Risks of Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) for Sustainable Water Recycling via Aquifers
Abstract
:1. Introduction
2. Role of MAR in Urban Water Management
3. PFAS in the Capture Zone and Urban Source Waters Used for MAR
3.1. PFAS in Urban Stormwater
3.2. PFAS in Treated Wastewater
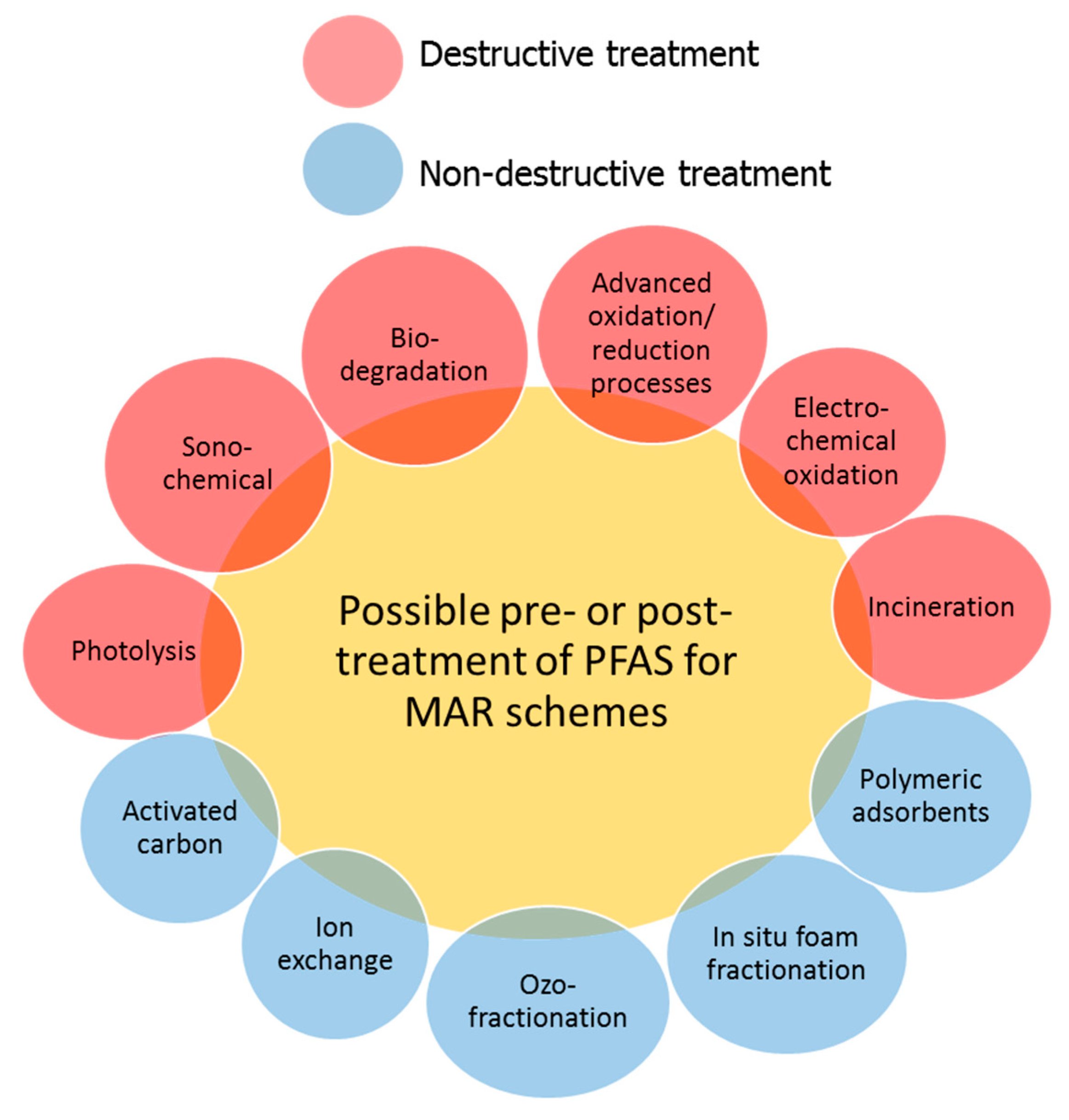
4. Engineered Treatment of PFAS
5. Recharge of the Aquifer
6. Subsurface Processes and PFAS in MAR
Key Potential Processes in the Natural Attenuation of PFAS in MAR
7. Recovery of Water from MAR
8. Post Treatment of Recovered Water
9. End Use of Water Recovered from MAR
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kauck, E.A.; Diesslin, A.R. Some properties of perfluorocarboxylic acids. Industr. Eng. Chem. 1986, 46, 2332–2334. [Google Scholar] [CrossRef]
- Shinoda, K.; Hato, M.; Hayashi, T. Physicochemical properties of aqueous solutions of fluorinated surfactants. J. Phys. Chem. 1972, 76, 909–914. [Google Scholar] [CrossRef]
- Lau, C.; Anitole, K.; Hodes, C.; Lai, D.; Pfahles-Hutchens, A.; Seed, J. Perfluoroalkyl Acids: A Review of Monitoring and Toxicological Findings. Toxicol. Sci. 2007, 99, 366–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, F. Emerging poly-and perfluoroalkyl substances in the aquatic environment: A review of current literature. Water Res. 2017, 124, 482–495. [Google Scholar] [CrossRef] [PubMed]
- Kissa, E. Fluorinated Surfactants and Repellants, 2nd ed.; Marcel Dekker Inc.: New York, NY, USA, 2001; ISBN 0-8247-0472-X. [Google Scholar]
- Interstate Technology Regulatory Council. Naming Conventions and Physical and Chemical Properties. Updated 16-3-18. Interstate Technology Regulatory Council Fact Sheet. 2018. Available online: https://pfas-1.itrcweb.org/wp-content/uploads/2018/03/pfas_fact_sheet_naming_conventions__3_16_18.pdf (accessed on 3 July 2019).
- Prevedouros, K.; Cousins, I.T.; Buck, R.C.; Korzeniowski, S.H. Sources, Fate and Transport of Perfluorocarboxylates. Environ. Sci. Technol. 2006, 40, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Vedagiri, U.K.; Anderson, R.H.; Loso, H.M.; Schwach, C.M. Ambient levels of PFOS and PFOA in multiple environmental media. Remediat. J. 2018, 28, 9–51. [Google Scholar] [CrossRef]
- Interstate Technology Regulator Council. Environmental Fate and Transport. Interstate Technology Regulatory Council Fact Sheet. 2018. Available online: https://pfas-1.itrcweb.org/wp-content/uploads/2018/03/pfas_fact_sheet_fate_and_transport__3_16_18.pdf (accessed on 3 July 2019).
- Giesy, J.P.; Naile, J.E.; Khim, J.S.; Jones, P.D.; Newsted, J.L. Aquatic Toxicology of Perfluorinated Chemicals. Rev. Environ. Contam. Toxicol. 2010, 202, 1–52. [Google Scholar] [PubMed]
- Giesy, J.P.; Kannan, K. Global Distribution of Perfluorooctane Sulfonate in Wildlife. Environ. Sci. Technol. 2001, 35, 1339–1342. [Google Scholar] [CrossRef]
- Gayland, S. Per and Polyfluorinated Alkyl Substances (PFAS) in the Marine Environment—Preliminary Ecological Findings, South Australian Environment Protection Authority, Adelaide, Australia. 2017. Available online: https://www.epa.sa.gov.au/files/12580_report_pfas_marine.pdf (accessed on 3 July 2019).
- McCarthy, C.; Kappleman, W.; DiGuiseppi, W. Ecological Considerations of Per- and Polyfluoroalkyl Substances (PFAS). Curr. Pollut. Rep. 2017, 3, 289–301. [Google Scholar] [CrossRef]
- Kannan, K.; Corsolini, S.; Falandysz, J.; Fillmann, G.; Kumar, K.S.; Loganathan, B.G.; Mohd, M.A.; Olivero, J.; Van Wouwe, N.; Yang, J.H.; et al. Perfluorooctanesulfonate and Related Fluorochemicals in Human Blood from Several Countries. Environ. Sci. Technol. 2004, 38, 4489–4495. [Google Scholar] [CrossRef]
- Weiss, P.T.; Gulliver, J.S.; Erickson, A.J. Cost and Pollutant Removal of Storm-Water Treatment Practices. J. Water Resour. Plan. Manag. 2007, 133, 218–229. [Google Scholar] [CrossRef]
- United Nations Environment Program. Stocknolm Convention on Persistent Organic Pollutants (POPs): The 16 New POPs. United Nations Environment, Stockholm. 2017. Available online: http://www.pops.int/TheConvention/ThePOPs/TheNewPOPs/tabid/2511/Default.aspx (accessed on 28 November 2018).
- United States Environmental Protection Agency. Lifetime Health Advisories and Health Effects Support Documents for Perfluorooctanoic Acid and Perfluorooctane Sulfonate; United States Environmental Protection Agency: Washington, DC, USA, 2016. Available online: https://www.epa.gov/ground-water-and-drinking-water/drinking-water-health-advisories-pfoa-and-pfos (accessed on 20 August 2019).
- Canadian Government. Canadian Environmental Protection Act, 1999; Federal Environmental Quality Guidelines, Perfluorooctane Sulfonate (PFOS), 2017. Available online: https://www.canada.ca/en/environment-climate-change/services/evaluating-existing-substances/federal-environmental-quality-guidelines perfluorooctane-sulfonate.html (accessed on 20 August 2019).
- Heads of Environmental Protection Agencies, PFAS. National Environmental Management Plan, January 2018 . Heads of EPA Australian and New Zealand (HEPA), 2018. Available online: http://www.environment.gov.au/protection/chemicals-management/pfas (accessed on 3 July 2019).
- Hu, X.C.; Andrews, D.Q.; Lindstrom, A.B.; Bruton, T.A.; Schaider, L.A.; Grandjean, P.; Lohmann, R.; Carignan, C.C.; Blum, A.; Balan, S.A.; et al. Detection of Poly-and Perfluoroalkyl Substances (PFASs) in U.S. Drinking Water Linked to Industrial Sites, Military Fire Training Areas, and Wastewater Treatment Plants. Environ. Sci. Technol. Lett. 2016, 3, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Winkens, K.; Vestergren, R.; Berger, U.; Cousins, I.T. Early life exposure to per-and polyfluoroalkyl substances (PFASs): A critical review. Emerg. Contam. 2017, 3, 55–68. [Google Scholar] [CrossRef]
- Valsecchi, S.; Conti, D.; Crebelli, R.; Polesello, S.; Rusconi, M.; Mazzoni, M.; Preziosi, E.; Carere, M.; Lucentini, L.; Ferretti, E.; et al. Deriving environmental quality standards for perfluorooctanoic acid (PFOA) and related short chain perfluorinated alkyl acids. J. Hazard. Mater. 2017, 323, 84–98. [Google Scholar] [CrossRef] [PubMed]
- New Zealand Ministry for the Environment. Impact of Per and Poly Fluoroalkyl Substances on Ecosystems, New Zealand Ministry for the Environment, (NZMftE). 2018; 130p. Available online: http://www.mfe.govt.nz/sites/default/files/media/Land/final-impact-of-pfas-on-ecosystems_0.pdf (accessed on 3 July 2019).
- Interstate Technology Regulatory Council. Remediation Technologies and Methods for Per-and Polyfluoroalkyl Substances (PFAS. Updated 16-3-18. Interstate Technology Regulatory Council Fact Sheet. Available online: https://pfas-1.itrcweb.org/wp-content/uploads/2018/03/pfas_fact_sheet_remediation_3_15_18.pdf (accessed on 3 July 2019).
- Ahrens, L.; Yeung, L.W.; Taniyasu, S.; Lam, P.K.; Yamashita, N. Partitioning of perfluorooctanoate (PFOA), perfluorooctane sulfonate (PFOS) and perfluorooctane sulfonamide (PFOSA) between water and sediment. Chemosphere 2011, 85, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Hu, J.; Tanaka, S.; Fujii, S. Perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) in sewage treatment plants. Water Res. 2009, 43, 2399–2408. [Google Scholar] [CrossRef] [PubMed]
- Dillon, P. Future management of aquifer recharge. Hydrogeol. J. 2005, 13, 313–316. [Google Scholar] [CrossRef]
- Page, D.; Bekele, E.; Vanderzalm, J.; Sidhu, J. Managed Aquifer Recharge (MAR) in Sustainable Urban Water Management. Water 2018, 10, 239. [Google Scholar] [CrossRef]
- Page, D.; Vanderzalm, J.; Dillon, P.; Gonzalez, D.; Barry, K. Stormwater Quality Review to Evaluate Treatment for Drinking Water Supply via Managed Aquifer Recharge. Water Air Soil Pollut. 2016, 227, 1–16. [Google Scholar] [CrossRef]
- Simcik, M.F.; Dorweiler, K.J. Ratio of Perfluorochemical Concentrations as a Tracer of Atmospheric Deposition to Surface Waters. Environ. Sci. Technol. 2005, 39, 8678–8683. [Google Scholar] [CrossRef]
- Zushi, Y.; Takeda, T.; Masunaga, S. Existence of nonpoint source of perfluorinated compounds and their loads in the Tsurumi River basin, Japan. Chemosphere 2008, 71, 1566–1573. [Google Scholar] [CrossRef]
- Murakami, M.; Shinohara, H.; Takada, H. Evaluation of wastewater and street runoff as sources of perfluorinated surfactants (PFSs). Chemosphere 2009, 74, 487–493. [Google Scholar] [CrossRef]
- Murakami, M.; Kuroda, K.; Sato, N.; Fukushi, T.; Takizawa, S.; Takada, H. Groundwater pollution by perfluorinated surfactants in Tokyo. Environ. Sci. Technol. 2009, 43, 3480–3486. [Google Scholar] [CrossRef]
- Xiao, F.; Simcik, M.F.; Gulliver, J.S. Perfluoroalkyl acids in urban stormwater runoff: Influence of land use. Water Res. 2012, 46, 6601–6608. [Google Scholar] [CrossRef]
- Houtz, E.F.; Sedlak, D.L. Oxidative conversion as a means of detecting precursors to perfluoroalkyl acids in urban runoff. Environ. Sci. Technol 2012, 46, 9342–9349. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Kannan, K. Perfluorinated acids in air, rain, snow, surface runoff, and lakes: Relative importance of pathways to contamination of urban lakes. Environ. Sci. Technol. 2007, 41, 8328–8334. [Google Scholar] [CrossRef] [PubMed]
- Loos, R.; Carvalho, R.; António, D.C.; Comero, S.; Locoro, G.; Tavazzi, S. EU-wide monitoring survey on emerging polar organic contaminants inwastewater treatment plant effluents. Water Res. 2013, 47, 6475–6487. [Google Scholar] [CrossRef]
- Schultz, M.M.; Higgins, C.P.; Huset, C.A.; Luthy, R.G.; Barofsky, D.F.; Field, J.A. Fluorochemical Mass Flows in a Municipal Wastewater Treatment Facility. Environ. Sci. Technol. 2006, 40, 7350–7357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, A.M.; Gerstmann, S.; Frank, H. Perfluorooctane surfactants in waste waters, the major source of river pollution. Chemosphere 2008, 72, 115–121. [Google Scholar] [CrossRef]
- Zareitalabad, P.; Siemens, J.; Hamer, M.; Amelung, W. Perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) in surface waters, sediments, soils and wastewater—A review on concentrations and distribution coefficients. Chemosphere 2013, 91, 725–732. [Google Scholar] [CrossRef]
- Radcliffe, J.C. Future directions for water recycling in Australia. Desalination 2006, 187, 77–87. [Google Scholar] [CrossRef]
- Sinclair, E.; Kannan, K. Mass Loading and Fate of Perfluoroalkyl Surfactants in Wastewater Treatment Plants. Environ. Sci. Technol. 2006, 40, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Loganathan, B.G.; Sajwan, K.S.; Sinclair, E.; Kumar, K.S.; Kannan, K. Perfluoroalkyl sulfonates and perfluorocarboxylates in two wastewater treatment facilities in Kentucky and Georgia. Water Res. 2007, 41, 4611–4620. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.; Eaglesham, G.; Reungoat, J.; Poussade, Y.; Bartkow, M.; Lawrence, M.; Mueller, J.F. Removal of PFOS, PFOA and other perfluoroalkyl acids at water reclamation plants in South East Queensland Australia. Chemosphere 2011, 82, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, U.; Haglund, P.; Kärrman, A. Contribution of precursor compounds to the release of per- and polyfluoroalkyl substances (PFASs) from waste water treatment plants (WWTPs). J. Environ. Sci. 2017, 61, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yeung, L.W.Y.; Xu, M.; Taniyasu, S.; Lam, P.K.; Yamashita, N.; Dai, J. Perfluorooctane sulfonate (PFOS) and other fluorochemicals in fish blood collected near the outfall of wastewater treatment plant (WWTP) in Beijing. Environ. Pollut. 2008, 156, 1298–1303. [Google Scholar] [CrossRef] [PubMed]
- Espana, V.A.A.; Mallavarapu, M.; Naidu, R. Treatment technologies for aqueous perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA): A critical review with an emphasis on field testing. Environ. Technol. Innov. 2015, 4, 168–181. [Google Scholar] [CrossRef]
- Kucharzyk, K.H.; Darlington, R.; Benotti, M.; Deeb, R.; Hawley, E. Novel treatment technologies for PFAS compounds: A critical review. J. Environ. Manag. 2017, 204, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Ross, I.; McDonough, J.; Miles, J.; Storch, P.; Kochunarayanan, P.T.; Kalve, E.; Hurst, J.; Dasgupta, S.S.; Burdick, J. A review of emerging technologies for remediation of PFASs. Remediat. J. 2018, 28, 101–126. [Google Scholar] [CrossRef]
- Vecitis, C.D.; Park, H.; Cheng, J.; Mader, B.T.; Hoffmann, M.R. Treatment technologies for aqueous perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA). Front. Environ. Sci. Eng. China 2009, 3, 129–151. [Google Scholar] [CrossRef]
- Ochoa-Herrera, V.; Luna-Velasco, A.; Field, J.A.; Sierra-Alvarez, R. Microbial toxicity and biodegradability of perfluorooctane sulfonate (PFOS) and shorter chain perfluoroalkyl and polyfluoroalkyl substances (PFASs). Environ. Sci. Process. Impacts 2016, 18, 1236–1246. [Google Scholar] [CrossRef] [PubMed]
- Rayne, S.; Forest, K. Perfluoroalkyl sulfonic and carboxylic acids: A critical review of physicochemical properties, levels and patterns in waters and wastewaters, and treatment methods. J. Environ. Sci. Health Part A 2009, 44, 1145–1199. [Google Scholar] [CrossRef] [PubMed]
- Boonya-Atichart, A.; Boontanon, S.K.; Boontanon, N. Removal of perfluorooctanoic acid (PFOA) in groundwater by nanofiltration membrane. Water Sci. Technol. 2016, 74, 2627–2633. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.Y.; Fu, Q.S.; Robertson, A.P.; Criddle, C.S.; Leckie, J.O. Use of reverse osmosis membranes to remove perfluorooctane sulfonate (PFOS) from semiconductor wastewater. Environ. Sci. Technol. 2006, 40, 7343–7349. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.Y.; Fu, Q.S.; Criddle, C.S.; Leckie, J.O. Effect of flux (transmembrane pressure) and membrane properties on fouling and rejection of reverse osmosis and nanofiltration membranes treating perfluorooctane sulfonate containing wastewater. Environ. Sci. Technol. 2007, 41, 2008–2014. [Google Scholar] [CrossRef] [PubMed]
- Gorenflo, A.; Velazquez-Padron, D.; Frimmel, F.H. Nano-filtration of a German groundwater of high hardness and NOM content: Performance and costs. Desalination 2003, 151, 253–265. [Google Scholar] [CrossRef]
- Schaep, J.; Van Der Bruggen, B.; Uytterhoeven, S.; Croux, R.; Vandecasteele, C.; Wilms, D.; Van Houtte, E.; Vanlerberghe, F. Removal of hardness from groundwater by nanofiltration. Desalination 1998, 119, 295–301. [Google Scholar] [CrossRef]
- Tang, H.; Xiang, Q.; Lei, M.; Yan, J.; Zhu, L.; Zou, J. Efficient degradation of perfluorooctanoic acid by UV–Fenton process. Chem. Eng. J. 2012, 184, 156–162. [Google Scholar] [CrossRef]
- Hori, H.; Yamamoto, A.; Hayakawa, E.; Taniyasu, S.; Yamashita, N.; Kutsuna, S.; Kiatagawa, H.; Arakawa, R. Efficient Decomposition of Environmentally Persistent Perfluorocarboxylic Acids by Use of Persulfate as a Photochemical Oxidant. Environ. Sci. Technol. 2005, 39, 2383–2388. [Google Scholar] [CrossRef]
- Hori, H.; Nagaoka, Y.; Murayama, M.; Kutsuna, S. Efficient decomposition of perfluorocarboxylic acids and alternative fluorochemical surfactants in hot water. Environ. Sci. Technol. 2008, 42, 7438–7443. [Google Scholar] [CrossRef]
- Kingshott, L.M. Remedial Approaches for Perfluorooctane Sulfonate. Master’s Thesis, Imperial College London, London, UK, 2008. [Google Scholar]
- Song, Z.; Tang, H.; Wang, N.; Zhu, L. Reductive defluorination of perfluorooctanoic acid by hydrated electrons in a sulfite-mediated UV photochemical system. J. Hazard. Mater. 2013, 262, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Hori, H.; Nagaoka, Y.; Yamamoto, A.; Sano, T.; Yamashita, N.; Taniyasu, S. Efficient decomposition of environmentally persistent perfluorooctanesulfonate and related fluorochemicals using zero valent iron in subcritical water. Environ. Sci. Technol. 2006, 40, 1049–1054. [Google Scholar] [CrossRef]
- Vecitis, C.D.; Wang, Y.; Cheng, J.; Park, H.; Mader, B.T.; Hoffmann, M.R. Sonochemical Degradation of Perfluorooctanesulfonate in Aqueous Film-Forming Foams. Environ. Sci. Technol. 2010, 44, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Collings, A.; Gwan, P.; Sosa-Pintos, A. Large scale environmental applications of high power ultrasound. Ultrason. Sonochem. 2010, 17, 1049–1053. [Google Scholar] [CrossRef]
- Cheng, J.; Vecitis, C.D.; Park, H.; Mader, B.T.; Hoffmann, M.R. Sonochemical Degradation of Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoate (PFOA) in Landfill Groundwater: Environmental Matrix Effects. Environ. Sci. Technol. 2008, 42, 8057–8063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez, N.A.; Rodriguez-Freire, L.; Keswani, M.; Sierra-Alvarez, R. Effect of chemical structure on the sonochemical degradation of perfluoroalkyl and polyfluoroalkyl substances (PFASs). Environ. Sci. Water Res. Technol. 2016, 2, 975–983. [Google Scholar] [CrossRef] [Green Version]
- Simonite, T. Sound Blaster Cleans Contaminated Soil. 2006. Available online: https://www.newscientist.com/article/dn10008-sound-blaster-cleans-contaminated-soil/. (accessed on 3 July 2019).
- Merino, N.; Qu, Y.; Deeb, R.A.; Hawley, E.L.; Hoffmann, M.R.; Mahendra, S. Degradation and Removal Methods for Perfluoroalkyl and Polyfluoroalkyl Substances in Water. Environ. Eng. Sci. 2016, 33, 615–649. [Google Scholar] [CrossRef] [Green Version]
- Conder, J.M.; Hoke, R.A.; Wolf, W.D.; Russell, M.H.; Buck, R.C. Are PFCAs bioaccumulative? A critical review and comparison with regulatory criteria and persistent lipophilic compounds. Environ. Sci. Technol. 2008, 42, 995–1003. [Google Scholar] [CrossRef]
- Australia New Zealand Guidelines. Australian and New Zealand Guidelines for Fresh and Marine Water Quality, Australian and New Zealand Governments. 2018. Available online: http://www.waterquality.gov.au/guidelines (accessed on 3 July 2019).
- Australian Government Department of Health. Health Based Guidance Values for PFAS for Use in Site Investigations in Australia. 2017. Available online: http://www.health.gov.au/internet/main/publishing.nsf/Content/2200FE086D480353CA2580C900817CDC/$File/fs-Health-Based-Guidance-Values.pdf (accessed on 3 July 2019).
- National Environmental Management Plan. 2018; (PFAS NEMP). Available online: http://www.environment.gov.au/protection/chemicals-management/pfas (accessed on 21 November 2018).
- Page, D.; Miotliński, K.; Gonzalez, D.; Barry, K.; Dillon, P.; Gallen, C. Environmental monitoring of selected pesticides and organic chemicals in urban stormwater recycling systems using passive sampling techniques. J. Contam. Hydrol. 2014, 158, 65–77. [Google Scholar] [CrossRef]
- Greskowiak, J.; Hamann, E.; Burke, V.; Massmann, G. The uncertainty of biodegradation rate constants of emerging organic compounds in soil and groundwater—A compilation of literature values for 82 substances. Water Res. 2017, 126, 122–133. [Google Scholar] [CrossRef]
- Tiehm, A.; Schmidt, N.; Page, D. Biodegradation of pharmaceuticals and endocrine disruptors with oxygen, nitrate, manganese (IV), iron (III) and sulfate as electron acceptors. J. Contam. Hydrol. 2017, 203, 62–69. [Google Scholar]
- Shareef, A.; Page, D.; Vanderzalm, J.; Williams, M.; Gupta, V.; Dillon, P.; Kookana, R. Biodegradation of two herbicides simazine and diuron during managed aquifer recharge of stormwater. Clean Soil Air Water 2013, 42, 745–752. [Google Scholar] [CrossRef]
- Ying, G.G.; Toze, S.; Hanna, J.; Yu, X.Y.; Dillon, P.J.; Kookana, R.S. Decay of endocrine-disrupting chemicals in aerobic and anoxic groundwater. Water Res. 2008, 42, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, M.; Patterson, B.; McKinley, A.; Reeder, A.; Furness, A.; Donn, M.; McKinley, A. Fate of benzotriazole and 5-methylbenzotriazole in recycled water recharged into an anaerobic aquifer: Column studies. Water Res. 2015, 70, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Patterson, B.; Pitoi, M.; Furness, A.; Bastow, T.; McKinley, A.; McKinley, A. Fate of N-Nitrosodimethylamine in recycled water after recharge into anaerobic aquifer. Water Res. 2012, 46, 1260–1272. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.C.; Szostek, B.; DeRito, C.; Madsen, E. Investigating the biodegradability of perfluorooctanoic acid. Chemosphere 2010, 80, 176–183. [Google Scholar] [CrossRef]
- Vaalgamaa, S.; Vähätalo, A.V.; Perkola, N.; Huhtala, S. Photochemical reactivity of perfluorooctanoic acid (PFOA) in conditions representing surface water. Sci. Total Environ. 2011, 409, 3043–3048. [Google Scholar] [CrossRef]
- Post, G.B.; Cohn, P.D.; Cooper, K.R. Perfluorooctanoic acid (PFOA), an emerging drinking water contaminant: A critical review of recent literature. Environ. Res. 2012, 116, 93–117. [Google Scholar] [CrossRef]
- AECOM. Stage 2C Environmental Investigation-Groundwater and Surface water monitoring: July 2017 to May 2018. Army Aviation Centre Oakey, Oakey QLD. Report Prepared by AECOM Australia Pty Ltd. 2018. Available online: www.defence.gov.au/Environment/PFAS/Docs/Oakey/Reports/201810OakeyGME.pdf (accessed on 3 July 2019).
- AECOM. Environmental Site Assessment, December 2017. RAAF Base Williamstown Stage 2B Environmental Investigation. Report Prepared by AECOM Australia Pty Ltd.; 2017. Available online: www.defence.gov.au/Environment/PFAS/./Williamtown/./20171205ESAFindings.pdf (accessed on 3 July 2019).
- Li, Y.; Oliver, D.P.; Kookana, R.S. A critical analysis of published data to discern the role of soil and sediment properties in determining sorption of per and polyfluoroalkyl substances (PFASs). Sci. Total. Environ. 2018, 110–120. [Google Scholar] [CrossRef]
- Ferrey, M.L.; Wilson, J.T.; Adair, C.; Su, C.; Fine, D.D.; Liu, X.; Washington, J.W. Behavior and Fate of PFOA and PFOS in Sandy Aquifer Sediment. Ground Water Monit. Remediat. 2012, 32, 63–71. [Google Scholar] [CrossRef]
- Higgins, C.P.; Luthy, R.G. Sorption of Perfluorinated Surfactants on Sediments. Environ. Sci. Technol. 2006, 40, 7251–7256. [Google Scholar] [CrossRef] [PubMed]
- Hellsing, M.J.; Josefsson, S.; Hughes, A.V.; Ahrens, L. Sorption of perfluroalkyl substances to two types of minerals. Chemosphere 2016, 159, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Vanderzalm, J.; Salle, C.L.G.L.; Dillon, P. Fate of organic matter during aquifer storage and recovery (ASR) of reclaimed water in a carbonate aquifer. Appl. Geochem. 2006, 21, 1204–1215. [Google Scholar] [CrossRef]
- Vanderzalm, J.; Dillon, P.; Barry, K.; Miotlinski, K.; Kirby, J.; Salle, C.L.G.L. Arsenic mobility and impact on recovered water quality during aquifer storage and recovery using reclaimed water in a carbonate aquifer. Appl. Geochem. 2011, 26, 1946–1955. [Google Scholar] [CrossRef]
- Vanderzalm, J.L.; Page, D.W.; Barry, K.E.; Dillon, P.J. A comparison of the geochemical response to different managed aquifer recharge operations for injection of urban storm water in a carbonate aquifer. Appl. Geochem. 2010, 25, 1350–1360. [Google Scholar] [CrossRef]
- Miotliński, K.; Dillon, P.J.; Pavelic, P.; Cook, P.G.; Page, D.W.; Levett, K. Recovery of injected freshwater to differentiate fracture flow in a low-permeability brackish aquifer. J. Hydrol. 2011, 409, 273–282. [Google Scholar] [CrossRef]
- Herczeg, A.L.; Rattray, K.J.; Dillon, P.J.; Pavelic, P.; Barry, K.E. Geochemical Processes During Five Years of Aquifer Storage Recovery. Ground Water 2004, 42, 438–445. [Google Scholar] [CrossRef]
- Barry, K.; Vanderzalm, J.; Miotlinski, K.; Dillon, P. Assessing the impact of recycled water quality and clogging on infiltration rates at a pioneering soil aquifer treatment (SAT) site in Alice Springs, Northern Territory (NT), Australia. Water 2017, 9, 179. [Google Scholar] [CrossRef]
- Gates, W.; Janik, L.; Pavelic, P.; Dillon, P.; Barry, K. Characterisation of the Physical and Geochemical Properties and Infrared Spectra of Five Soil Cores at the AZRI Site near Alice Springs, Northern Territory. CSIRO: Canberra, Australia. Available online: http://www.clw.csiro.au/publications/waterforahealthycountry/2009/wfhc-characterisation-AZRI-site.pdf (accessed on 19 August 2019).
- Prommer, H.; Descourvieres, C.D.; Handyside, M.; Johnston, K.; Harris, B.; Li, Q.; Fang, H.; Costello, P.; Seibert, S.; Martin, M. Final Report—Aquifer storage and Recovery of Potable Water in the Leederville Aquifer. Water for a Healthy Country National Research Flagship; CSIRO: Perth, Australia, 2013; Available online: https://publications.csiro.au/rpr/pub?pid=csiro:EP136027 (accessed on 3 July 2019).
- Page, D.W.; Miotlinski, K.; Dillon, P.; Taylor, R.; Wakelin, S.; Levett, K.; Barry, K.; Pavelic, P. Stormwater pre-treatment options for sustaining aquifer storage and recovery in low permeability fractured rock aquifer. J. Environ. Manag. 2011, 92, 2410–2418. [Google Scholar] [CrossRef]
- Bekele, E.; Toze, S.; Patterson, B.; Higginson, S. Managed aquifer recharge of treated wastewater: Water quality changes resulting from infiltration through the vadose zone. Water Res. 2011, 45, 5764–5772. [Google Scholar] [CrossRef]
- Bekele, E.; Toze, S.; Patterson, B.; Fegg, W.; Shackleton, M.; Higginson, S. Evaluating two infiltration gallery designs for managed aquifer recharge using secondary treated wastewater. J. Environ. Manag. 2013, 117, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Bekele, E.; Patterson, B.; Toze, S.; Furness, A.; Higginson, S.; Shackleton, M. Aquifer residence times for recycled water estimated using chemical tracers and the propagation of temperature signals at a managed aquifer recharge site in Australia. Hydrogeol. J. 2014, 22, 1383–1401. [Google Scholar] [CrossRef]
- Liu, J.; Avendaño, S.M. Microbial degradation of polyfluoroalkyl chemicals in the environment: A review. Environ. Int. 2013, 61, 98–114. [Google Scholar] [CrossRef] [PubMed]
- Regnery, J.; Gerba, C.P.; Dickenson, E.R.V.; Drewes, J.E. The importance of key attenuation factors for microbial and chemical contaminants during managed aquifer recharge: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1409–1452. [Google Scholar] [CrossRef]
- Parsons, J.R.; Sáez, M.; Dolfing, J.; De Voogt, P. Biodegradation of perfluorinated compounds. Rev. Environ. Contam. Toxicol. 2008, 196, 53–71. [Google Scholar] [PubMed]
- Patterson, B.M.; Bekele, E.B. A novel technique for estimating wetting front migration rates through the vadose zone based on changes in groundwater velocity. J. Hydrol. 2011, 409, 538–544. [Google Scholar] [CrossRef]
- Kodešová, R.; Grabic, R.; Kočárek, M.; Klement, A.; Golovko, O.; Fér, M.; Nikodem, A.; Jakšík, O. Pharmaceuticals’ sorptions relative to properties of thirteen different soils. Sci. Total Environ. 2015, 511, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Christiano, E.; Hu, Y.J.; Siegried, M.; Nitsche, H. A comparison of point of zero charge measurement methodology. Clays Clay Miner. 2011, 59, 107–115. [Google Scholar] [CrossRef]
- Johnson, R.; Anschutz, A.; Smolen, J.; Simcik, M.; Lee Penn, R. The Adsorption of Perfluorooctane Sulfonate onto Sand, Clay, and Iron Oxide Surfaces. J. Chem. Eng. Data 2007, 52, 1165–1170. [Google Scholar] [CrossRef]
- Somasundaran, P.; Agar, G. The zero point of charge of calcite. J. Colloid Interface Sci. 1967, 24, 433–440. [Google Scholar] [CrossRef]
- Natural Resource Ministerial Management Council; Environmental Protection Heritage Council; National Health Medical Research Council. Australian Guidelines for Water Recycling: Managing Health And Environmental Risks (Phase 2), Augmentation of Drinking Water Supplies. Natural Resource Ministerial Management Council; Environment Protection and Heritage Council; National Health and Medical Research Council: Canberra, Australia. Available online: https://www.nhmrc.gov.au/file/2686/download?token=3_6bfluL (accessed on 3 July 2019).
- Stahl, T.; Heyn, J.; Thiele, H.; Hüther, J.; Failing, K.; Georgii, S.; Brunn, H. Carryover of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) from soil to plants. Arch. Environ. Contam. Toxicol. 2009, 57, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Lechner, M.; Knapp, H. Carryover of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) from soil to plant and distribution to the different plant compartments studied in cultures of carrots (Daucus carota ssp. sativus), potatoes (Solanum tuberosum), and cucumbers (Cucumis sativus). J. Agric. Food Chem. 2011, 59, 11011–11018. [Google Scholar] [PubMed]
- Scher, D.P.; Kelly, J.E.; Huset, C.A.; Barry, K.M.; Hoffbeck, R.W.; Yingling, V.L.; Messing, R.B. Occurrence of perfluoroalkyl substances (PFAS) in garden produce at homes with a history of PFAS-contaminated drinking water. Chemosphere 2018, 196, 548–555. [Google Scholar] [CrossRef] [PubMed]
- National Ground Water Association. Groundwater and PFAS: State of Knowledge and Practice. National Groundwater Association Press. Available online: https://www.ngwa.org/publications-and-news/Newsroom/2018-press-releases/ngwa-releases-groundwater-and-pfas (accessed on 3 July 2019).

| Process | Destruction Mechanism | Condition | PFAS Destruction | Reference |
|---|---|---|---|---|
| Light-activated persulfateS2O82− | Oxidative | Light-activated persulfate at 50 mM, and 4 h of radiation | 100% (PFOA 559 mg/L) | [59] |
| Heat-activated persulfate | Oxidative | Persulfate at 50 mM, activated by heating at 80 °C for 6 h | PFOA decreased from 155 to 0.63 mg/L | [60] |
| Fenton’s reagent-activated persulfate | Oxidative | Not available | >97.5% PFOS destruction | [61] |
| H2O2 activated persulfate | Oxidative | Not available | >97.5% PFOS destruction | [61] |
| UV-Fenton | Oxidative | H2O2 30.0 mM, Fe2+ 2.0 mM, pH 3.0. A 9 W UV lamp (λmax = 254 nm) | >95% PFOA destruction from 8.2 mg/L and defluorination efficiency of 53.2% | [58] |
| Sulfite/UV | Reductive | UV at 254 nm, 25 °C, 10 mM sulfite, 20 µM PFOA, anaerobic conditions, 1 h reaction time | 100% PFOA removal after 1 hour, and defluorination of 88.5% after 24 h under N2 atmosphere | [62] |
| Subcritical water catalyzed by zero-valent iron | Oxidative | Subcritical water (350 °C) catalyzed by zero-valent iron. Adsorption of PFOS onto Fe3O4 precipitate followed by oxidation to O2 and F−. | 97.6–99.4% removal of PFOS to below <1.1 mg/L | [63] |
| Sonochemical | Sonolysis | Solutions containing various PFOS concentrations (65 μg/L to 13,100 μg/L) were treated with ultrasonic irradiation (frequency 505 kHz, at power density 187.5 W/L) | 73% degradation of PFOS during 120 min treatment | [64] |
| PFOS | PFOA | Exposure Scenario | Notes |
|---|---|---|---|
| Groundwater Environmental Values | |||
| 0.07 µg/L | 0.56 µg/L | Drinking | Drinking water supply |
| 0.7 µg/L | 5.6 µg/L | Ingestion | Recreation |
| Downstream Environmental Receptors a | |||
| 0.00023 µg/L (freshwater) | 19 µg/L (freshwater) | 99% species protection concentration (PC99)—high conservation value systems | Notes: Draft [71]— The PC99 for PFOS is close to the limit of detection. The PC99 would generally be adopted for chemicals that bioaccumulate and bio-magnify in wildlife. The PC95 is the default guideline value for most toxicants. |
| 0.29 µg/L (marine) | 3000 µg/L (marine) | ||
| 0.13 µg/L (freshwater) | 220 µg/L (freshwater) | 95% species protection concentration (PC95)—slightly to moderately disturbed systems | |
| 7.8 µg/L (marine) | 8500 µg/L (marine) | ||
| 2 µg/L (freshwater) | 632 µg/L (freshwater) | 90% species protection concentration (PC90)—highly disturbed systems | |
| 32 µg/L (marine) | 14,000 µg/L (marine) | ||
| 31 µg/L (freshwater) | 1820 µg/L (freshwater) | 80% species protection concentration (PC80)—highly disturbed systems | |
| 130 µg/L (marine) | 22,000 µg/L (marine) | ||
| Aquifer Type | Recharge Type | Porosity | Mineralogy | Organic Carbon (%) | Clay Content (%) | CEC (meq/100g) | pH * | Fe2+ (mg/L) * | Ca2+ (mg/L) * | Redox State | Temperature (°C) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| limestone | injection | 0.47 | calcite (74%), quartz (18%), ankerite (5%), hematite, microcline, albite | 0.2 | 2.0 | 7.3 | 0.56 | 152 | anoxic | 26 | [90,91] | |
| limestone | injection | 0.34 | calcite (65%), quartz (22%), ankerite, goethite, hematite, pyrite, albite, microcline | <0.5 | 1.8 | 6.9 | 1.54 | 135 | anoxic | 26 | [92,93] | |
| limestone | injection | - | quartz (55%), calcite (40%), chlorite, smectite, K-feldspar, pyrite, hematite, magnetite | - | - | - | 7.6 | - | 146 | anoxic | - | [94] |
| alluvium | infiltration | - | quartz (20–60%), albite (4–12%), orthoclase, kaolinite, smectite, calcite, hematite | <0.1–4 | 1–58 | 2–27 | 6.9 | - | 242 | oxic | - | [91,95,96] |
| alluvium | injection | - | quartz (>60%), goethite, smectite, kaolinite | <0.4 | - | 4.4 | 7.8 | <0.5 | 32 | anoxic | 19 | [74] |
| sandstone | injection | - | quartz (72%), k-feldspar (24%), pyrite Na-feldspar | 0.32 | <1% | - | 6.5–7.3 | 5–20 | 11–21 | anoxic | - | [80,97] |
| fractured siltstone-sandstone | injection | 0.22 | quartz (48–73%), kaolinite (13–23%), muscovite, siderite, chlorite, pyrite. | <0.3 | - | 3.2 | 7.7 | - | - | anoxic | - | [93,98] |
| calcareous sand over limestone | infiltration | 0.35 (unsat zone) | quartz, calcite, microclilne, anorthite, iron oxides | - | <2% | - | 7.0 | 0.44 | 99 | oxic | 22 | [99,100,101] |
| Reaction a | ΔG (kJ/mol) | |
|---|---|---|
| Defluorination | De-Chlorination | |
| 2-Halobenzoate + H2 → Benzoate + H+ + halide- | −132 | −145 |
| 3-Halobenzoate + H2 → Benzoate + H+ + halide- | −138 | −137 |
| 4-Halobenzoate + H2 → Benzoate + H+ + halide- | −142 | −144 |
| Halomethane → Methane + H+ + halide- | −156 | −164 |
| Dihalomethane → Halomethane + H+ + halide- | −107 | −161 |
| Trihalomethane → Dihalomethane + H+ + halide- | −85 | −170 |
| Tetrahalomethane → Trihalomethane + H+ + halide- | −89 | −188 |
| Mineral | Point of Zero Charge (pHZPC) | Kd (L/kg) | Surface Area (m2/g) | Reference |
|---|---|---|---|---|
| goethite (α-FeOOH) | 7.5–9.5 | 7.88 | 58 | [107,108] |
| calcite | 8–9.5 | 1–2 | [109] | |
| alumina | 6 | [89] | ||
| kaolinite | 4.6 | 5.31 | 10 | [107] |
| silica | 2 | no sorption | [89] |
| Dose (mL) | Frequency (n/year) | PFOS (µg/L) | PFOA (µg/L) | ||
|---|---|---|---|---|---|
| Garden irrigation 1 | Ingestion of sprays | 0.1 | 90 | 700 | 5600 |
| Routine ingestion | 1 | 90 | 70 | 560 | |
| Accidental ingestion | 100 | 1 | 0.7 | 5.6 | |
| Municipal irrigation | Ingestion | 1 | 50 | 490 | 3920 |
| Golf course irrigation | Ingestion | 1 | 33 | ||
| Toilet flushing | Ingestion of sprays | 0.01 | 1100 | 7000 | 56,000 |
| Washing machine use | Ingestion of sprays | 0.01 | 100 | 7000 | 56,000 |
| Industrial (dust suppression) | Routine ingestion | 1 | 90 | 70 | 560 |
| Car washing | Ingestion and Inhalation | 20 | 50 | 3.5 | 28 |
| Fishing activity | Ingestion | 3 | 18 | 23 | 187 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Page, D.; Vanderzalm, J.; Kumar, A.; Cheng, K.Y.; Kaksonen, A.H.; Simpson, S. Risks of Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) for Sustainable Water Recycling via Aquifers. Water 2019, 11, 1737. https://doi.org/10.3390/w11081737
Page D, Vanderzalm J, Kumar A, Cheng KY, Kaksonen AH, Simpson S. Risks of Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) for Sustainable Water Recycling via Aquifers. Water. 2019; 11(8):1737. https://doi.org/10.3390/w11081737
Chicago/Turabian StylePage, Declan, Joanne Vanderzalm, Anupama Kumar, Ka Yu Cheng, Anna H. Kaksonen, and Stuart Simpson. 2019. "Risks of Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) for Sustainable Water Recycling via Aquifers" Water 11, no. 8: 1737. https://doi.org/10.3390/w11081737
APA StylePage, D., Vanderzalm, J., Kumar, A., Cheng, K. Y., Kaksonen, A. H., & Simpson, S. (2019). Risks of Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) for Sustainable Water Recycling via Aquifers. Water, 11(8), 1737. https://doi.org/10.3390/w11081737









