Abstract
New photocatalytic membranes based on polylactic acid (PLA)/TiO2 hybrid nanofibers deposited on fiberglass supports were prepared and tested for the removal of ampicillin from aqueous solutions. The electrospinning technique was used to obtain hybrid nanofibers that were deposited on three types of fiberglass with different structures, resulting in three distinct photocatalytic membranes namely fiberglass fabric plain woven-type membrane, fiberglass mat-type membrane, and fiberglass fabric one-fold edge-type membrane. The results of the photocatalytic tests showed that the highest efficiency of ampicillin removal from aqueous solution is obtained with the fiberglass fabric plain woven-type membrane. Although it has been shown that the rate of photocatalytic degradation of ampicillin is high, being practically eliminated within the first 30 min of photocatalysis, the degree of mineralization of the aqueous solution is low even after two hours of photocatalysis due to the degradation of PLA from the photocatalytic membrane. The instability of PLA in the reactive environment of the photocatalytic reactor, evidenced by morphological, mineralogical and spectroscopic analyzes as well as by kinetic studies, is closely related to the structure of the fiberglass membrane used as a support for PLA/TiO2 hybrid nanofibers.
Keywords:
photocatalysis; fiberglass; polylactic acid; anatase; electrospining; hybrid nanofibers; ampicillin 1. Introduction
The emergence of an increasing number of antibiotic resistant bacteria is one of the biggest challenges of current medicine. This phenomenon is associated with the significant decrease in the number of new types of antibiotics introduced in the last decades, as well as with the increasing consumption of conventional antibiotics in both human and animal medicine worldwide [1]. It is well known that many of these antibiotics, once arrived in municipal wastewater, cannot be completely removed in conventional wastewater treatment plants being an important source of contamination of natural water bodies [2,3]. These can affect indigenous microbial populations by disrupting the natural balance of that environment, and may even increase the resistance of certain species of bacteria to these antibiotics. This could be a serious risk to human health because it is known that the most antibiotic resistant bacteria come from the natural environment [4].
Therefore, considerable effort has been made in recent years to identify an optimal treatment method to remove antibiotics from wastewater. Thus, the possibility of removing the antibiotics through adsorption [5,6], advanced oxidation [7,8], and biological processes has been intensively studied [9,10,11]. Of all these methods, photocatalytic oxidation, and especially heterogeneous photocatalysis with TiO2, has received particular attention due to the advantages that this method offers [12]. Regarding the application of this method for treating wastewater contaminated with antibiotics, the main advantage is the possibility of simultaneous removal of antibiotics and antibiotic resistant bacteria [13,14,15].
Electrospinning is a versatile, cost-effective, and environmental friendly process currently used to produce continuous nanofibers for tissue engineering, sensors, water and air purification, etc. [16]. Also, electrospinning has been successfully used for synthesis of TiO2 nanofibers by using solutions that contained poly (vinyl pyrrolidone) (PVP) and Ti(IV)-isopropoxide [17,18], polyvinyl acetate (PVAc) and titanium tetraisopropoxide [19], polylactic acid (PLA), tetrabutyl titanate, and hexafluoroisopropanol [20]. The latter, PLA/TiO2 (anatase) nanofibers, have also been tested for photocatalytic degradation of methyl orange with excellent results. Also, hybrid PLA/TiO2 fibrous membrane containing up to 1.75 wt. % TiO2 nanoparticles (NPs) was tested for its photocatalytic and antibacterial activities. It was found that about 97% of methyl orange degrades within 150 min of irradiation, at room temperature [21]. Other studies on the photocatalytic degradation of rhodamine B in the presence of PLA/TiO2 (anatase) composite have shown that increasing the amount of TiO2 in the composite leads to an increase in PLA degradation with consequences on the stability and efficacy of the photocatalytic membrane [22]. However, more studies need to be conducted in different working conditions to prove the photocatalytic efficacy of this composite, and subsequently the opportunity to be used for the removing of refractory organic contaminants from different environments.
Therefore, the objective of this paper is to prepare, characterize and test new photocatalytic membranes based on PLA/TiO2 (anatase) hybrid nanofibers deposited on different types of fiberglass supports. Aqueous ampicillin solution is used to test the photocatalytic potential of the membranes obtained.
2. Materials and Methods
2.1. Materials
Polylactic acid, PLA (IngeoTM Biopolymer 2003D), of thermoplastic grade derived from 100% renewable resources was purchased from NatureWorks LLC. TiO2 nanoparticles (anatase crystalline form) were previously synthesizes by electrospinning of polyvinyl pyrrolidone (PVP)/Ti(IV) isopropoxide solution and calcination at 500 °C [23]. Three types of commercial fiberglass purchased from SC POLYDIS SRL, Romania, namely fabric plain woven-type (product code—EWR), mat-type (product code—EMC), and fabric one fold edge-type (product code—TEX) were used as supports for the deposition of PLA/TiO2 hybrid nanofibers. Acetone and dimethylformamide (DMF) (Scahrlau S.L.) with different boiling points namely 56 °C and 153 °C, were used as solvents for spinning solution. Ampicillin AtbTM 1000 mg of pharmaceutical grade was used to prepare the ampicillin aqueous solutions at a concentration of 130 mg ampicillin/L, which is equivalent to a chemical oxygen demand (COD) of 300 mg/L. This concentration was chosen to simulate a residual effluent derived from the pharmaceutical industry with a very high loading of ampicillin, which is twice as big comparative with the concentration of ampicillin in a residual effluent derived from the pharmaceutical industry that we have found in the literature [24]. Hydrogen peroxide solution of 30% from Sigma-Aldrich was used in the photocatalysis experiments. The photocatalyzed samples were treated with MnO2 (manganese dioxide) powder purchased from Sigma-Aldrich. The pH of aqueous solutions was adjusted to an optimal value of 3 by using 1N H2SO4 (sulfuric acid). Distilled water was used in all experiments.
2.2. Preparation of the PLA/TiO2 Solutions
PLA solution at a concentration of 10% (wt.) was prepared by dissolving PLA polymer in a mixture of acetone/DMF (at a volume ratio of 3:2) at temperature of 60 °C, under magnetic stirring at 600 rpm for 4 h until a homogeneous solution was obtained. Then, TiO2 nanoparticles (anatase crystalline form) was added to the PLA solution in order to prepare a PLA/TiO2 solution with a TiO2 concentration of 1% (wt.), and the mixing continued for 1 h. In order to remove the air bubbles, the ultrasonic treatment was applied to the PLA/TiO2 solution for 1 h by using a Digital Heated Ultrasonic Cleaner, HBM Machines, The Netherlands.
2.3. Pretreatment of Fiberglass Supports with Polylactic Acid (PLA) Solution
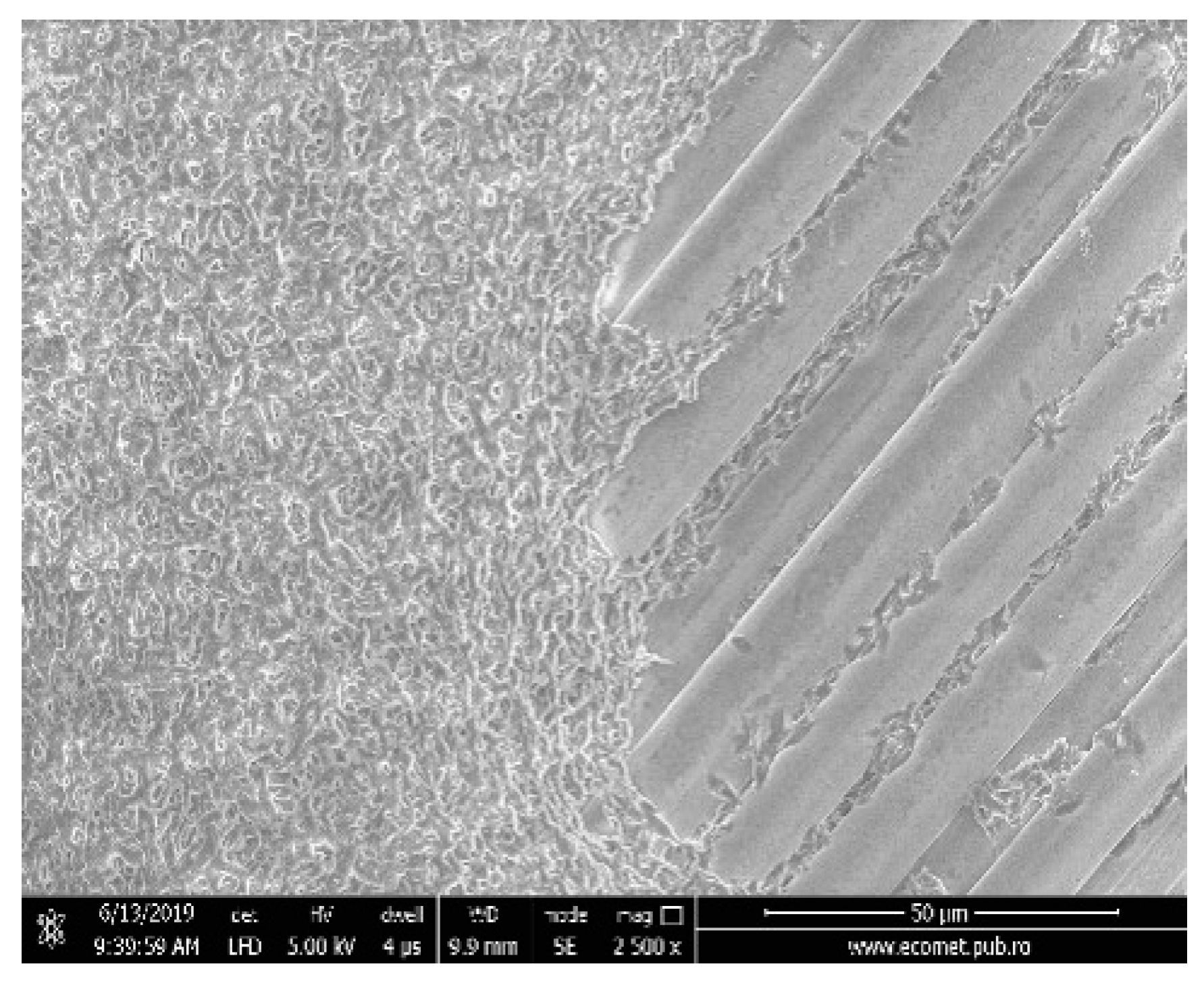
Before electrospinning process the surface of all materials was painted (covered) with PLA solution as prepared above. This technique will assure the better adhesion of electrospun nanofibers onto fiberglass surface. The Figure 1 shows the scanning electron microscopy (SEM) image of initial fiberglass membrane as alignments (right side) before to be covered with PLA solution (left side).
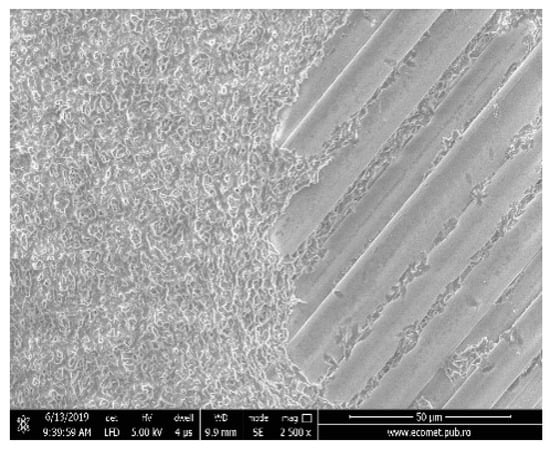
Figure 1.
Scanning electron microscopy (SEM) image of fiberglass (right side) and painted (covered) with polylactic acid (PLA) solution (left side).
2.4. Electrospinning
The prepared PLA/TiO2 spinning solution was loaded into a syringe with a 21-gauge stainless steel needle and ejected at a controllable feed rate (3 mL/h) using a syringe pump; 6 mL of PLA/TiO2 solution were used for each fiberglass membrane (corresponding to a TiO2 content of 0.06 g per each fiberglass membrane) during 2 h of electrospinning. A high voltage (24.4 kV) was applied to the needle tip through a high-voltage supply (TL-Pro-BM Electrospinning, Tong Li Tech Co., Ltd., Bao An, Shenzhen, China) equipped with a syringe pump Tong Li Tech device (Figure S1, Supplementary Materials). The PLA/TiO2 hybrid nanofibers were deposited on a grounded metallic covered with the pretreated fiberglass supports under a rotating roller at 50 rpm, which was positioned 11 cm from the tip of the needle. The scanning rate was fixed at 10 mm/s. The environmental conditions were: temperature 22.2 ± 2.5 °C and humidity 23 ± 1%. The obtained membranes, ready for testing their photocatalytic potential, are presented in Figure S2, Supplementary Materials.
2.5. Photocatalytic Experiments
The photocatalytic experiments were conducted in a cylindrical ultraviolet (UV) photocatalytic reactor provided with a water cooling jacket and with an external centrifugal pump used for recirculation of the ampicillin working solution throughout irradiation period. The UV lamp and photocatalytic membrane (cylindrical shape 10 cm × 30 cm) were coaxially positioned in a reactor, the UV lamp being inside the photocatalytic membrane (Figure S3, Supplementary Materials). The experimental conditions are presented in Table 1. It is worth mentioning that the continuous recirculation of the working solution through the centrifugal pump involves its passage through a recirculating vessel which requires an additional volume of working solution. This is why the volume of the working solution is greater than the volume of the reactor (Figure S3, Supplementary Materials). The photocatalytic degradation process was studied by monitoring the changes in the organic content as a function of irradiation time. For this purpose, samples of approximately 10 mL were taken from the reactor at irradiation times of 5, 15, 30, 60, 90 and 120 min, and treated with MnO2 powder to quickly decompose the unreacted hydrogen peroxide initially added to the aqueous ampicillin solution as hydroxyl radicals precursor (MnO2 catalyzes the decomposition of hydrogen peroxide). The MnO2 powder was added to the photocatalysed aqueous solution until no more O2 bubble formation was observed. Next, the samples were filtered and subjected to COD analysis in order to determine the organic content according to the APHA 5220 D standard method (closed reflux, colorimetric method) [25], by using a Hach Lange LT 200 thermostat and Hach Lange DR 3800 spectrophotometer. Although the ampicillin solution was prepared so as to have an initial COD equivalent concentration of 300 mg/L, its concentration was verified by COD analysis after each preparation, and these obtained values were used in the calculations. The experiments were conducted under the optimal conditions previously established [26], namely a molar ratio of hydrogen peroxide/ampicillin of 1.5 and a pH 3 of the ampicillin working solution. The control sample was performed in the absence of ampicillin (only distilled water was used) in the presence of fiberglass fabric plain woven-type membrane, and in the same working conditions with that for ampicillin samples. Cycle I and cycle II refer to two consecutive experiments carried out under identical conditions for each photocatalytic membrane. Between the two photocatalytic experiments the membranes were dried and weighed to determine the mass loss. Each photocatalytic experiment was performed in triplicate.

Table 1.
Photocatalytic experimental conditions.
2.6. Photocatalytic Membranes Characterization
The morphology of the membranes obtained was examined by means of a scanning electron microscopy (SEM) coupled with energy-dispersive X-ray (EDX) using a QUANTA 450 FEG scanning electron microscope (FEI, Eindhoven, The Netherlands), equipped with a field emission gun and a 1.2 nm resolution X-ray energy dispersive spectrometer, with a resolution of 133 eV (Everhart-Thornley conventional detector (SED) for signal detection of secondary electrons (SE) and EDS detector with silicon drift technology (SDD)-EDAX Octane Plus). The specimens were gold sputtered prior to microscopy. In order to characterize the purity and size of crystallites of the studied powder materials, a Panalytical X’Pert PRO MPD X-ray diffractometer (PANalytical, Almelo, The Netherlands) (Goniometer: vertical theta-theta; Detector: proportional with a channel) with high-intensity Cu-Kα radiation (wavelength of 1.54065 Å) and 2θ range from 10° to 90° was used for obtaining the X-ray diffraction (XRD) patterns. The mid-infrared spectra of membranes were investigated by using an INTERSPEC 200-X spectrophotometer (Interspectrum, Tartumaa, Estonia) (Interferometer type: Michelson, 30 mm beam diameter, IR source: High-intensity ceramic source), with a device for attenuated total reflectance (ATR). All spectra collected in triplicate were obtained in the wavenumber range 2000 cm−1–700 cm−1, at a resolution of 2 cm−1. The ATR crystal was carefully cleaned with pure ethanol between measurements. SEM, EDX, XRD and Fourier transform infrared (FT–IR) analyses were performed before and after photocatalytic experiments.
3. Results and Discussion
3.1. Photocatalytic Degradation of Ampicillin
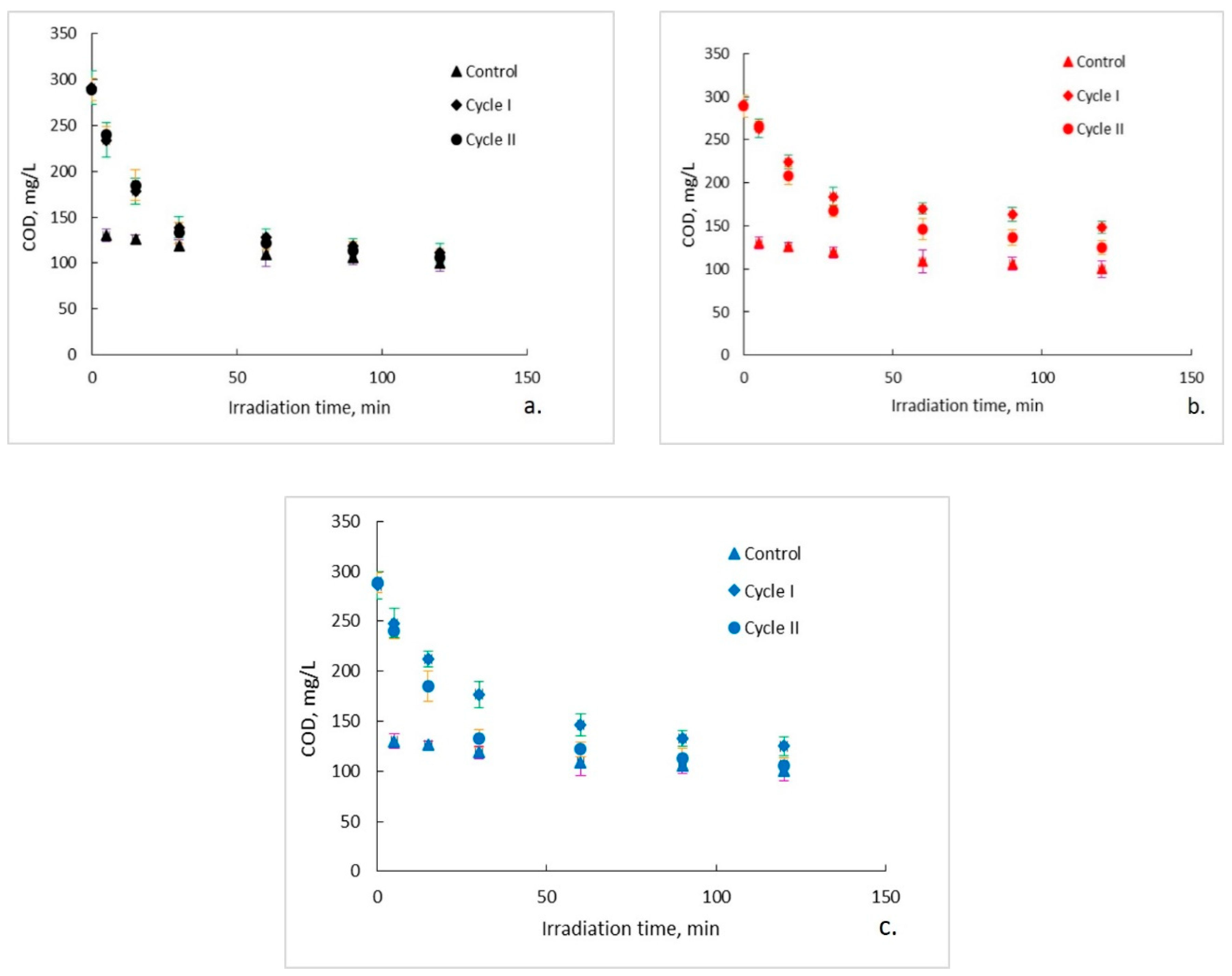
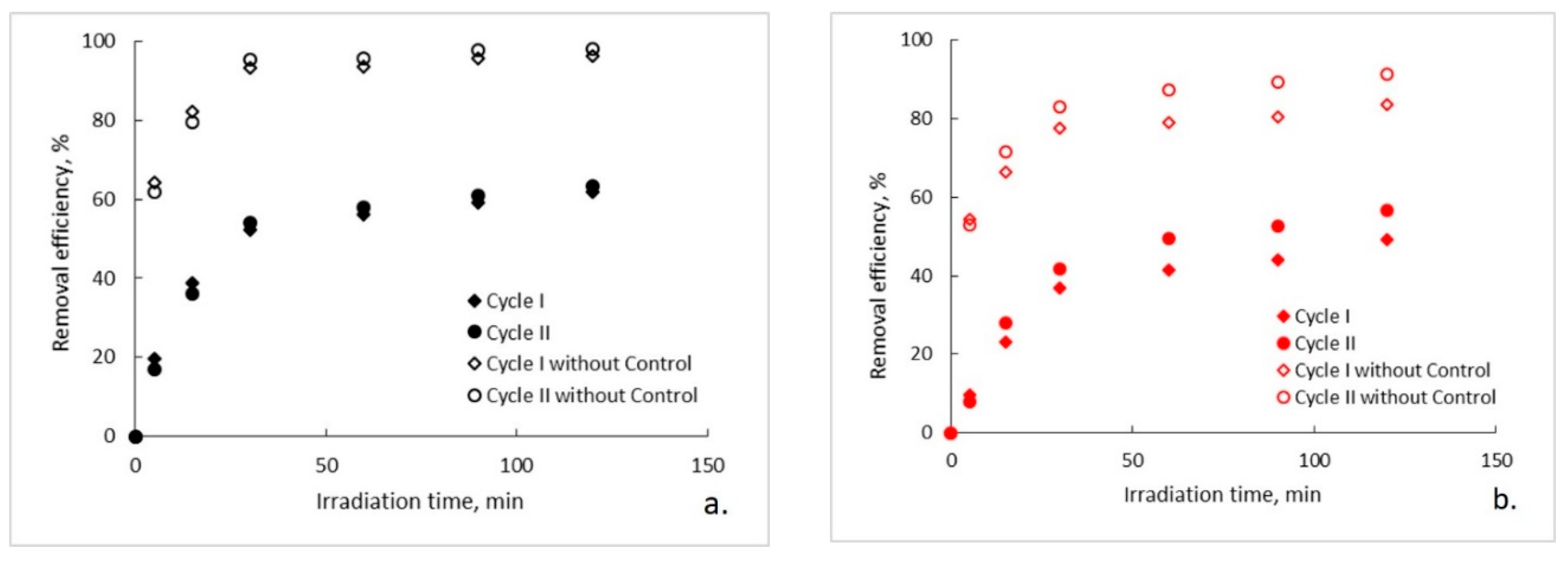
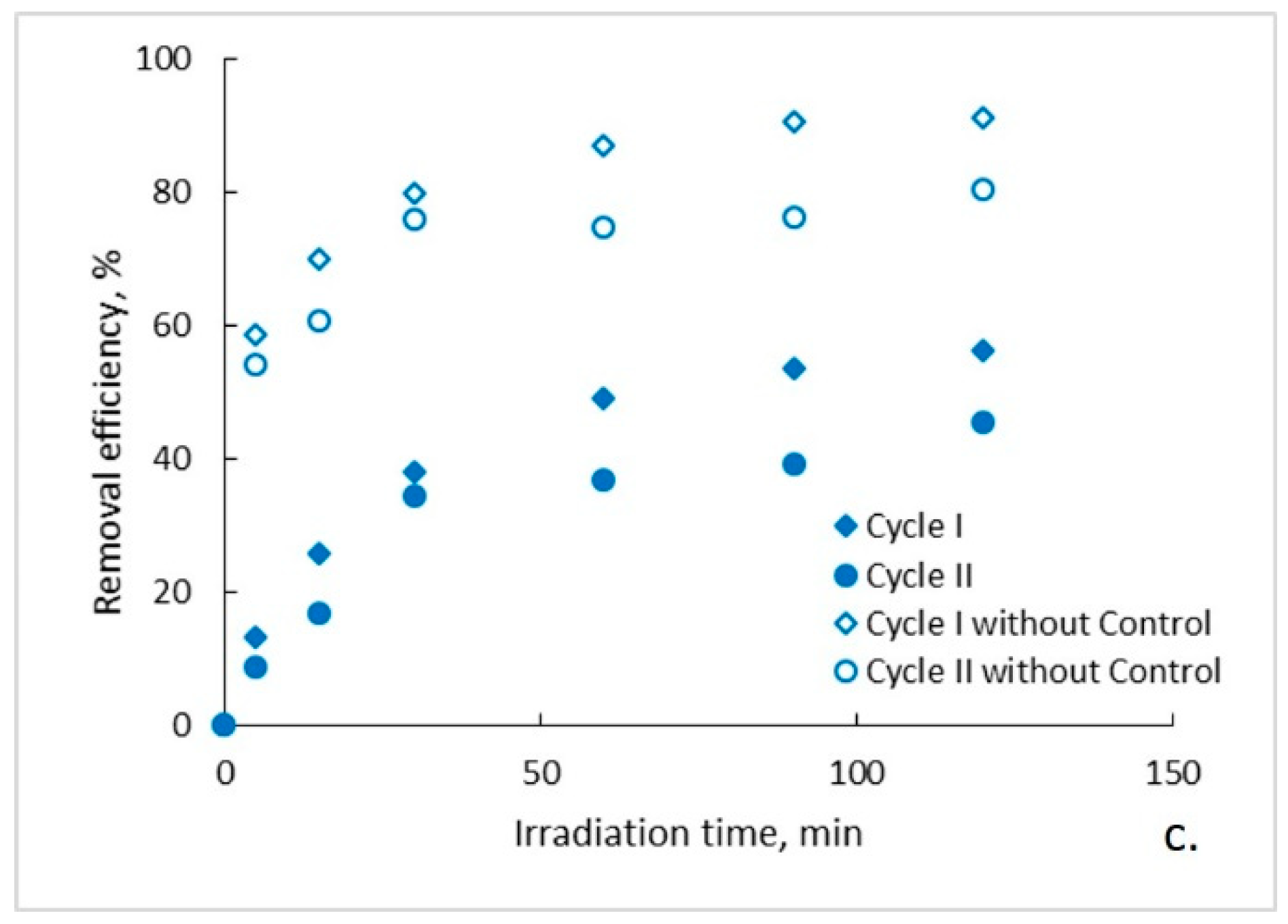
The evolution of the organic content of the aqueous solutions of ampicillin during the photocatalytic process is presented in Figure 2a–c. It can be observed that, regardless of the photocatalytic membranes used, after two hours of photocatalytic oxidation a complete mineralization of the aqueous solutions of ampicillin does not occur. Moreover, it seems that after a marked decrease of the organic content in the first 30 min of photocatalysis, its rate of degradation subsequently decreases significantly and tends to reach a plateau. This trend is specific to both photocatalytic cycles, with some differences regarding the degree of mineralization obtained after the two hours of irradiation. To explain this trend, the results obtained for the ampicillin solutions studied were plotted together with the results obtained for the control sample in which ampicillin was absent and fiberglass fabric plain woven-type membrane (EWR) was used (Figure 2a). It can be observed that the plateau, which tends to align the trend of the organic content of the photocatalyzed ampicillin samples after 30 min of irradiation, corresponds to the trend of the organic content of the control sample. It is also surprising that, in the absence of ampicillin, which theoretically represents the sole available source of organic material of the studied aqueous solutions, the organic content of the control sample is quite high even at low irradiation times. The organic content in the control sample most likely results from PLA degradation under the action of UV radiation. The similarity between the trend of the organic content of the ampicillin samples after 30 min of irradiation, with the trend of the organic content of the control sample, suggests that the mineralization of the ampicillin is almost completed after the first 30 min of irradiation, further slowly degrading the PLA from the photocatalytic membranes. If this is true, then the efficiency of removing the organic content due to ampicillin at the end of 30 min of irradiation should be much higher than that indicated by the obtained results. Figure 3 shows the removal efficiency of organic content for ampicillin samples calculated from the experimental results comparative with the removal efficiency of organic content for ampicillin samples from which the organic content attributed to PLA in the control sample was subtracted. It can be observed that the efficiency of removing the organic content after 30 min of irradiation for which the contribution of the PLA was taken into account is much higher than that for which it was not taken. For example, the efficiency of removing the organic content of the ampicillin samples after 30 min of irradiation in the presence of the fiberglass fabric plain woven-type membrane (EWR) is only about 52% (cycle I), while that for the ampicillin samples calculated taking into account the PLA contribution is about twice as big (Figure 3a). If the results obtained in the photocatalysis of the control sample in the presence of the fiberglass fabric plain woven-type membrane (EWR) are used to calculate the theoretical efficiency of removing of the organic content from the ampicillin samples photocatalized with the other two membranes, namely fiberglass mat-type membrane (EMC) (Figure 3b) and fiberglass fabric one fold edge-type membrane (TEX) (Figure 3c), approximately the same difference is recorded. A comparative analysis of removal efficiency of organic content of the ampicillin aqueous solution is shown in Table 2.
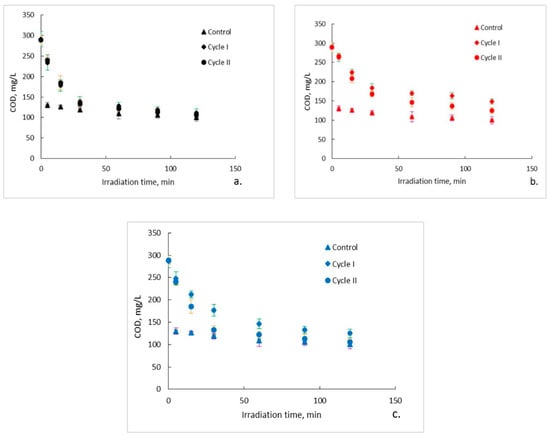
Figure 2.
Photocatalytic degradation of ampicillin: (a) fiberglass fabric plain woven-type membrane (EWR); (b) fiberglass mat-type membrane (EMC); (c) fiberglass fabric one fold edge-type membrane (TEX). Error bars represent the calculated standard deviation for the experimental data. Cycle I—green error bars; Cycle II—orange error bars; Control—purple error bars.
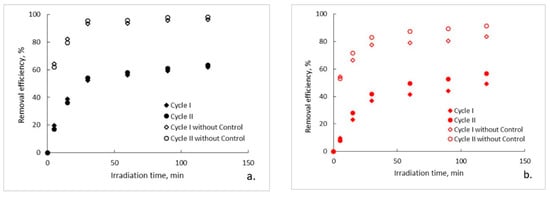
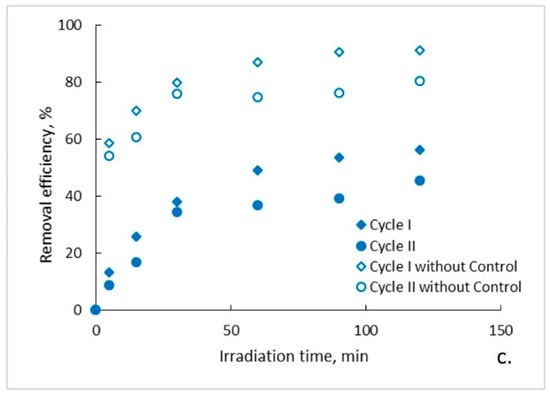
Figure 3.
Removal efficiency of organic content: (a) fiberglass fabric plain woven-type membrane (EWR); (b) fiberglass mat-type membrane (EMC); (c) fiberglass fabric one fold edge-type membrane (TEX).

Table 2.
Comparison of the photocatalytic degradation efficiency after 30 min of irradiation.
The experimental results indicate that there is a significant difference between the photocatalytic oxidation potentials of the membranes used (demonstrated by statistical analysis in the following). Thus, the efficiency of removing the organic content after 30 min of irradiation in the presence of fiberglass fabric plain woven-type membrane (EWR) is 1.42 (cycle I) and 1.29 (cycle II) times higher than that in the presence of fiberglass mat-type membrane (EMC), and 1.37 (cycle I) and 1.57 (cycle II) times higher than that in the presence of fiberglass fabric one fold edge-type membrane (TEX) (Table 2). The different network of the fiberglass support may have a significant influence on the structure of the PLA layer initially deposited on its surface with subsequent repercussions on the adhesion of the PLA/TiO2 hybrid nanofibers to the pretreated membranes. The lower the adhesion, the smaller the amount of TiO2 on the membrane surface available for the photocatalytic process. Moreover, it is expected that the addition of TiO2 nanoparticles to PLA polymeric matrix will improve its stability under UV radiation action which would lead to a decrease in its degree of degradation [27]. Therefore, a weak adhesion of the PLA/TiO2 hybrid nanofibers to the pretreated surface of the fiberglass substrate leads to an increase in the PLA unprotected surface, which is much more vulnerable to UV radiation. The proposed mechanism for degradation of PLA into lactic acid indicates the further oxidation of monomer to generate the hydroperoxides, the global process being self-catalytic [28]. The oxidation induction times caused by the scissions of C–O bonds decreased as adhesion of the PLA/TiO2 hybrid nanofibers is weaker.
The loss of mass specific to each type of membrane after the first cycle of use (1.05% for fiberglass fabric plain woven-type membrane (EWR), 2.03% for fiberglass mat-type membrane (EMC), and 7.45% for fiberglass fabric one fold edge-type membrane (TEX), respectively) could be associated with both the stability of the polymer matrix on the fiberglass support and the PLA degradation depending on the degree of protection offered by the PLA/TiO2 hybrid nanofibers deposited on the surface of the pretreated membrane.
Regarding the efficiency of removal of the organic content in cycle II by using of photocatalytic membranes, it can be observed that this is slightly higher compared to cycle I in the case of fiberglass fabric plain woven-type membrane (EWR) (1.03 times higher) and fiberglass mat-type membrane (EMC) (1.14 times higher), and lower for fiberglass fabric one fold edge-type membrane (TEX) (0.9 times lower) (Table 2). In the case of the two membranes with higher values of the removal efficiency of the organic content in cycle II than in cycle I it can be assumed that the photocatalytic potential of these two membranes increased as a result of the activation of TiO2 in the first two hours of irradiation in cycle I. Although this explanation should be valid in the case of fiberglass fabric one fold edge-type membrane (TEX), the weak adhesion of the polymeric matrix to the surface of the glass fiber substrate led to the release of a significant amount of PLA/TiO2 composite from it (7.45% mass loss) and, therefore, to the decrease of the photocatalytic potential of the membrane.
3.2. Degradation Kinetics
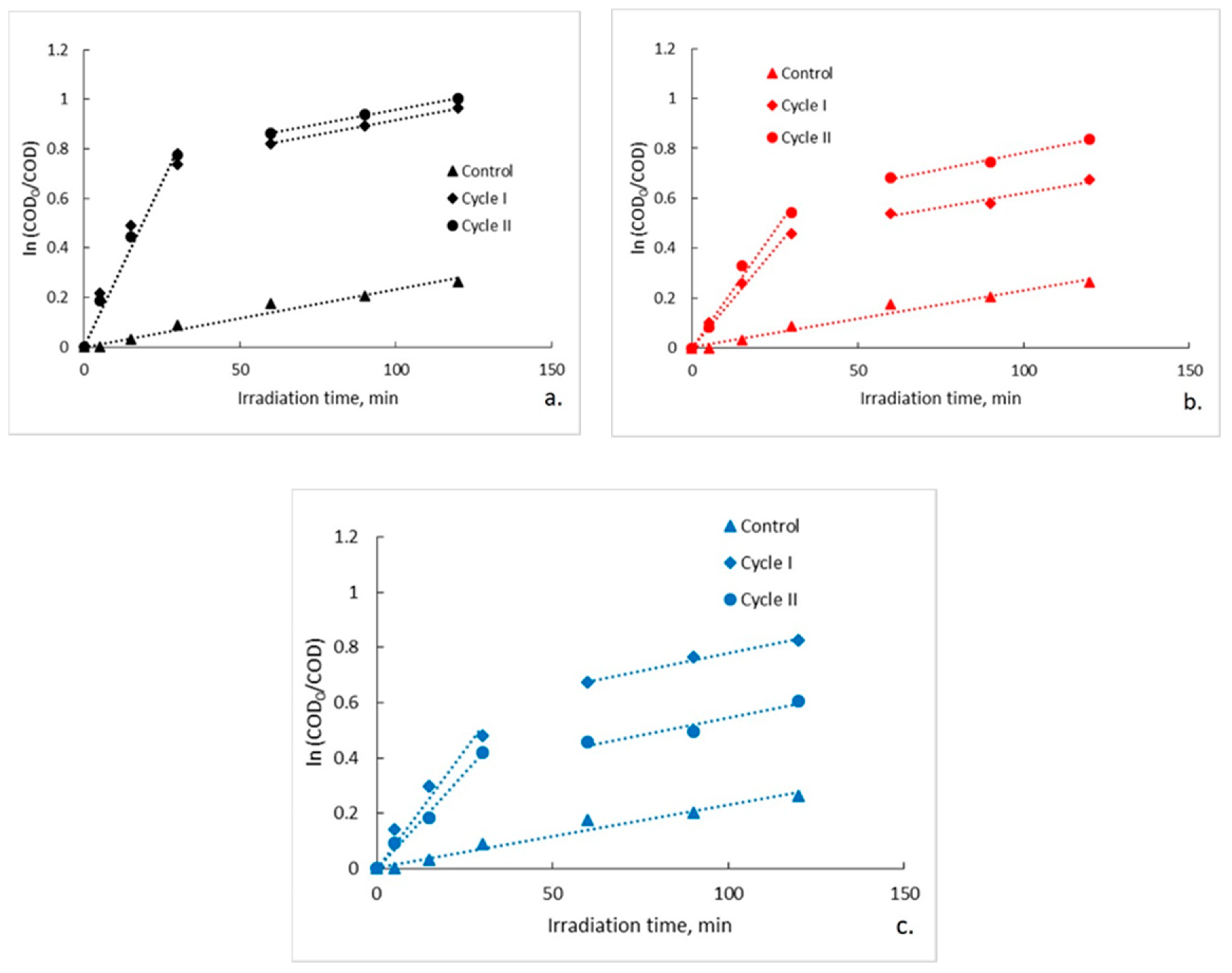
Pseudo-first order kinetic model was used to describe the photocatalytic degradation rate of ampicillin by plotting ln(C0/C) versus irradiation time for each photocatalytic membrane used [29]. The pseudo-first order kinetic model and its linearized form are presented in Equations (1) and (2):
where C0 is the initial concentration of organic material (ampicillin) in the aqueous solution (mg/L), C is the residual concentration of the organic material after irradiation at time t (mg/L), kobs is the observed pseudo-first order rate constant (related to the total reaction) (min−1), and t is the irradiation time (min). kobs is determined from the slope of the straight line of the plots (Figure 4). The kinetic parameters are presented in Table 3. The plots highlight the fact that a straight line cannot be obtained throughout the data set, these being divided into two distinct slope intervals (0–30 min and 30–120 min). This evolution suggests at first sight the existence at the beginning of the photocatalytic process of two distinct organic compounds that subsequently degrade with different degradation rates. Considering that after 5 min of irradiation of the control sample an organic content of 110 mg O2/L is recorded, it is assumed that the slope interval corresponding to a lower degradation rate describes the degradation of the PLA deposited on the fiberglass support. If, by contrast, this does not happen and the organic content recorded in the control sample derives from a possible contamination of it with ampicillin accidentally left in the recirculation pump and on the feed hoses of the photocatalytic reactor, and if it is also assumed that the degradation is described by a pseudo zero order kinetic, the initial rate of degradation of ampicillin should be approximately the same for any initial concentration of it. Therefore, to test this hypothesis, the initial rate of the photocatalytic degradation process is calculated by assuming for the two distinct slope intervals a pseudo-zero order kinetic [30].
where r0 is the initial rate of degradation of the organic material, (mg/L·min) kobs is the observed pseudo-first order rate constant (determined from the pseudo-first order kinetic model) (min−1), and k0 is the pseudo-zero order rate constant (min−1). The calculated initial rates are presented in Table 3.
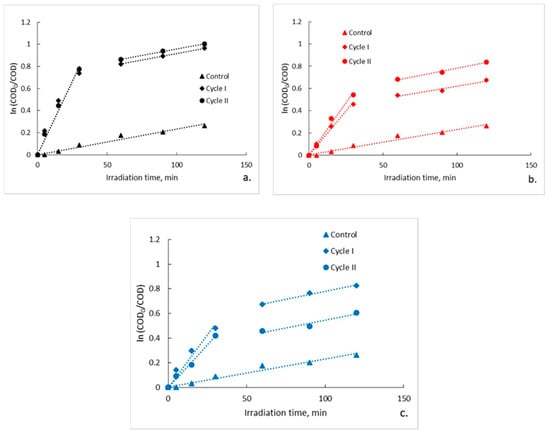
Figure 4.
Pseudo-first-order kinetic model for photocatalytic degradation of ampicillin: (a) fiberglass fabric plain woven-type membrane (EWR); (b) fiberglass mat-type membrane (EMC); (c) fiberglass fabric one fold edge-type membrane (TEX).

Table 3.
Kinetic parameters of photocatalytic degradation.
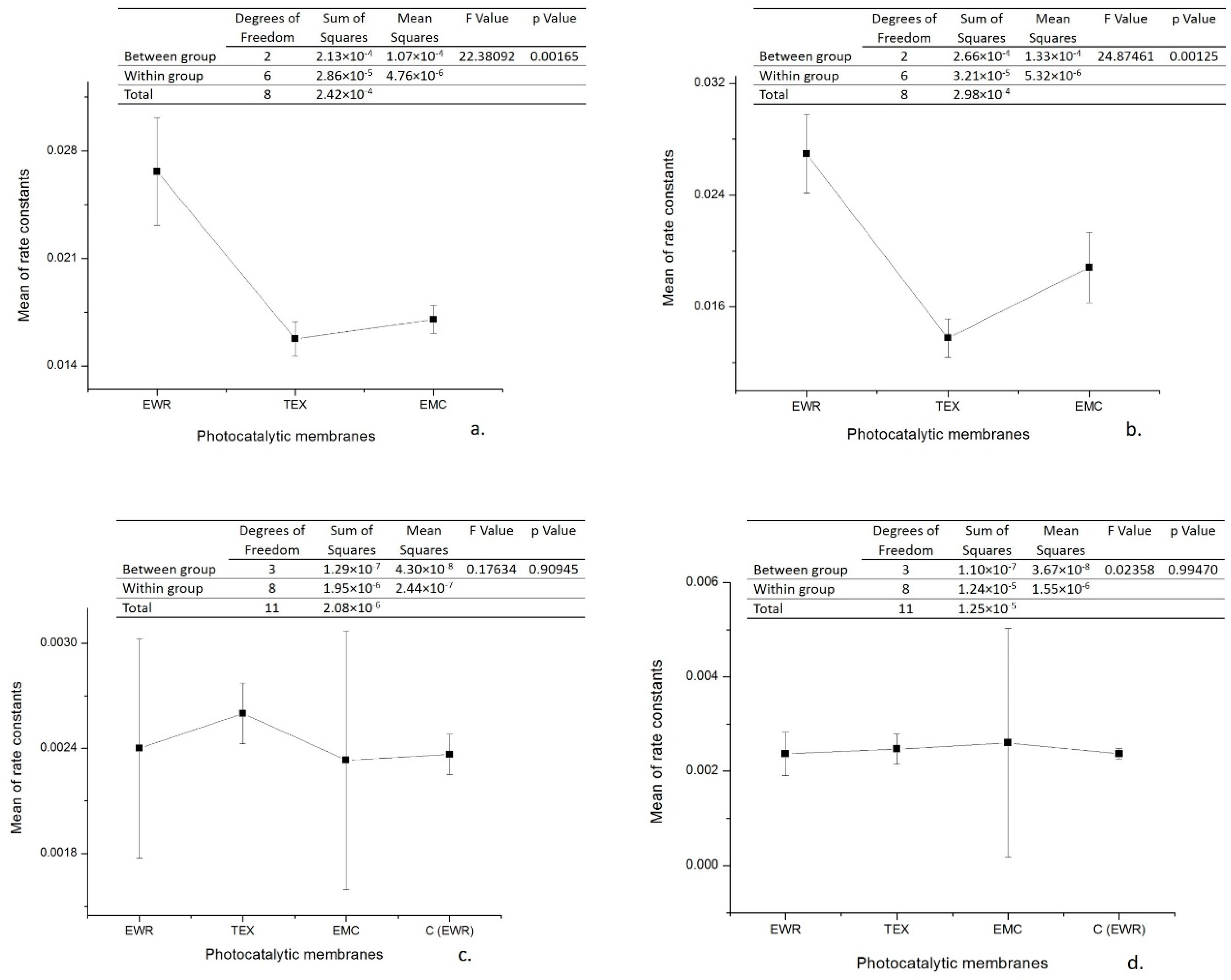
As can be seen, the initial rate calculated for the interval 0–30 min is, in all cases, with an order of magnitude greater than that calculated for the interval 30–120 min. Moreover, the observed pseudo-first order kinetic constant determined from the slope of the interval 30–120 min is in all cases approximately the same with observed pseudo-first order kinetic constant determined from the slope of entire interval (5–120 min) corresponding to the control sample (kobs = 0.0023 min−1). The kinetic parameters of photocatalytic degradation are presented in Table 3. The data were statistically analyzed with one-way analysis of variance (one-way ANOVA) at a significance level of α = 0.05 by using OriginPro 8 software. In order to establish which groups of data differ from each other, the means of the pseudo-first order kinetic constants were compared between them by conducting a Tukey post hoc test. The results obtained (Figure 5) highlight that for the interval 0–30 min the differences between the means of the pseudo-first order kinetic constants corresponding to the three types of photocatalytic membranes are statistically significant (p < 0.05), while for the interval 30–120 min the differences are not statistically significant (p > 0.05). It is worth noting that for the irradiation interval 30–120 the statistical analysis also included the data regarding the control test. Tukey post hoc test results confirm these findings. As an example, in Table 4 are presented the results obtained for data collected from experiments carried out in Cycle I. In this regard, it seems that there is a statistically significant difference in terms of means of the pseudo-first order kinetic constants for irradiation interval 0–30 min only between fiberglass fabric plain woven-type membrane (EWR) and fiberglass fabric one fold edge-type membrane (TEX), and also between fiberglass fabric plain woven-type membrane (EWR) and fiberglass mat-type membrane (EMC). Similar results were also obtained for data collected from experiments carried out in Cycle II (data not shown). These results are in agreement with the differences recorded between the photocatalytic membranes used in terms of the efficiency of organic material degradation (Figure 3 and Table 2). It should also be mentioned that the results of the statistical analysis confirm the hypothesis that at the beginning of the photocatalytic process, there are two available sources of organic material, namely ampicillin and the PLA deposited by the fiberglass support. Therefore, it is obvious that in the first 30 min of irradiation, ampicillin is almost completely degraded, further slowly degrading the PLA from the fiberglass support.
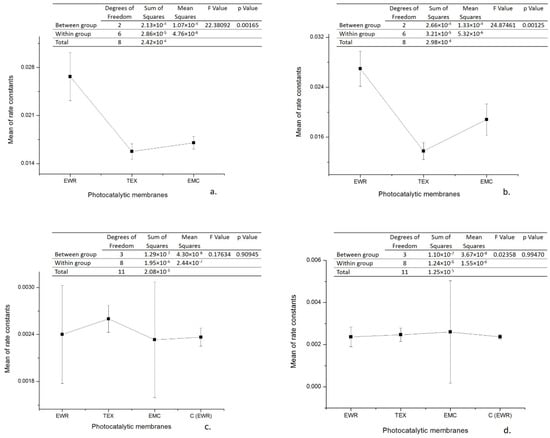
Figure 5.
One-way analysis of variance (ANOVA) test results (significance level: α = 0.05): (a) irradiation interval 0–30 min (Cycle I); (b) irradiation interval 0–30 min (Cycle II); (c) irradiation interval 30–120 min (Cycle I); (d) irradiation interval 30–120 min (Cycle II).

Table 4.
Tukey test results for data collected from experiments carried out in Cycle I.
Regarding the rate of degradation of the organic material depending on the photocatalytic membrane used (evaluated on the interval 0–30 min), the highest rate of degradation of the organic material is obtained in the presence of fiberglass fabric plain woven-type membrane (EWR) which exhibits a reaction rate of 1.69 (cycle I) and 1.42 (cycle II) times higher than that obtained in the presence of fiberglass mat-type membrane (EMC), and 1.59 (cycle I) and 1.93 (cycle II) times higher in the presence of fiberglass fabric one fold edge-type membrane (TEX).
In order to highlight the photocatalytic performance of the membranes used in this work for the photocatalytic degradation of ampicillin in terms of both removal efficiency and degradation rate, a comparative study with other photocatalytic systems used for the ampicillin degradation reported in the literature is presented in Table 5.

Table 5.
Performance of different photocatalytic systems for ampicillin removal from aqueous solutions. The data presented correspond to the best operating conditions of the photocatalytic reactors.
3.3. Characterization of Photocatalytic Membranes
3.3.1. Scanning Electron Microscopy–Energy-Dispersive X-ray (SEM–EDX) Results
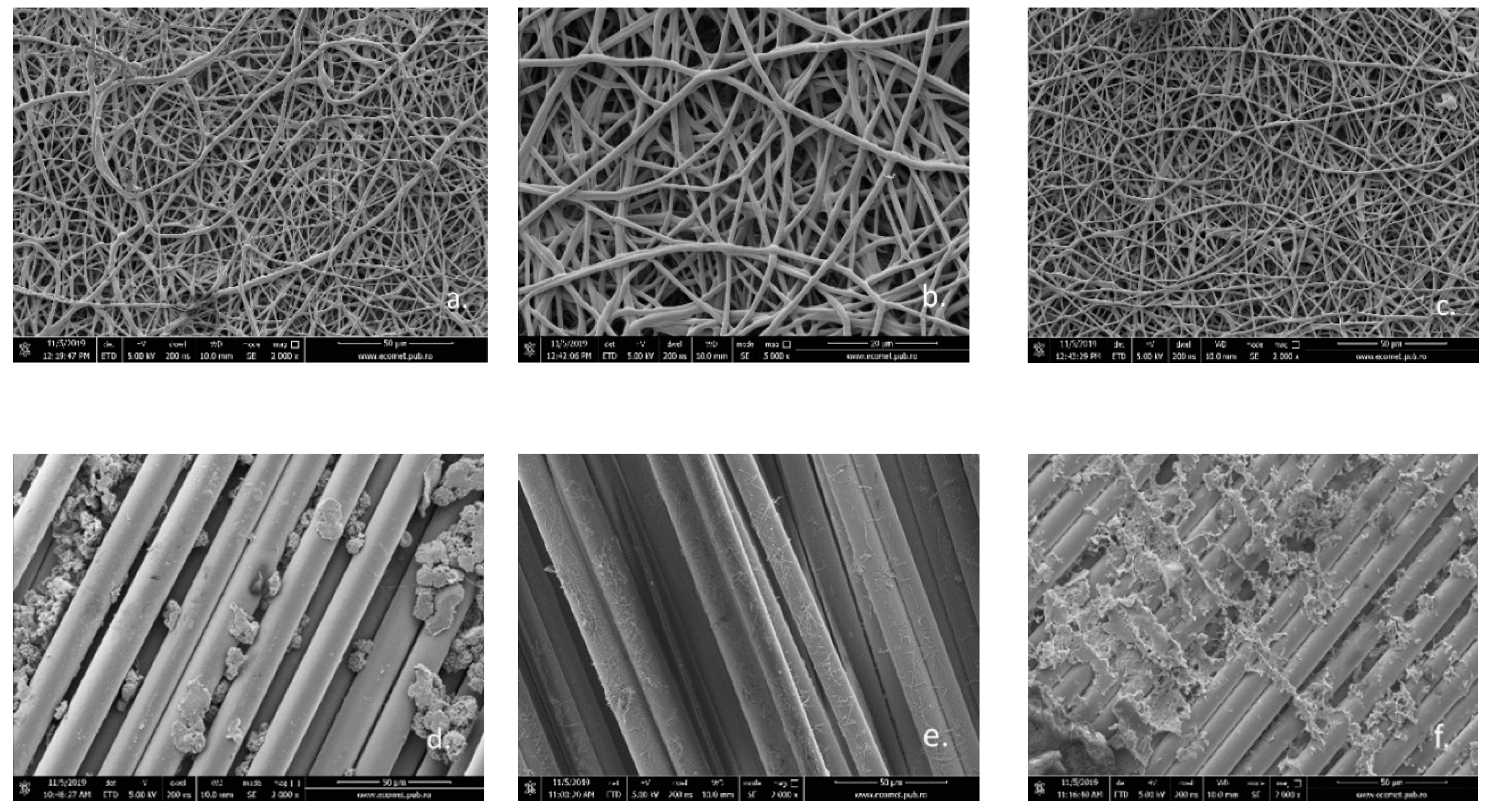
Figure 6 shows the SEM images of fiberglass membranes before (Figure 6a–c) and after photocatalysis (Figure 6d–f). Major changes in the structure of the membranes can be clearly observed after they have been used in the photocatalytic process. If initially the three membranes have a dense nanoporous structure due to PLA/TiO2 hybrid nanofibers deposited on their surface, after exposure to UV radiation in the reactive environment in which the photocatalytic process takes place, this structure practically disappears. What appears as parallel oriented linear fibers represents the fiberglass structure left uncoated with PLA/TiO2 hybrid nanofibers. This significant change in morphology can be attributed to PLA degradation during the photocatalytic process.
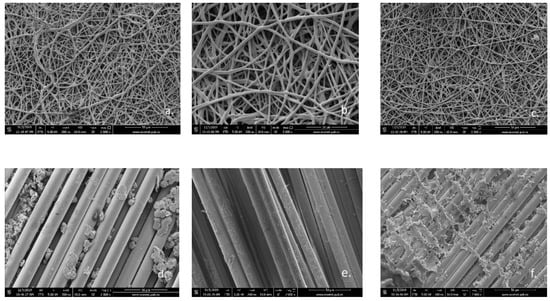
Figure 6.
SEM images for fiberglass covered with PLA/TiO2 hybrid nanofibers: (a) fabric plain woven-type (EWR)—initial (2000× magnification); (b) fabric mat-type (EMC)—initial (5000× magnification); (c) fabric one fold edge-type (TEX)—initial (2000× magnification); (d) fabric plain woven-type (EWR)—after photocatalysis (2000× magnification); (e) fabric mat-type (EMC)—after photocatalysis (2000× magnification); (f) fabric one fold edge-type (TEX)—after photocatalysis (2000× magnification).
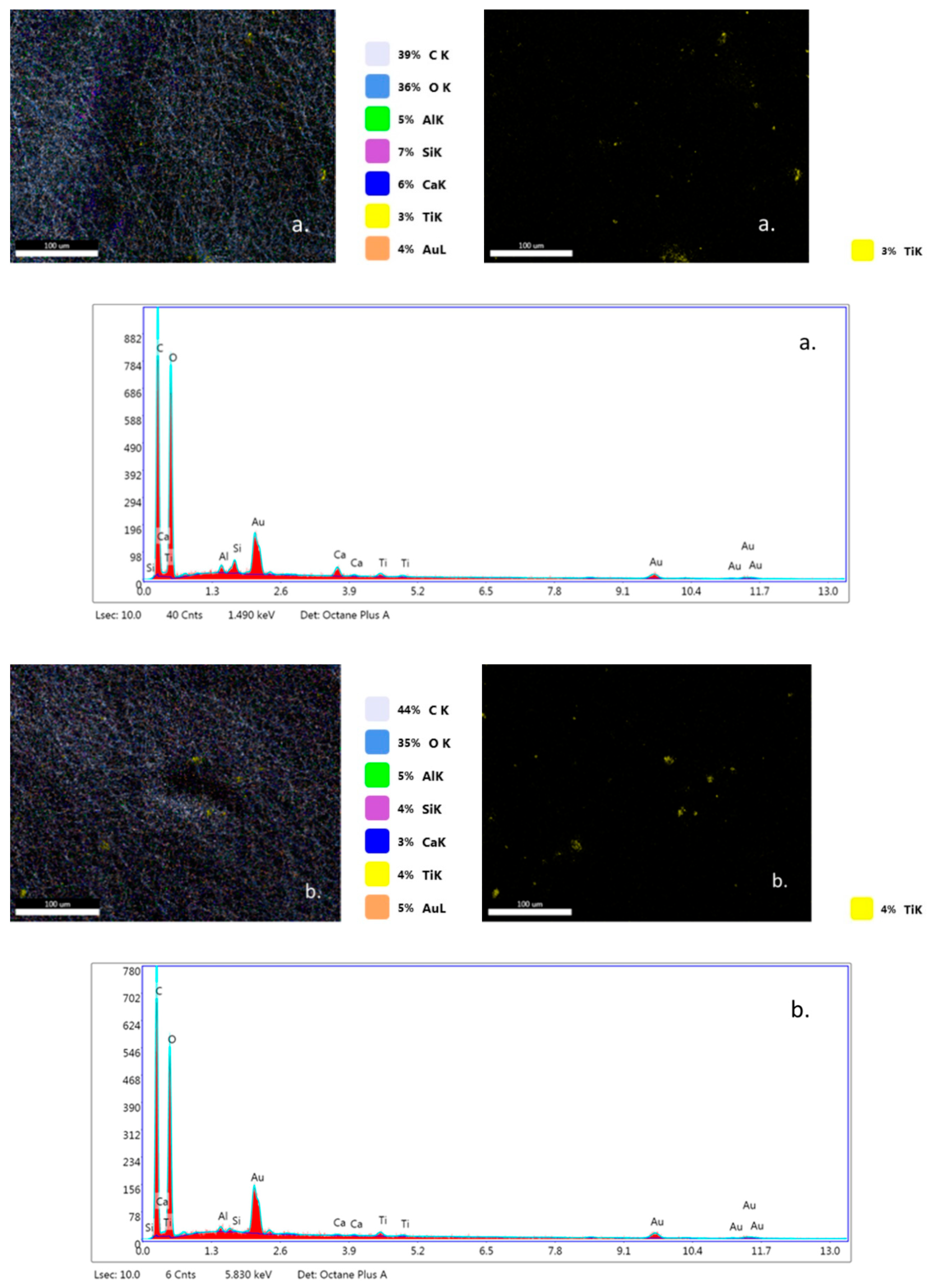
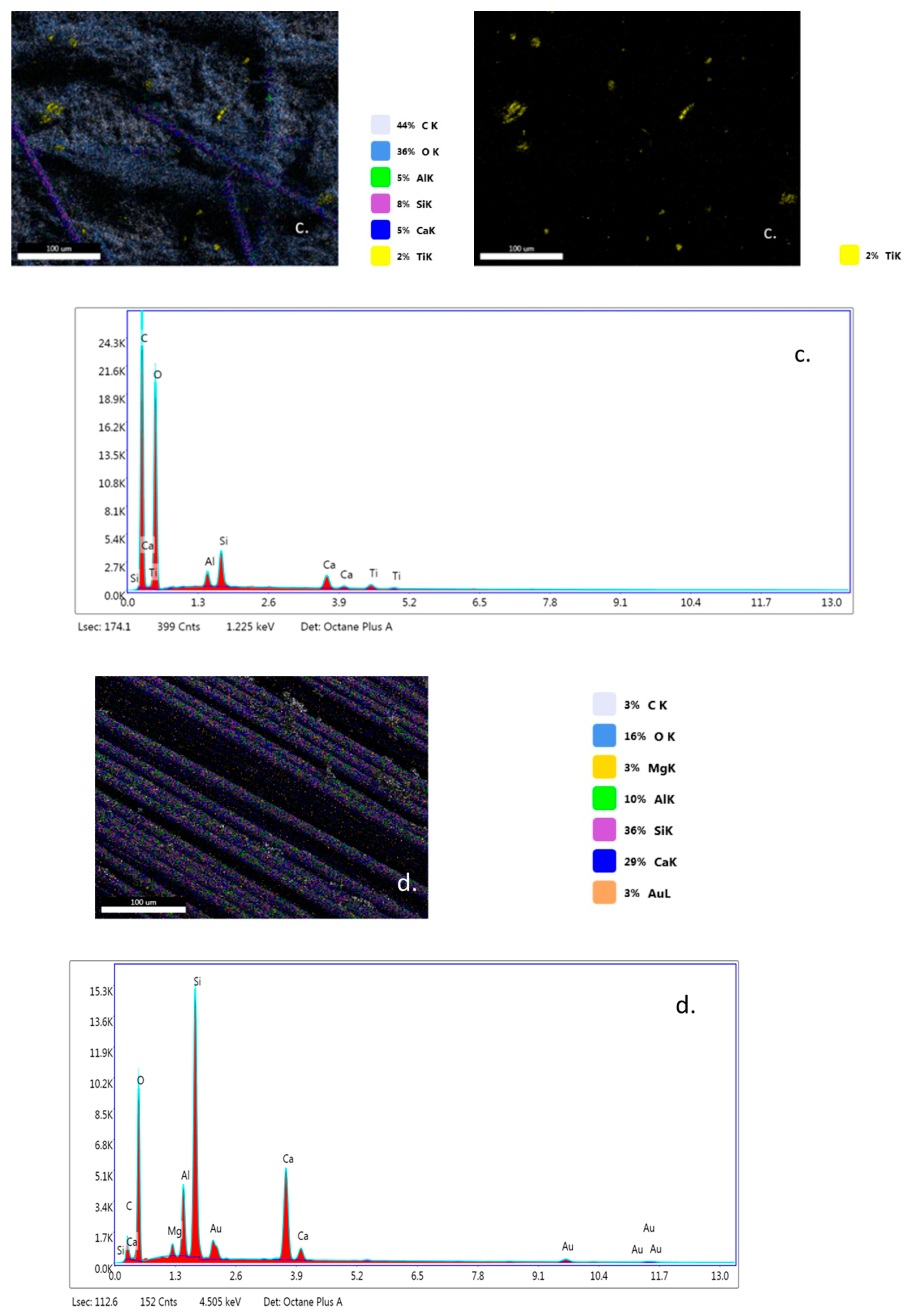
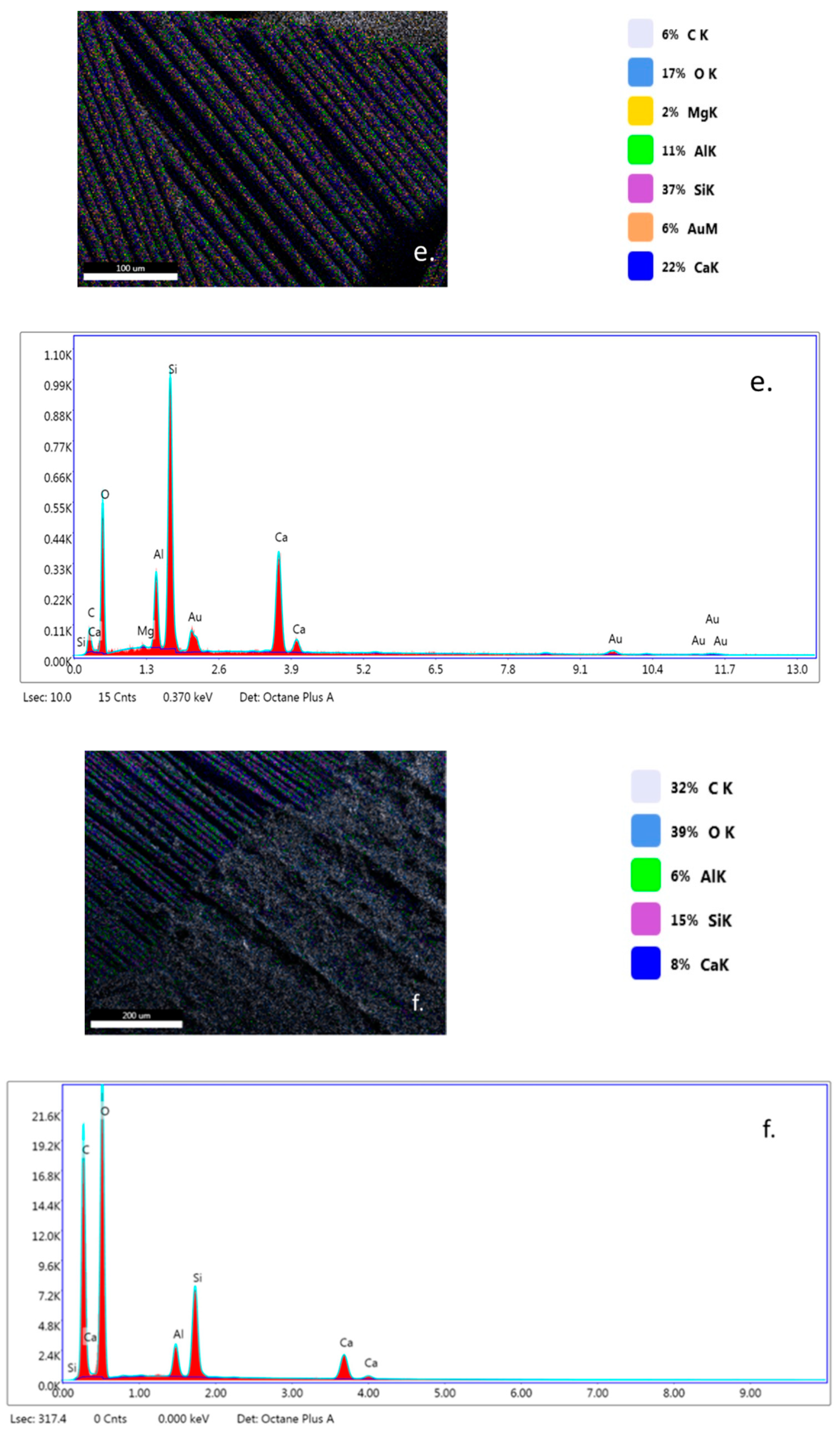
EDX analysis (Figure 7a–c) highlights that TiO2 nanoparticles were initially distributed randomly on the surface of the fibers (yellow color), due to the agglomeration of TiO2 nanoparticles onto the surface of hybrid PLA/TiO2 fiberglass membranes. After photocatalysis it is observed that all EDX spectra do not contain titanium (Ti), which may be a new evidence that shows the degradation of PLA during the photocatalytic process. If the PLA degrades during the photocatalytic process, then the TiO2 deposited on its nanofibers reaches the aqueous solution of ampicillin subjected to photocatalysis, forming a suspension whose concentration is likely to gradually decrease during the continuous recirculation of the working solution in the photocatalytic reactor. Also, the significant decrease in the carbon weight ratio (i.e., Figure 7d) may indicate the degradation of PLA.
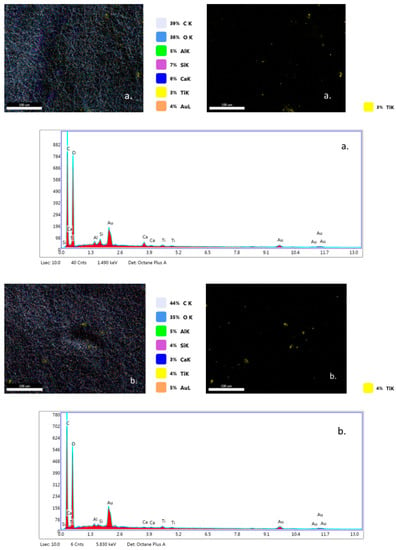
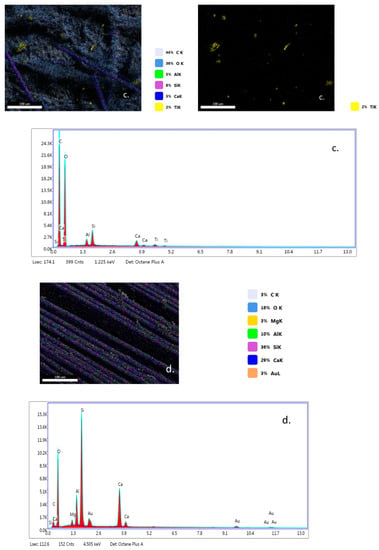
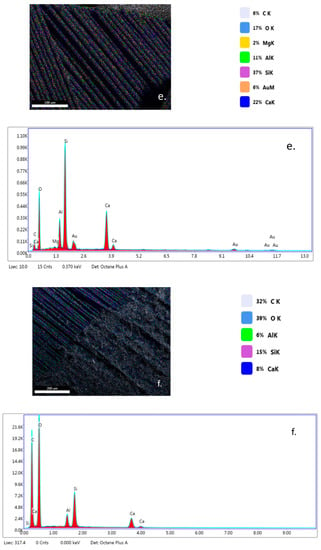
Figure 7.
Energy-dispersive X-ray (EDX) patterns for fibersglass covered with PLA/TiO2 hybrid nanofibers: (a) fabric plain woven-type (EWR); (b) fabric mat-type (EMC); (c) fabric one fold edge-type (TEX); (d) fabric plain woven-type (EWR)—after photocatalysis; (e) fabric mat-type (EMC)—after photocatalysis; (f) fabric one fold edge-type (TEX)—after photocatalysis.
3.3.2. X-ray Diffraction (XRD) Results
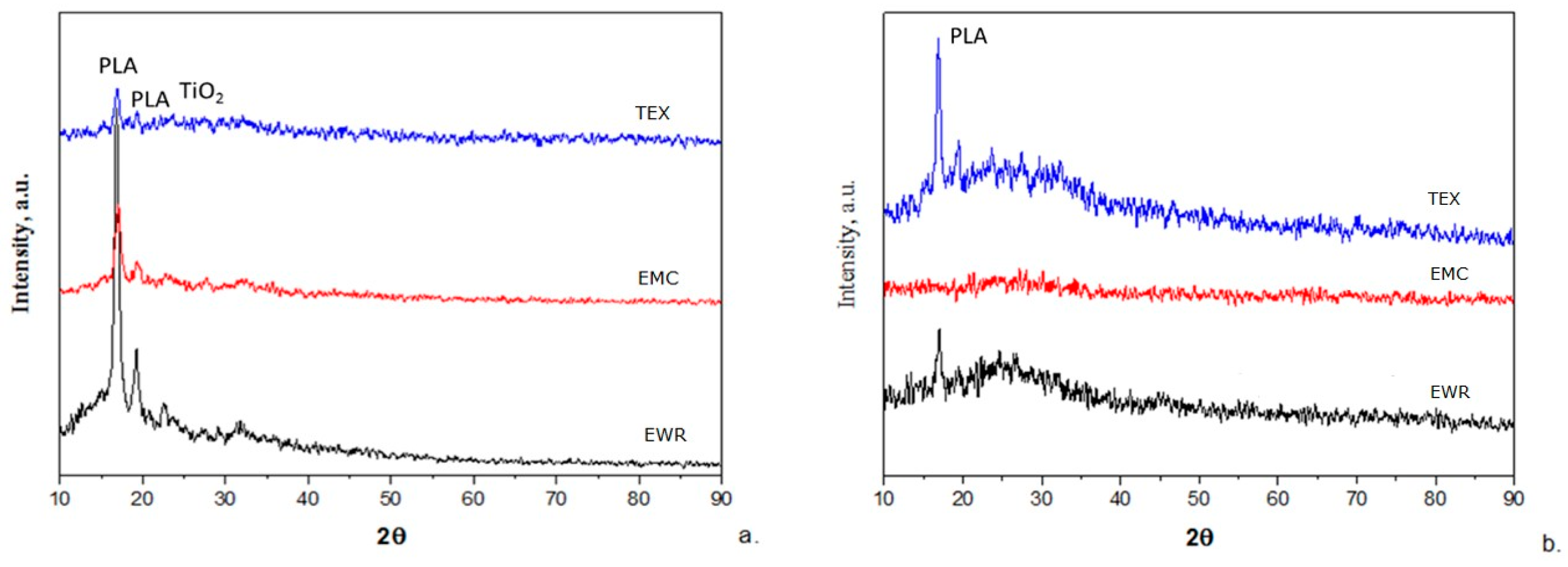
The XRD patterns of fiberglass membranes before and after photocatalysis are shown in Figure 8. The patterns of the membranes before use (Figure 8a) show a strong peak at 16.81° 2θ and one of a lower intensity at 19.23° 2θ, both being attributed to the α PLA crystals. Also, a very low intensity peak appears at 25.35° 2θ, which is attributed to TiO2 in anatase form [22]. The patterns of the membranes after photocatalysis (Figure 8b) indicate a marked decrease in the intensity of the peaks attributed to α PLA crystals (even their disappearance in the case of the fiberglass mat-type membrane) and the absence of TiO2 in anatase form. These results are consistent with those obtained from SEM–EDX and indicate the degradation of PLA during photocatalysis with the loss of TiO2 photocatalyst.
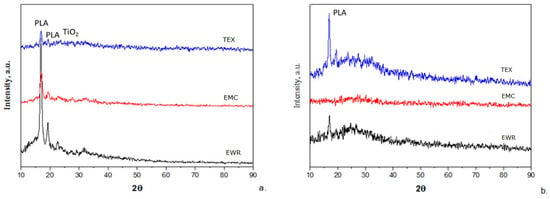
Figure 8.
X-ray diffraction (XRD) patterns of membranes: (a) before photocatalysis; (b) after photocatalysis.
3.3.3. Attenuated Total Reflectance (ATR) Fourier Transform Infrared (FT–IR) Results
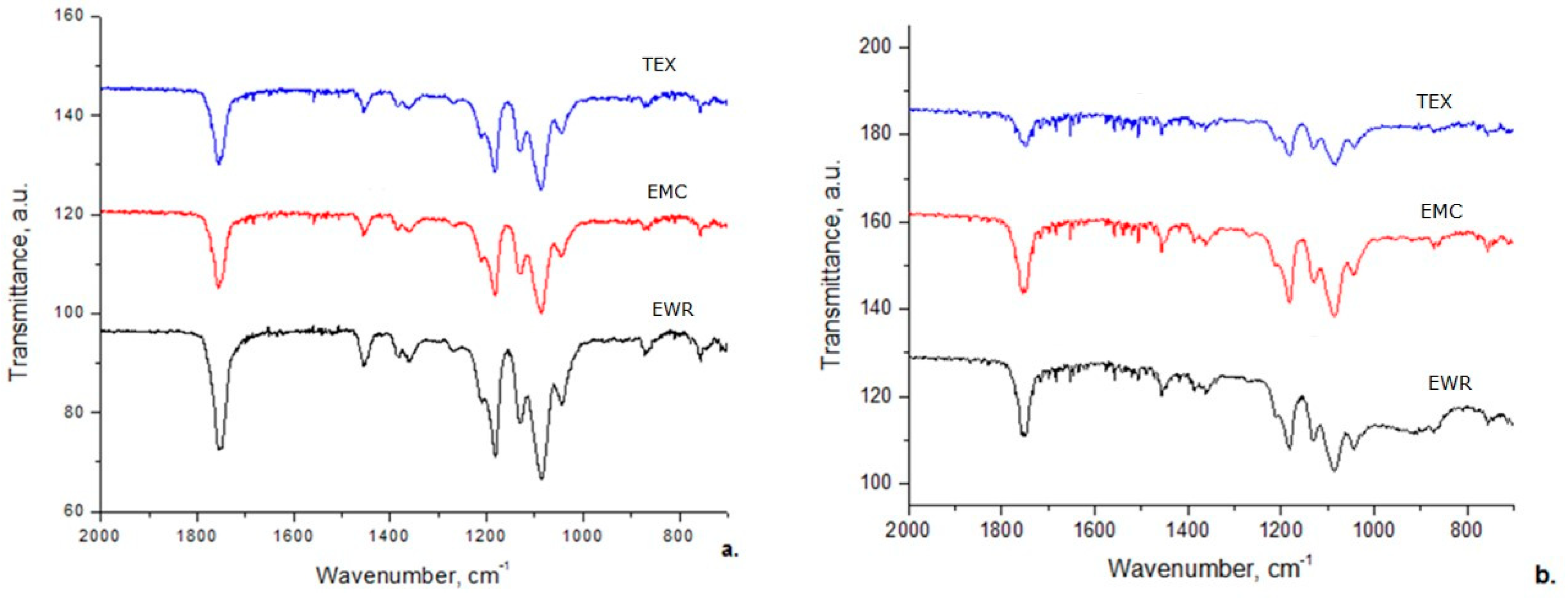
The FT–IR spectra for prepared membranes show the characteristic adsorption bands reported for PLA (Figure 9a) namely υC = O stretching vibration position of the COOR group at 1755 cm−1, –CH3 antisymmetric bending vibration at 1452 cm−1, –C–O–C stretching vibration at 1041–1181 cm−1, the amorphous and crystalline phases of PLA at 866 cm−1 and 755 cm−1, respectively [36,37].
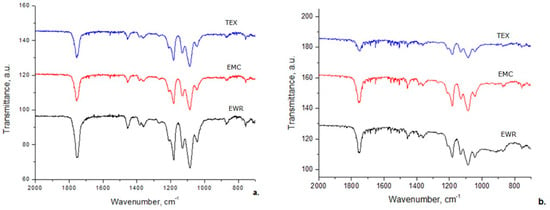
Figure 9.
Fourier transform infrared (FT–IR) spectra of membranes: (a) before photocatalysis; (b) after photocatalysis.
After photocatalytic experiments (Figure 9b) all FT–IR spectra of membranes recorded a decrease of intensity of all FT–IR adsorption bands. In addition, after photocatalytic tests, the adsorption peak from 1755 cm−1 is shifted to 1748 cm−1, demonstrating that many ester groups from membranes were destroyed and some oligomers resulted.
4. Conclusions
The purpose of the present paper was to prepare, characterize and test new photocatalytic membranes based on PLA/TiO2 hybrid nanofibers deposited on different types of fiberglass structures. The membranes obtained were tested for the removal of ampicillin from aqueous solutions by photocatalytic oxidation under predetermined working conditions. The experimental results highlighted the following:
- The kinetic results showed that after 30 min of photocatalysis almost all ampicillin is removed from the aqueous solution, the best results being recorded for fiberglass fabric plain woven-type membrane.
- PLA/TiO2 hybrid nanofibers exhibit reduced stability under the reactive conditions of the photocatalytic reactor, their instability being closely related to the type of fiberglass used as a support.
- The tendency of PLA degradation during photocatalysis, evidenced both by SEM-EDX, XRD, FT–IR and kinetic studies, has a direct effect on the photocatalytic oxidation potential of the membranes used.
- The main limitations of using PLA/TiO2 photocatalyst are the degradation of PLA under the photocatalytic conditions used (UV radiation, pH 3, hydrogen peroxide/ampicillin molar ratio of 1.5) and the need to work at low pH values compared to the pH value of the natural waters in order to obtain high degradation efficiency of the ampicillin (as previous photocatalytic studies have demonstrated the decrease of the efficiency of degradation of the organic material with the increase of the pH value of the aqueous system subjected to treatment).
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4441/12/1/176/s1: Figure S1: TL-Pro-BM Electrospinning equipment, Figure S2: Photocatalytic membranes based on PLA/TiO2 hybrid nanofibers coated on different types of fiberglass: (a) fiberglass fabric one fold edge-type membrane (EWR); (b) fiberglass mat-type membrane (EMC); (c) fiberglass fabric plain woven-type membrane (TEX), Figure S3: UV photocatalytic reactor.
Author Contributions
Conceptualization, C.O. and E.M.; methodology, C.O., C.B., and E.M.; investigation, C.B., L.B., M.R., and A.M.P.; writing—original draft preparation, C.B. and M.R. All authors reviewed and edited the article. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a grant of the Romanian Ministry of Research and Innovation, CCCDI-UEFISCDI, project number 26PCCDI/01.03.2018, “Integrated and sustainable processes for environmental clean-up, wastewater reuse and waste valorization” (SUSTENVPRO), within PNCDI III and by POC Program, Grant no. 49/05.09.2016, Project ID P_37_649. The FTIR analysis (on INTERSPEC 200-X Spectrophotometer) was possible due to European Regional Development Fund through Competitiveness Operational Program 2014–2020, Priority axis 1, Project No. P_36_611, MySMIS code 107066, Innovative Technologies for Materials Quality Assurance in Health, Energy and Environmental—Center for Innovative Manufacturing Solutions of Smart Biomaterials and Biomedical Surfaces—INOVABIOMED.
Acknowledgments
The authors recognize the support of Ignazio Renato Bellobono, R&D Team Leader of B.I.T. s.r.l., Milan (Italy), who generously offered the testing installation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pazda, M.; Kumirska, J.; Stepnowski, P.; Mulkiewicz, E. Antibiotic resistance genes identified in wastewater treatment plant systems—A review. Sci. Total Environ. 2019, 697, 134023. [Google Scholar] [CrossRef] [PubMed]
- Osińska, A.; Korzeniewska, E.; Harnisz, M.; Felis, E.; Bajkacz, S.; Jachimowicz, P.; Niestępski, S.; Konopka, I. Small-scale wastewater treatment plants as a source of the dissemination of antibiotic resistance genes in the aquatic environment. J. Hazard. Mater. 2020, 381, 121221. [Google Scholar] [CrossRef] [PubMed]
- Voigt, A.M.; Zacharias, N.; Timm, C.; Wasser, F.; Sib, E.; Skutlarek, D.; Parcina, M.; Schmithausen, R.M.; Schwartz, T.; Hembach, N.; et al. Association between antibiotic residues, antibiotic resistant bacteria and antibiotic resistance genes in anthropogenic wastewater—An evaluation of clinical influences. Chemosphere 2020, 241, 125032. [Google Scholar] [CrossRef]
- Almakki, A.; Jumas-Bilak, E.; Marchandin, H.; Licznar-Fajardo, P. Antibiotic resistance in urban runoff. Sci. Total Environ. 2019, 667, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ai, S.; Zhou, Y.; Luo, Z.; Dai, C.; Yang, Y.; Zhang, J.; Huang, H.; Luo, S.; Luo, L. Adsorption of agricultural wastewater contaminated with antibiotics, pesticides and toxic metals by functionalized magnetic nanoparticles. J. Environ. Chem. Eng. 2018, 6, 6468–6478. [Google Scholar] [CrossRef]
- Nasseh, N.; Barikbin, B.; Taghavi, L.; Nasseri, M.A. Adsorption of metronidazole antibiotic using a new magnetic nanocomposite from simulated wastewater (isotherm, kinetic and thermodynamic studies). Compos. Part B Eng. 2019, 159, 146–156. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, J.; Fang, W.; Xu, M.; Qin, Z.; Jiang, Z.; Shangguan, W. Photocatalytic hydrogen evolution with simultaneous antibiotic wastewater degradation via the visible-light-responsive bismuth spheres-g-C3N4 nanohybrid: Waste to energy insight. Chem. Eng. J. 2019, 358, 944–954. [Google Scholar] [CrossRef]
- Fiorentino, A.; Esteban, B.; Garrido-Cardenas, J.A.; Kowalska, K.; Rizzo, L.; Aguera, A.; Pérez, J.A.S. Effect of solar photo-Fenton process in raceway pond reactors at neutral pH on antibiotic resistance determinants in secondary treated urban wastewater. J. Hazard. Mater. 2019, 378, 120737. [Google Scholar] [CrossRef]
- Leng, L.; Wei, L.; Xiong, Q.; Xu, S.; Li, W.; Lv, S.; Lu, Q.; Wan, L.; Wen, Z.; Zhou, W. Use of microalgae based technology for the removal of antibiotics from wastewater: A review. Chemosphere 2020, 238, 124680. [Google Scholar] [CrossRef]
- Kebede, T.G.; Dube, S.; Nindi, M.M. Removal of multi-class antibiotic drugs from wastewater using water-soluble protein of Moringa stenopetala seeds. Water 2019, 11, 595. [Google Scholar] [CrossRef]
- Hou, J.; Chen, Z.; Gao, J.; Xie, Y.; Li, L.; Qin, S.; Wang, Q.; Mao, D.; Luo, Y. Simultaneous removal of antibiotics and antibiotic resistance genes from pharmaceutical wastewater using the combinations of up-flow anaerobic sludge bed, anoxic-oxic tank, and advanced oxidation technologies. Water Res. 2019, 159, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Phan, T.-D.; Shin, E.W. Morphological effect of TiO2 catalysts on photocatalytic degradation of methylene blue. J. Ind. Eng. Chem. 2011, 17, 397–400. [Google Scholar] [CrossRef]
- Jiménez-Tototzintle, M.; Ferreira, I.J.; da Silva Duque, S.; Barrocas, P.R.G.; Saggioro, E.M. Removal of contaminants of emerging concern (CECs) and antibiotic resistant bacteria in urban wastewater using UVA/TiO2/H2O2 photocatalysis. Chemosphere 2018, 210, 449–457. [Google Scholar] [CrossRef]
- Biancullo, F.; Moreira, N.F.F.; Ribeiro, A.R.; Manaia, C.M.; Faria, J.L.; Nunes, O.C.; Castro-Silva, S.M.; Silva, A.M.T. Heterogeneous photocatalysis using UVA-LEDs for the removal of antibiotics and antibiotic resistant bacteria from urban wastewater treatment plant effluents. Chem. Eng. J. 2019, 367, 304–313. [Google Scholar] [CrossRef]
- Moreira, N.F.F.; Narciso-da-Rocha, C.; Polo-López, M.I.; Pastrana-Martínez, L.M.; Faria, J.L.; Manaia, C.M.; Fernández-Ibáñez, P.; Nunes, O.C.; Silva, A.M.T. Solar treatment (H2O2, TiO2-P25 and GO-TiO2 photocatalysis, photo-Fenton) of organic micropollutants, human pathogen indicators, antibiotic resistant bacteria and related genes in urban wastewater. Water Res. 2018, 135, 195–206. [Google Scholar] [CrossRef]
- Han, D.; Steckl, A.J. Coaxial electrospinning formation of complex polymer fibers and their applications. Chempluschem 2019, 84, 1453–1497. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.H.; Kim, J.Y.; Kim, S.S. Synthesis of aligned TiO2 nanofibers using electrospinning. Appl. Sci. 2018, 8, 309. [Google Scholar]
- Tekmen, C.; Suslu, A.; Cocen, U. Titania nanofibers prepared by electrospinning. Mater. Lett. 2008, 62, 4470–4472. [Google Scholar] [CrossRef]
- Park, J.Y.; Choi, S.W.; Asokan, K.; Kim, S.S. Controlling the size of nanograins in TiO2 nanofibers. Met. Mater. Int. 2010, 16, 785–788. [Google Scholar] [CrossRef]
- Liu, M.; Cheng, Z.; Yan, J.; Qiang, L.; Ru, X.; Liu, F.; Ding, D.; Li, J. Preparation and characterization of TiO2 nanofibers via using polylactic acid as template. J. Appl. Polym. Sci. 2013, 128, 1095–1100. [Google Scholar] [CrossRef]
- Wang, Z.; Pan, Z.; Wang, J.; Zhao, R. A novel hierarchical structured poly(lactic acid)/titania fibrous membrane with excellent antibacterial activity and air filtration performance. J. Nanomater. 2016, 2016, 39. [Google Scholar] [CrossRef]
- Costa, R.G.F.; Brichi, G.S.; Ribeiro, C.; Mattoso, L.H.C. Nanocomposite fibers of poly(lactic acid)/titanium dioxide prepared by solution blow spinning. Polym. Bull. 2016, 73, 2973–2985. [Google Scholar] [CrossRef]
- Tarcea, C.I.; Predescu, C.; Matei, E.; Predescu, A.M.; Rapa, M.; Turcanu, A. Use of nano-anatase in degradation of heterocyclic dyes from waters. UPB Sci. Bull. Ser. B 2019, 81, 169–178. [Google Scholar]
- Abou-Elela, S.I.; El-Khateeb, M.A. Performance evaluation of activated sludge process for treating pharmaceutical wastewater contaminated with β-lactam antibiotics. J. Ind. Pollut. Control 2015, 31, 1–5. [Google Scholar]
- American Public Health Association; American Water Works Association; Water Environment Federation. Standard Methods for the Examination of Water and Wastewater, 18th ed.; American Public Health Association: Washington, DC, USA, 1992. [Google Scholar]
- Orbeci, C.; Modrogan, C.; Dăncilă, A.M. Degradation of pharmaceutical effluents by photo-assisted techniques. Rev. Chim. 2016, 67, 166–170. [Google Scholar]
- Man, C.; Zhang, C.; Liu, Y.; Wang, W.; Ren, W.; Jiang, L.; Reisdorffer, F.; Nguyen, T.P.; Dan, Y. Poly (lactic acid)/titanium dioxide composites: Preparation and performance under ultraviolet irradiation. Polym. Degrad. Stab. 2012, 97, 856–862. [Google Scholar] [CrossRef]
- Zaharescu, T.; Rapa, M.; Marinescu, V. Chemiluminescence kinetic analysis on the oxidative degradation of poly(lactic acid). J. Therm. Anal. Calorim. 2017, 128, 185–191. [Google Scholar] [CrossRef]
- Lee, K.M.; Abd Hamid, S.B.; Lai, C.W. Mechanism and kinetics study for photocatalytic oxidation degradation: A case study for phenoxyacetic acid organic pollutant. J. Nanomater. 2015, 2015, 9. [Google Scholar] [CrossRef]
- Ollis, D. Kinetic analysis of liquid phase photocatalysis and photolysis: A frequent disguise! Catal. Today 2020, 340, 7–11. [Google Scholar] [CrossRef]
- Elmolla, E.S.; Chaudhuri, M. Photocatalytic degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution using UV/TiO2 and UV/H2O2/TiO2 photocatalysis. Desalination 2010, 252, 46–52. [Google Scholar] [CrossRef]
- Elmolla, E.S.; Chaudhuri, M. Degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution by the UV/ZnO photocatalytic process. J. Hazard. Mater. 2010, 173, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Alalm, M.G.; Tawfik, A.; Ookawara, S. Enhancement of photocatalytic activity of TiO2 by immobilization on activated carbon for degradation of pharmaceuticals. J. Environ. Chem. Eng. 2016, 4, 1929–1937. [Google Scholar] [CrossRef]
- Sharma, G.; Gupta, V.K.; Agarwal, S.; Bhogal, S.; Naushad, M.; Kumar, A.; Stadler, F.J. Fabrication and characterization of trimetallic nano-photocatalyst for remediation of ampicillin antibiotic. J. Mol. Liq. 2018, 260, 342–350. [Google Scholar] [CrossRef]
- Raizada, P.; Kumari, J.; Shandilya, P.; Dhiman, R.; Singh, V.P.; Singh, P. Magnetically retrievable Bi2WO6/Fe3O4 immobilizedon graphene sand composite for investigation of photocatalytic mineralization of oxytetracycline and ampicillin. Process Saf. Environ. Prot. 2017, 106, 104–116. [Google Scholar] [CrossRef]
- Rapa, M.; Stefan, L.M.; Preda, P.; Darie-Nita, R.N.; Gaspar-Pintiliescu, A.; Seciu, A.M.; Vasile, C.; Matei, E.; Predescu, A.M. Effect of hydrolyzed collagen on thermal, mechanical and biological properties of poly(lactic acid) bionanocomposites. Iran. Polym. J. 2019, 28, 271–282. [Google Scholar] [CrossRef]
- Ramos, M.; Fortunati, E.; Peltzer, M.; Dominici, F.; Jiménez, A.; Garrigós, M.C.; Kenny, J.M. Influence of thymol and silver nanoparticles on the degradation of poly (lactic acid) based nanocomposites: Thermal and morphological properties. Polym. Degrad. Stab. 2014, 108, 158–165. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).