholar] [CrossRef]
- Cerco, C.F. Phytoplankton kinetics in the Chesapeake Bay eutrophication model. Water Qual. Ecosyst. Model. 2000, 1, 5–49. [Google Scholar] [CrossRef]
- Harding, L.W.; Mallonee, M.E.; Perry, E.S.; Miller, W.D.; Adolf, J.E.; Gallegos, C.L.; Paerl, H.W. Long-term trends, current status, and transitions of water quality in Chesapeake Bay. Sci. Rep. 2019, 9, 6709. [Google Scholar] [CrossRef]
- Langland, M.J.; Hainly, R.A. Changes IN Bottom-Surface Elevations IN Three Reservoirs on the Lower Susquehanna River, Pennsylvania and Maryland, Following the January 1996 Flood; Implications for Nutrient and Sediment Loads to Chesapeake Bay; US Geological Survey: Reston, VA, USA, 1997.
- Wang, S.; Li, J.; Zhang, B.; Shen, Q.; Zhang, F.; Lu, Z. A simple correction method for the MODIS surface reflectance product over typical inland waters in China. Int. J. Remote Sens. 2016, 37, 6076–6096. [Google Scholar]
- Shen, L.; Xu, H.; Guo, X. Satellite remote sensing of harmful algal blooms (HABs) and a potential synthesized framework. Sensors 2012, 12, 7778–7803. [Google Scholar] [CrossRef]
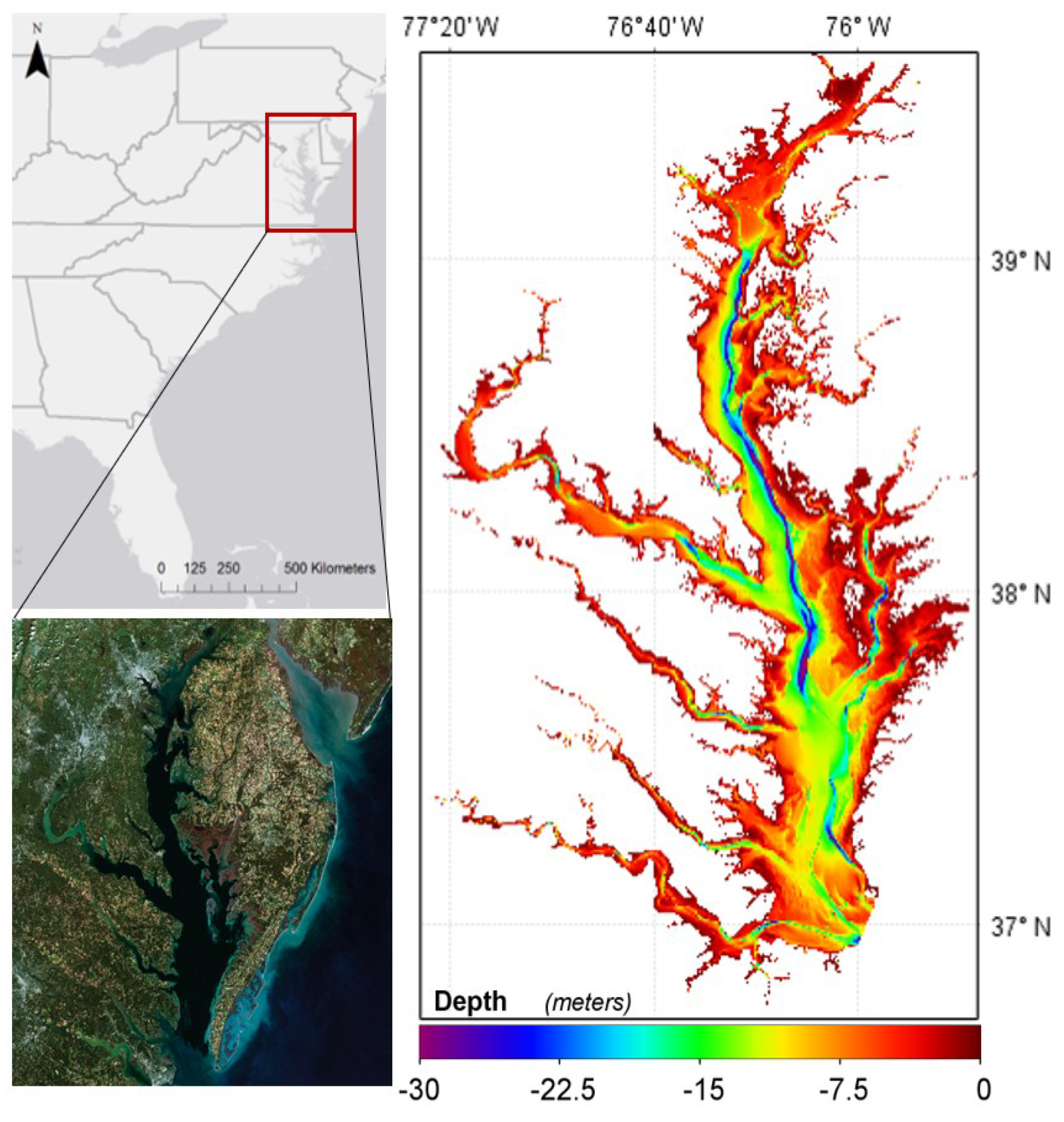





| Location | Purpose | Sampling Locations | Samples | Period | Chl-a (mg m−3) | ||
|---|---|---|---|---|---|---|---|
| Max | Min | Mean | |||||
| Chesapeake Bay | Validation of the OC3M and development of the GROC4 algorithm | 47 | 134 | 2012–16 | 71.782 | 2.674 | 16.462 |
| Validation of the GROC4 algorithm | 38 | 110 | 2017 | 38.021 | 1.736 | 10.698 | |
| Sargasso Sea | Validation of the OC3M Algorithm | 25 | 25 | 2004–05 | 0.062 | 0.030 | 0.047 |
| Season | Number of Samples | Chl-a (mg m−3) | ||
|---|---|---|---|---|
| Max | Min | Mean | ||
| Spring | 34 | 29.477 | 1.736 | 10.392 |
| Summer | 29 | 38.021 | 4.410 | 13.109 |
| Autumn | 34 | 23.191 | 2.274 | 9.745 |
| Winter | 13 | 15.379 | 3.632 | 8.619 |
| Water Type | Algorithm | R2 | p-Value | Slope | Intercept | RMSE | MAE | MAPE |
|---|---|---|---|---|---|---|---|---|
| Case 1 | OC3M | 0.518 | <0.001 | 0.97 | 0.00 | 0.007 | 0.005 | 12.171 |
| Case 2 | OC3M | 0.009 | 0.356 | 0.10 | 21.05 | 23.217 | 16.527 | 162.251 |
| Algorithm | Band Ratio | Log Base | a0 | a1 | a2 | a3 | a4 |
|---|---|---|---|---|---|---|---|
| OC3M | Xbg | 10 | 0.2424 | −2.7423 | 1.8017 | 0.0015 | −1.2280 |
| GROC4 | Xgr | e | 4.1579 | −1.9875 | −1.5994 | 2.1028 | −0.6595 |
| Algorithm | R2 | p-Value | Slope | Intercept | RMSE | MAE | MAPE |
|---|---|---|---|---|---|---|---|
| GROC4 | 0.444 | <0.001 | 0.67 | 3.29 | 4.924 | 3.921 | 46.401 |
| OC3M | <0.001 | 0.780 | −0.07 | 25.64 | 24.783 | 16.904 | 243.870 |
| RGCI | 0.405 | <0.001 | 0.67 | 4.69 | 5.410 | 4.456 | 54.764 |
| RG | 0.495 | <0.001 | 4.71 | −30.79 | 37.413 | 17.181 | 132.306 |
| Season | R2 | p-Value | Slope | Intercept | RMSE | MAE | MAPE |
|---|---|---|---|---|---|---|---|
| Spring | 0.578 | <0.001 | 0.85 | −0.12 | 4.685 | 3.873 | 44.751 |
| Summer | 0.637 | <0.001 | 0.72 | 2.49 | 4.644 | 3.603 | 28.163 |
| Autumn | 0.176 | 0.014 | 0.42 | 6.57 | 5.546 | 4.424 | 61.511 |
| Winter | 0.067 | 0.392 | 0.32 | 7.93 | 4.387 | 3.439 | 51.897 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, M.M.; Melesse, A.M.; Scinto, L.J.; Rehage, J.S. Satellite Estimation of Chlorophyll-a Using Moderate Resolution Imaging Spectroradiometer (MODIS) Sensor in Shallow Coastal Water Bodies: Validation and Improvement. Water 2019, 11, 1621. https://doi.org/10.3390/w11081621
Abbas MM, Melesse AM, Scinto LJ, Rehage JS. Satellite Estimation of Chlorophyll-a Using Moderate Resolution Imaging Spectroradiometer (MODIS) Sensor in Shallow Coastal Water Bodies: Validation and Improvement. Water. 2019; 11(8):1621. https://doi.org/10.3390/w11081621
Chicago/Turabian StyleAbbas, Mohd Manzar, Assefa M. Melesse, Leonard J. Scinto, and Jennifer S. Rehage. 2019. "Satellite Estimation of Chlorophyll-a Using Moderate Resolution Imaging Spectroradiometer (MODIS) Sensor in Shallow Coastal Water Bodies: Validation and Improvement" Water 11, no. 8: 1621. https://doi.org/10.3390/w11081621
APA StyleAbbas, M. M., Melesse, A. M., Scinto, L. J., & Rehage, J. S. (2019). Satellite Estimation of Chlorophyll-a Using Moderate Resolution Imaging Spectroradiometer (MODIS) Sensor in Shallow Coastal Water Bodies: Validation and Improvement. Water, 11(8), 1621. https://doi.org/10.3390/w11081621






