The Quality of Stored Rainwater for Washing Purposes
Abstract
:1. Introduction
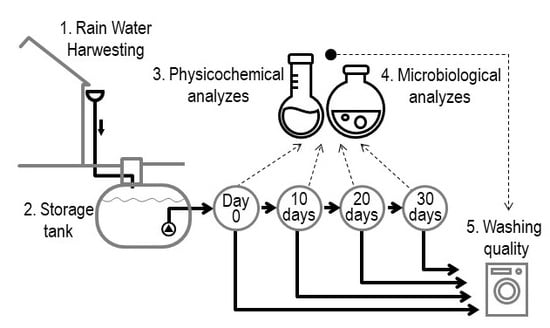
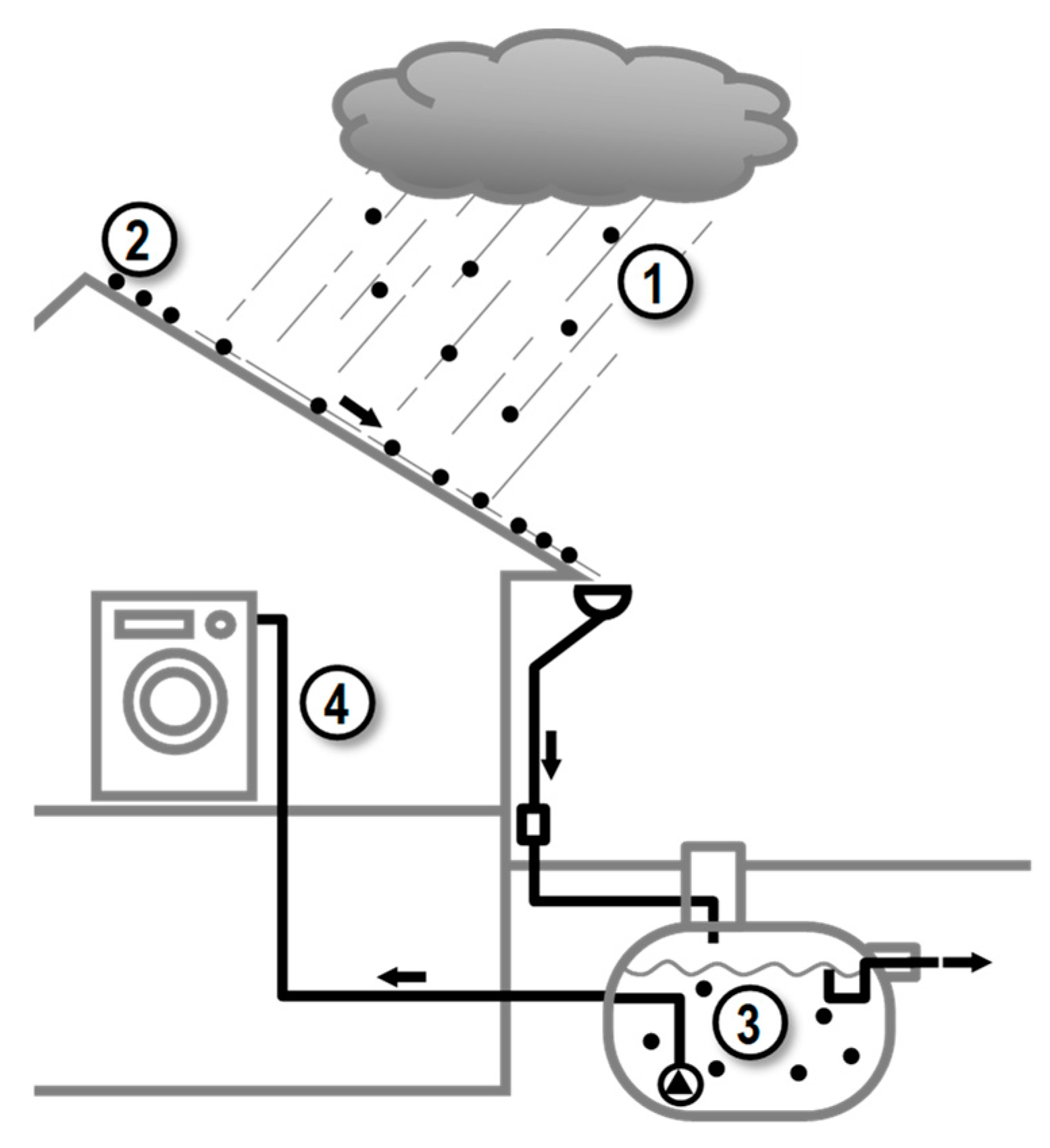
2. Materials and Methods
3. Results
3.1. Weather Conditions
3.2. Rainwater
4. Conclusions
- ▪
- The stored rainwater contains pollutants flushed from roofing and atmosphere. This is in accordance with the research results available in literature. The detected contaminants have been classified as safe for the process and quality of washing clothes.
- ▪
- The multi-day storage of rainwater process changes water parameters in a safe range. It remains of good quality and is suitable for washing clothes. Underground tanks which are closed in a safe way can store water and help to maintain its good quality.
- ▪
- Storage of water in the RWH system for 30 days was observed: Conductivity has more than doubled with the biggest drop occurring between 10 and 20 days. An inverse regularity was observed in the case of TDS and TSS whose highest increase occurred in the first 10 days after rainfall and between 20 and 30 days of storage respectively. This indicates changes in the composition of water stored in the RWH system in the underground tank, but these changes do not cause a critical deterioration in water quality. The stored water still corresponds to the quality of water intended for human consumption except for the microbiological parameters TVC of heterotrophic bacteria incubated in 22 °C and 37 °C (CFU/mL) which do not disqualify the water as suitable for laundry use.
- ▪
- During the entire test period the water met BWD requirements which confirms its suitability for washing.
- ▪
- Throughout the entire test period the tested water met the general requirements of three factors of stored rainwater suitability for washing purposes (Figure 2) guaranteeing safety and good washing quality. The indicated parameters of water affecting the quality of washing such as hardness, pH, color, turbidity met the requirements and did not change adversely during the test period.
- ▪
- The results indicated that harvested and stored rainwater can be directly used for washing purposes even after 30 days of storage. The experiment carried out and the results obtained prove that disinfection process is not necessary.
- ▪
- The ecological and economic benefits of using rainwater for laundry should be highlighted in terms of reducing the use of tap water and washing detergents.
- ▪
- Based on the experiment results the myth of a drastic decrease in rainwater quality during storage can finally be dispelled. Appropriate design and materials of RWH system guarantees the maintenance of its quality, prevents water bloom and mosquitoes from growing. The favorable factor is low temperature in the underground tank. We are convinced that that the results will dispel doubts and myths about inferior rainwater quality which will result in the growing popularity of such solutions in Poland.
- ▪
- For the safe use of collected rainwater on a large scale a legal framework for water quality and RWH systems design is needed.
Author Contributions
Funding
Conflicts of Interest
References
- García-Montoya, M.; Bocanegra-Martínez, A.; Nápoles-Rivera, F.; Serna-González, M.; Ponce-Ortega, J.M.; El-Halwagi, M.M. Simultaneous design of water reusing and rainwater harvesting systems in a residential complex. Comput. Chem. Eng. 2015, 76, 104–116. [Google Scholar] [CrossRef]
- Velasco-Muñoz, J.F.; Aznar-Sánchez, J.A.; Batlles-delaFuente, A.; Fidelibus, M.D. Rainwater Harvesting for Agricultural Irrigation: An Analysis of Global Research. Water 2019, 11, 1320. [Google Scholar] [CrossRef] [Green Version]
- Steffen, J.; Jensen, M.; Pomeroy, C.A.; Burian, S.J. Water supply and stormwater management benefits of residential rainwater harvesting in U.S. cities. J. Am. Water Resour. Assoc. 2012, 49, 810–824. [Google Scholar] [CrossRef]
- Wanjiru, E.; Xia, X. Sustainable energy-water management for residential houses with optimal integrated grey and rain water recycling. J. Clean. Prod. 2018, 170, 1151–1166. [Google Scholar] [CrossRef] [Green Version]
- Stec, A.; Zeleňáková, M. An Analysis of the Effectiveness of Two Rainwater Harvesting Systems Located in Central Eastern Europe. Water 2019, 11, 458. [Google Scholar] [CrossRef] [Green Version]
- Ghaffarian Hoseini, A.; Tookey, J.; Ghaffarian Hoseini, A.; Yusoff, S.M.; Hassan, N.B. State of the art of rainwater harvesting systems towards promoting green built environments: A review Desalinat. Water Treat. 2016, 57, 95–104. [Google Scholar] [CrossRef]
- Chun Ding, G.K. Recycling and Reuse of Rainwater and Stormwater, Reference Module in Earth Systems and Environmental Sciences. Encycl. Sustain. Technol. 2017, 69–76. [Google Scholar] [CrossRef]
- Yannopoulos, S.; Giannopoulou, I.; Kaiafa-Saropoulou, M. Investigation of the Current Situation and Prospects for the Development of Rainwater Harvesting as a Tool to Confront Water Scarcity Worldwide. Water 2019, 11, 2168. [Google Scholar] [CrossRef] [Green Version]
- Freni, G.; Liuzzo, L. Effectiveness of Rainwater Harvesting Systems for Flood Reduction in Residential Urban Areas. Water 2019, 11, 1389. [Google Scholar] [CrossRef] [Green Version]
- Teston, A.; Teixeira, C.A.; Ghisi, E.; Cardoso, E.B. Impact of Rainwater Harvesting on the Drainage System: Case Study of a Condominium of Houses in Curitiba, Southern Brazil. Water 2018, 10, 1100. [Google Scholar] [CrossRef] [Green Version]
- Amos, C.C.; Rahman, A.; Gathenya, J.M. Economic Analysis and Feasibility of Rainwater 871 Harvesting Systems in Urban and Peri-Urban Environments: A Review of the Global Situation with 872 a Special Focus on Australia and Kenya. Water 2016, 8, 149. [Google Scholar] [CrossRef]
- Gilliom, R.L.; Bell, C.D.; Hogue, T.S.; McCray, J.E. A Rainwater Harvesting Accounting Tool for Water Supply Availability in Colorado. Water 2019, 11, 2205. [Google Scholar] [CrossRef] [Green Version]
- Campisano, A.; Butler, D.; Ward, S.; Burns, M.J.; Friedler, E.; DeBusk, K.; Fisher-Jeffes, L.N.; Ghisi, E.; Rahman, A.; Furumai, H.; et al. Urban rainwater harvesting systems: Research, implementation and future perspectives. Water Res. 2017, 115, 195–209. [Google Scholar] [CrossRef]
- Devkota, J.; Schlachter, H.; Apul, D. Life cycle based evaluation of harvested rainwater use in toilets and for irrigation. J. Clean. Prod. 2015, 95, 311–321. [Google Scholar] [CrossRef]
- Morales-Pinzón, T.; Lurueña, R.; Gabarrell, X.; Gasol, C.M.; Rieradevall, J. Financial and environmental modelling of water hardness—Implications for utilising harvested rainwater in washing machines. Sci. Total Environ. 2014, 470–471, 1257–1271. [Google Scholar] [CrossRef]
- Melville-Shreeve, P.; Ward, S.; Butler, D. Developing a methodology for appraising rainwater harvesting with integrated source control using a case study from south-west England. In Proceedings of the 13th International Conference on Urban Drainage (ICUD2014), Kuching, Malaysia, 7–12 September 2014; IWA: Publishing, UK, 2014. [Google Scholar]
- Schuetze, T. Rainwater harvesting and management—policy and regulations in Germany. Water Sci. Technol. 2013, 13, 376–385. [Google Scholar] [CrossRef]
- Domènech, L.; Saurí, D. A comparative appraisal of the use of rainwater harvesting in single and multi-family buildings of the Metropolitan Area of Barcelona (Spain): Social experience, drinking water savings and economic costs. J. Clean. Prod. 2011, 19, 598–608. [Google Scholar] [CrossRef]
- De Gouvello, B.; Gerolin, A.; Le Nouveau, N. Rainwater harvesting in urban areas: How can foreign experiences enhance the French approach? Water Sci. Technol. 2014, 14, 569–576. [Google Scholar] [CrossRef] [Green Version]
- UNI (Italian National Unification). Installations for the Collection and Use of Rainwater for Uses other than Human Consumption–Design, Installation and Maintenance; Guideline; UNI/TS: Trieste, Italy, 2012. (In Italian) [Google Scholar]
- Godskesen, B.; Hauschild, M.; Rygaard, M.; Zambrano, K.; Albrechtsen, H.J. Life-cycle and freshwater withdrawal impact assessment of water supply technologies. Water Res. 2013, 47, 2363–2374. [Google Scholar] [CrossRef] [Green Version]
- Iveroth, S.P.; Johansson, S.; Brandt, N. The potential of the infrastructural system of Hammerby Sjöstad in Stockholm, Sweden. Energ. Policy 2013, 59, 716–726. [Google Scholar] [CrossRef]
- Ringelstein, O. Now we can shower with Rain Water. GWF Wasser-Abwasser 2015, 156, 58–61. [Google Scholar]
- Umapathi, S.; Pezzaniti, D.; Beecham, S.; Whaley, D.; Sharma, A. Sizing of Domestic Rainwater Harvesting Systems Using Economic Performance Indicators to Support Water Supply Systems. Water 2019, 11, 783. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, T.; Abbasi, S.A. Sources of pollution in rooftop rainwater harvesting systems and their control. Crit. Rev. Environ. Sci. Technol. 2011, 41, 2097–2167. [Google Scholar] [CrossRef]
- Gee, K.; Hunt, W. Enhancing stormwater management benefits of rainwater harvesting via innovative technologies. J. Environ. Eng. 2016, 142, 04016039. [Google Scholar] [CrossRef]
- DIN (Deutsches Institut für Normung). Rainvater Harvesting; Guideline; DIN: Berlin, Germany, 2001. (In German) [Google Scholar]
- BS (British Standards). Rainwater Harvesting Systems-Code of Practice; Guideline; BS: London, UK, 2009. [Google Scholar]
- Council directive. Drinking Water Directive on the quality of water intended for human consumption. 98/83/EC. Off. J. Eur. Commun. 1998, 330, 32–54. [Google Scholar]
- Bathing Water Directive concerning the management of bathing water quality. In Directive of the European Parlament and of the Concil; European Environment Agency: Copenhagen, Denmark, 2006; 2006/7/EC.
- Lee, J.Y.; Yang, J.S.; Han, M.; Choi, J. Comparison of the microbiological and chemical characterization of harvested rainwater and reservoir water as alternative water resources. Sci. Total Environ. 2010, 408, 896–905. [Google Scholar] [CrossRef]
- Sammut, G.; Sinagra, E.; Helmus, R.; de Voogt, P. Perfluoroalkyl substances in the Maltese environment—(I) surface water and rain water. Sci. Total Environ. 2017, 589, 182–190. [Google Scholar] [CrossRef]
- Al-Batsh, N.; Al-Khatib, I.A.; Ghannam, S.; Anayah, F.; Jodeh, S.; Hanbali, G.; Khalaf, B.; Van der Valk, M. Assessment of Rainwater Harvesting Systems in Poor Rural Communities: A Case Study from Yatta Area, Palestine. Water 2019, 11, 585. [Google Scholar] [CrossRef] [Green Version]
- Hofman-Caris, R.; Bertelkamp, C.; de Waal, L.; van den Brand, T.; Hofman, J.; van der Aa, R.; van der Hoek, J.P. Rainwater Harvesting for Drinking Water Production: A Sustainable and Cost-Effective Solution in The Netherlands? Water 2019, 11, 511. [Google Scholar] [CrossRef] [Green Version]
- Al-Khatib, I.A.; Arafeh, G.A.; Al-Qutob, M.; Jodeh, S.; Hasan, A.R.; Jodeh, D.; van der Valk, M. Health Risk Associated with Some Trace and Some Heavy Metals Content of Harvested Rainwater in Yatta Area, Palestine. Water 2019, 11, 238. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, A.S.; Cohim, E.; Kalid, R.A. A review on physicochemical and microbiological contamination of roof-harvested rainwater in urban areas. Sustain. Water Qual. Ecol. 2015, 6, 119–137. [Google Scholar] [CrossRef]
- Leong, J.Y.C.; Oh, K.S.; Poh, P.E.; Chong, M.N. Prospects of hybrid rainwater-greywater decentralised system for water recycling and reuse: A review. J. Clean. Prod. 2017, 142, 3014–3027. [Google Scholar] [CrossRef]
- World Health Organization. Regulation on the Quality of Water Intended for Human Consumption, Regulation of the Polish Minister of Health; Panthera Design: Tappernøje, Denmark, 2017; 2017/2294. (In Polish) [Google Scholar]
- World Health Organization (WHO). Guideline for Drinking Water Quality, 3rd ed.; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Yaziz, M.I.; Gunting, H.; Sapari, N.; Ghazali, A.W. Variations in rainwater quality from roof catchments. Water Res. 1989, 23, 761–765. [Google Scholar] [CrossRef]
- Simmons, G.; Hope, V.; Lewis, G.; Whitmore, J.; Gao, W. Contamination of potable roof-collected rainwater in Auckland, New Zealand. Water Res. 2001, 35, 1518–1524. [Google Scholar] [CrossRef]
- Huston, R.; Chan, Y.; Gardner, T.; Shaw, G.; Chapman, H. Characterisation of atmospheric deposition as a source of contaminants in urban rainwater tanks. Water Res. 2009, 43, 1630–1640. [Google Scholar] [CrossRef]
- Magyar, M.; Ladson, A.; Mitchell, V.; Diaper, C. The effect of rainwater tank design on sediment re-suspension and subsequent outlet water quality. Aust. J. Water Resour. 2011, 15, 71–84. [Google Scholar] [CrossRef]
- Huston, R.; Chan, Y.; Chapman, H.; Gardner, T.; Shaw, G. Source apportionment of heavy metals and ionic contaminants in rainwater tanks in a subtropical urban area in Australia. Water Res. 2012, 46, 1121–1132. [Google Scholar] [CrossRef]
- Meera, V.; Ahammed, M.M. Water quality of rooftop rainwater harvesting systems: A review. J. Water Supply Res. Technol. 2006, 55, 257–268. [Google Scholar] [CrossRef]
- Daoud, A.K.; Swaileh, K.M.; Hussein, R.M.; Matani, M. Quality assessment of roof harvested rainwater in West Bank, Palestinian Authority. J. Water Health 2011, 9, 525–533. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.K.; Grant, A.L.; Grant, T.; Pamminger, F.; Opray, L. Environmental and economic assessment of urban water services for a green field development. Environ. Eng. Sci. 2009, 26, 921–934. [Google Scholar] [CrossRef]







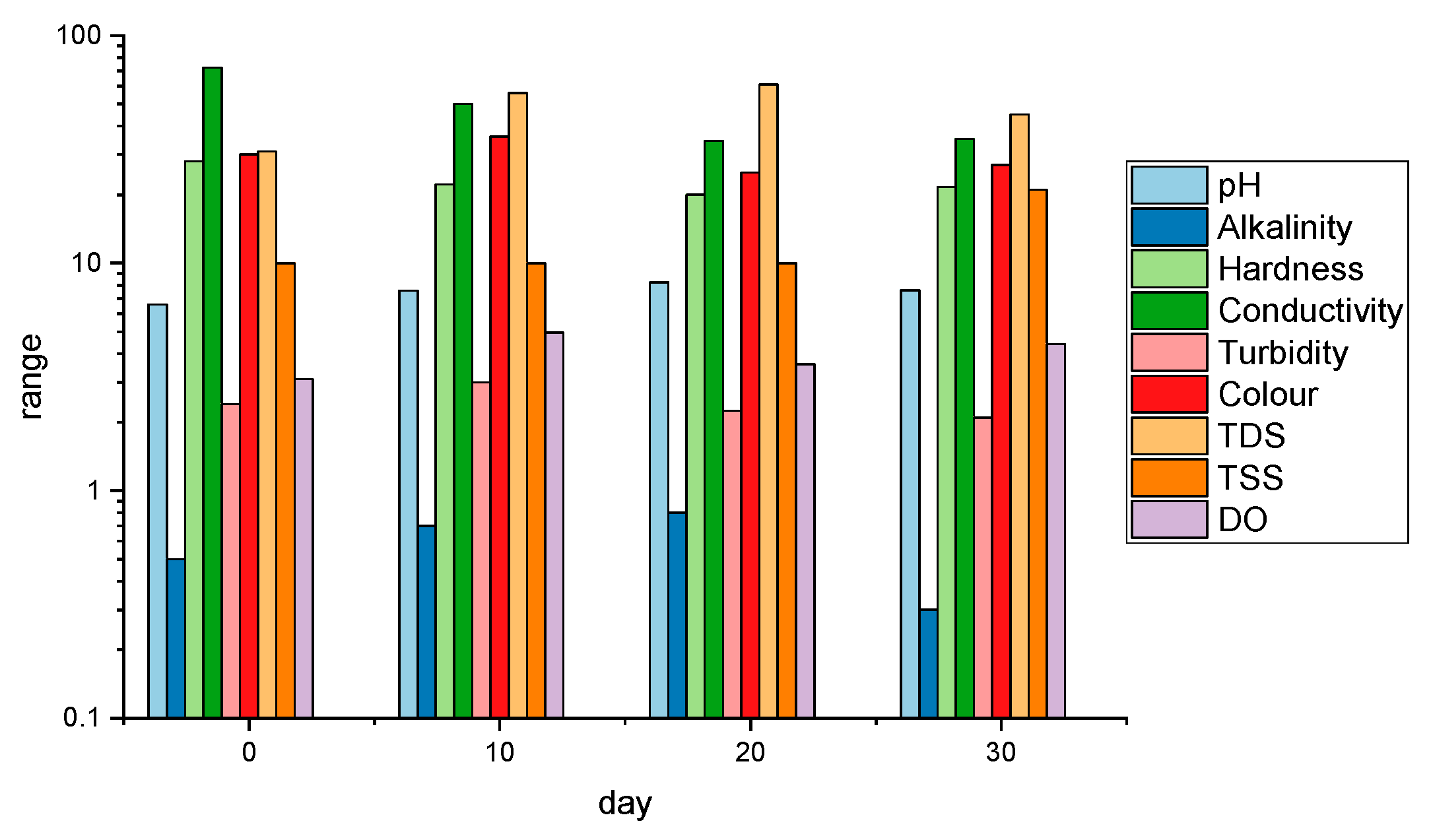
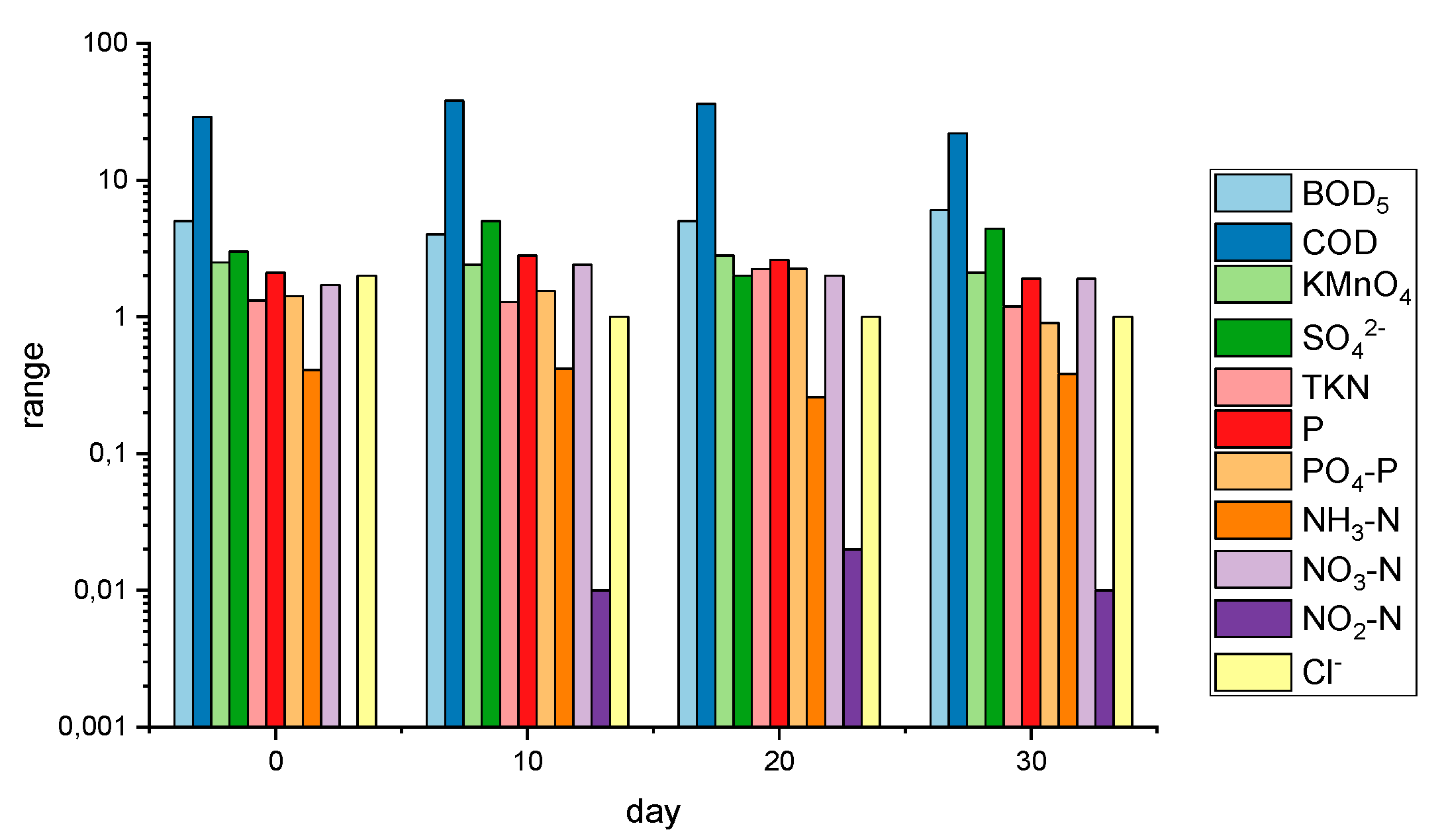



| Parameter | Unit | After … Days | DWS [38] | WHO [39] | DWD [29] | BWD [30] | |||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | ||||||
| Water temperature each time it was sampled | °C | 13.0 | 13.5 | 12.3 | 11.8 | – | – | – | – |
| pH | - | 6.60 | 7.58 | 8.26 | 7.61 | 6.5–9.5 | 6.5–8.5 | 6.5–9.5 | – |
| Alkalinity | mg·dm−3 | 0.50 | 0.70 | 0.80 | 0.30 | – | – | – | – |
| Hardness | mg·dm−3 | 28.02 | 22.16 | 20.02 | 21.60 | 60.0–500.0 | – | – | – |
| Conductivity at 25 °C | µS·cm−1 | 72.40 | 50.00 | 34.40 | 35.16 | 2500.0 | 2000.0 | 2500.0 at 20 °C | – |
| Turbidity | NTU | 2.40 | 3.00 | 2.25 | 2.10 | 1.0 | 5.0 | acceptable | – |
| Colour | Pt-Co | 30.00 | 36.00 | 25.00 | 27.00 | acceptable | – | acceptable | – |
| Total dissolved solids (TDS) | mg·dm−3 | 31.00 | 56.00 | 61.00 | 45.00 | – | 500.0 | – | – |
| Total suspended solids (TSS) | mg·dm−3 | 10.00 | 10.00 | 10.00 | 21.00 | – | – | – | – |
| Dissolved oxygen (DO) | mg·dm−3 | 3.10 | 4.96 | 3.60 | 4.41 | – | – | – | – |
| Biological Oxygen Demand (BOD5) | mg·dm−3 | 5.00 | 4.00 | 5.00 | 6.00 | – | – | – | – |
| Chemical Oxygen Demand (COD) | mg·dm−3 | 29.00 | 38.00 | 36.00 | 22.00 | – | – | – | – |
| Oxidizable (KMnO4) | mg·dm−3 | 2.50 | 2.40 | 2.80 | 2.10 | 5.0 | – | 5.0 | – |
| Sulfate (SO42−) | mg·dm−3 | 3.00 | 5.00 | 2.00 | 4.40 | 250.0 | – | 250.0 | – |
| Total Kiedjahl nitrogen (TKN) | mg·dm−3 | 1.31 | 1.28 | 2.23 | 1.19 | – | – | – | – |
| Total phosphorous (P) | mg·dm−3 | 2.10 | 2.80 | 2.60 | 1.90 | – | – | – | – |
| Phosphates (PO4-P) | mg·dm−3 | 1.41 | 1.54 | 2.24 | 0.90 | – | – | – | – |
| Ammoniacal-nitrogen (NH3−N) | mg·dm−3 | 0.41 | 0.42 | 0.26 | 0.38 | – | – | – | – |
| Nitrate-nitrogen (NO3−N) | mg·dm−3 | 1.71 | 2.40 | 2.00 | 1.90 | 50.0 | – | 50.0 | – |
| Nitrite-nitrogen (NO2−N) | mg·dm−3 | 0.00 | 0.01 | 0.02 | 0.01 | 0.5 | – | 0.5 | – |
| Chloride (Cl−) | mg·dm−3 | 2.00 | 1.00 | 1.00 | 1.00 | 250.0 | 250.0 | 250.0 | – |
| Cadmium (Cd) | µg·dm−3 | 0.00 | 0.02 | 0.02 | 0.01 | 5.0 | – | 5.0 | – |
| Calcium (Ca) | mg·dm−3 | 4.2442 | 4.2810 | 3.5006 | 3.6801 | – | – | – | – |
| Chromium (Cr) | µg·dm−3 | 0.0198 | 0.0221 | 0.0290 | 0.0271 | 50.0 | – | 50.0 | – |
| Copper (Cu) | mg·dm−3 | 0.0126 | 0.0063 | 0.0151 | 0.0583 | 2.0 | – | 2.0 | – |
| Iron (Fe) | µg·dm−3 | 0.1405 | 0.1215 | 0.1488 | 0.1533 | 200.0 | – | 200.0 | – |
| Lead (Pb) | µg·dm−3 | 0.0306 | 0.0295 | 0.0281 | 0.0297 | 10.0 | – | 10.0 | – |
| Magnesium (Mg) | mg·dm−3 | 0.0925 | 0.1311 | 0.0802 | 0.1103 | 30.0–125.0 | – | – | – |
| Manganese (Mn) | µg·dm−3 | 0.0023 | 0.0002 | 0.0006 | 0.0017 | 50.0 | – | 50.0 | – |
| Nickel (Ni) | µg·dm−3 | 0.0415 | 0.0341 | 0.0414 | 0.0366 | 20.0 | – | 20.0 | – |
| Potassium (K) | µg·dm−3 | 1.8386 | 2.0873 | 1.2937 | 1.8953 | – | – | – | – |
| Zinc (Zn) | µg·dm−3 | 0.0557 | 0.0138 | 0.0306 | 0.0432 | – | – | – | – |
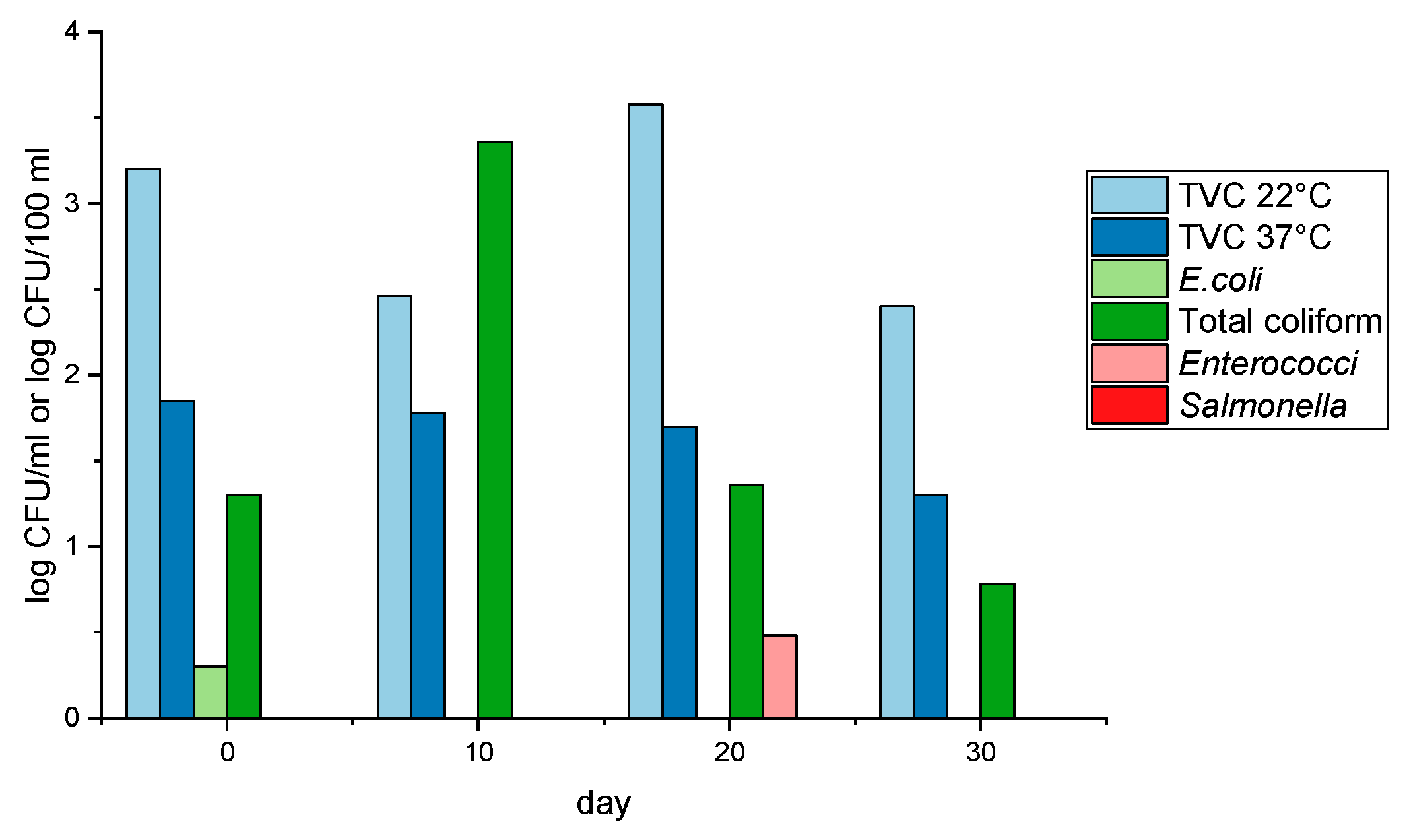
| Indicator | Unit | After … Days | DWD [29] | BWD [30] | |||
|---|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | ||||
| TVC 22 °C | CFU/mL | 1.6 × 103 | 2.9 × 102 | 3.8 × 103 | 2.5 × 102 | 100 | - |
| TVC 37 °C | CFU/mL | 7.0 × 101 | 6.0 × 101 | 5.0 × 101 | 2.0 × 101 | 20 | - |
| E. coli | CFU/100 mL | 2.0 × 100 | 0 | 0 | 0 | 0 | 500 excellent quality * |
| Total coliform | CFU/100 mL | 2.0 × 101 | 2.3 × 103 | 2.3 × 101 | 6.0 × 100 | - | - |
| Enterococci | CFU/100 mL | 0 | 0 | 3.0 × 100 | 0 | 0 | 200 excellent quality * |
| Salmonella | CFU/100 mL | 0 | 0 | 0 | 0 | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Struk-Sokołowska, J.; Gwoździej-Mazur, J.; Jadwiszczak, P.; Butarewicz, A.; Ofman, P.; Wdowikowski, M.; Kaźmierczak, B. The Quality of Stored Rainwater for Washing Purposes. Water 2020, 12, 252. https://doi.org/10.3390/w12010252
Struk-Sokołowska J, Gwoździej-Mazur J, Jadwiszczak P, Butarewicz A, Ofman P, Wdowikowski M, Kaźmierczak B. The Quality of Stored Rainwater for Washing Purposes. Water. 2020; 12(1):252. https://doi.org/10.3390/w12010252
Chicago/Turabian StyleStruk-Sokołowska, Joanna, Joanna Gwoździej-Mazur, Piotr Jadwiszczak, Andrzej Butarewicz, Piotr Ofman, Marcin Wdowikowski, and Bartosz Kaźmierczak. 2020. "The Quality of Stored Rainwater for Washing Purposes" Water 12, no. 1: 252. https://doi.org/10.3390/w12010252
APA StyleStruk-Sokołowska, J., Gwoździej-Mazur, J., Jadwiszczak, P., Butarewicz, A., Ofman, P., Wdowikowski, M., & Kaźmierczak, B. (2020). The Quality of Stored Rainwater for Washing Purposes. Water, 12(1), 252. https://doi.org/10.3390/w12010252