Challenges in Biodiversity Conservation in a Highly Modified Tropical River Basin in Sri Lanka
Abstract
:1. Introduction
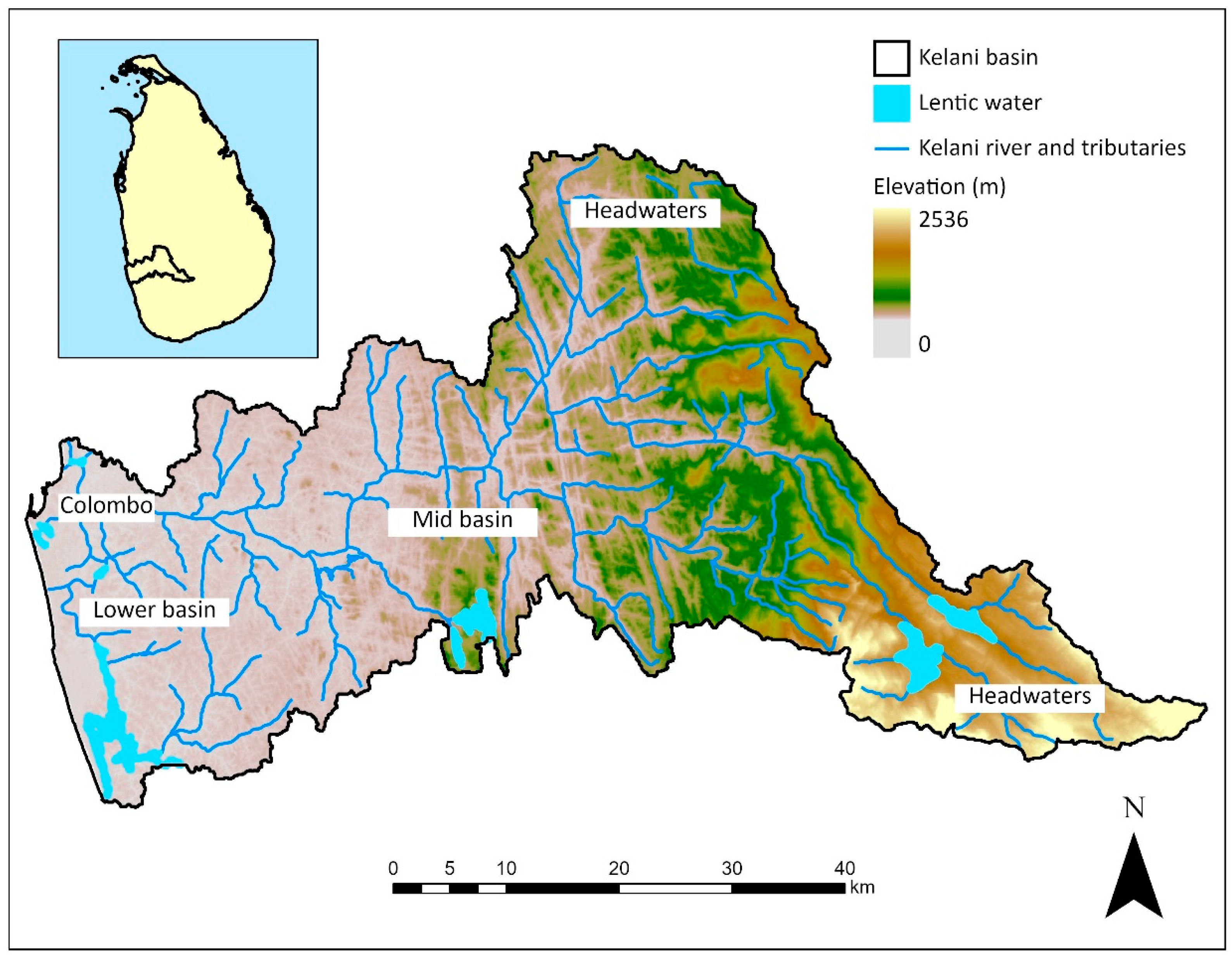
2. Threats for the Kelani River
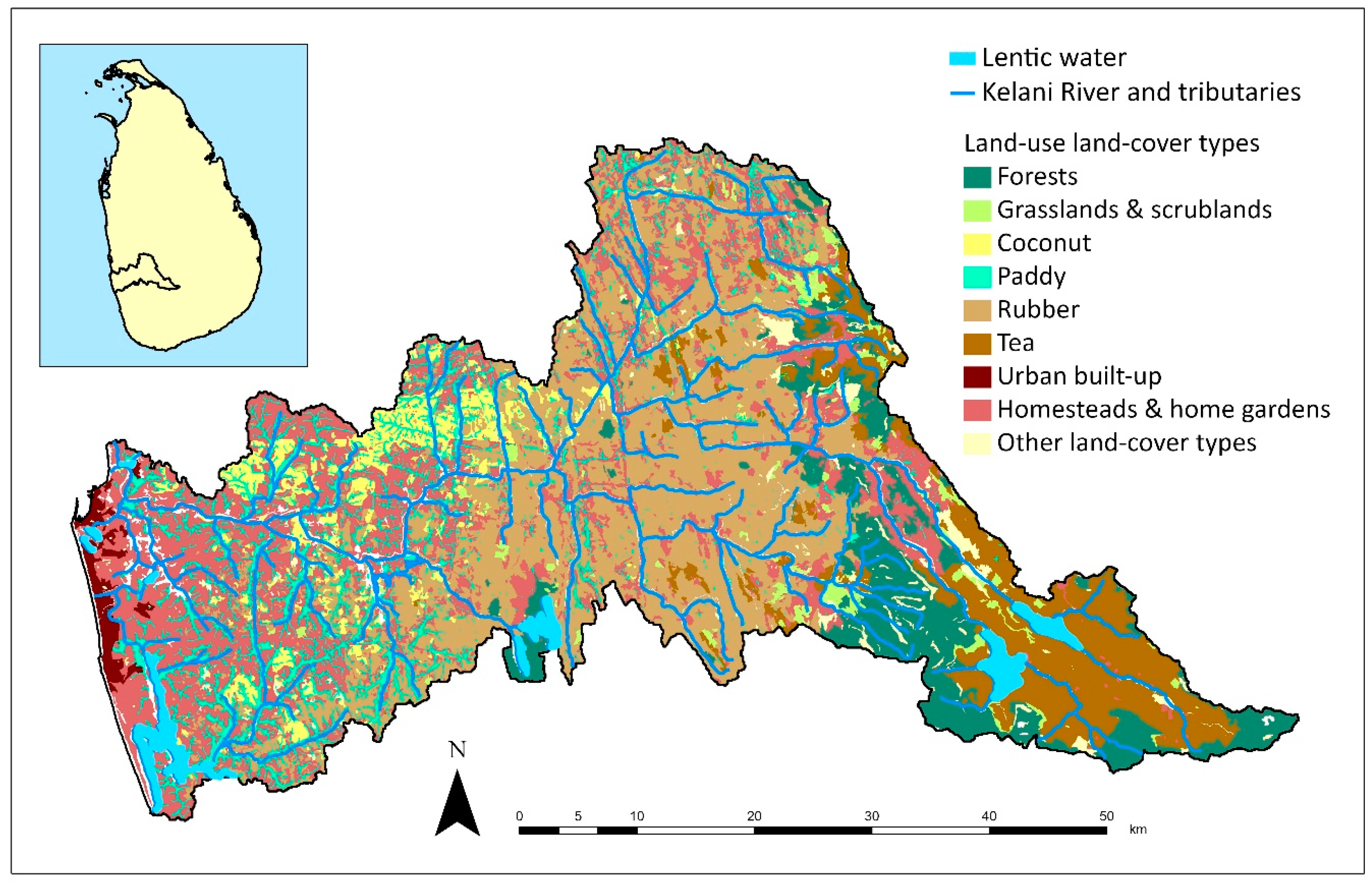
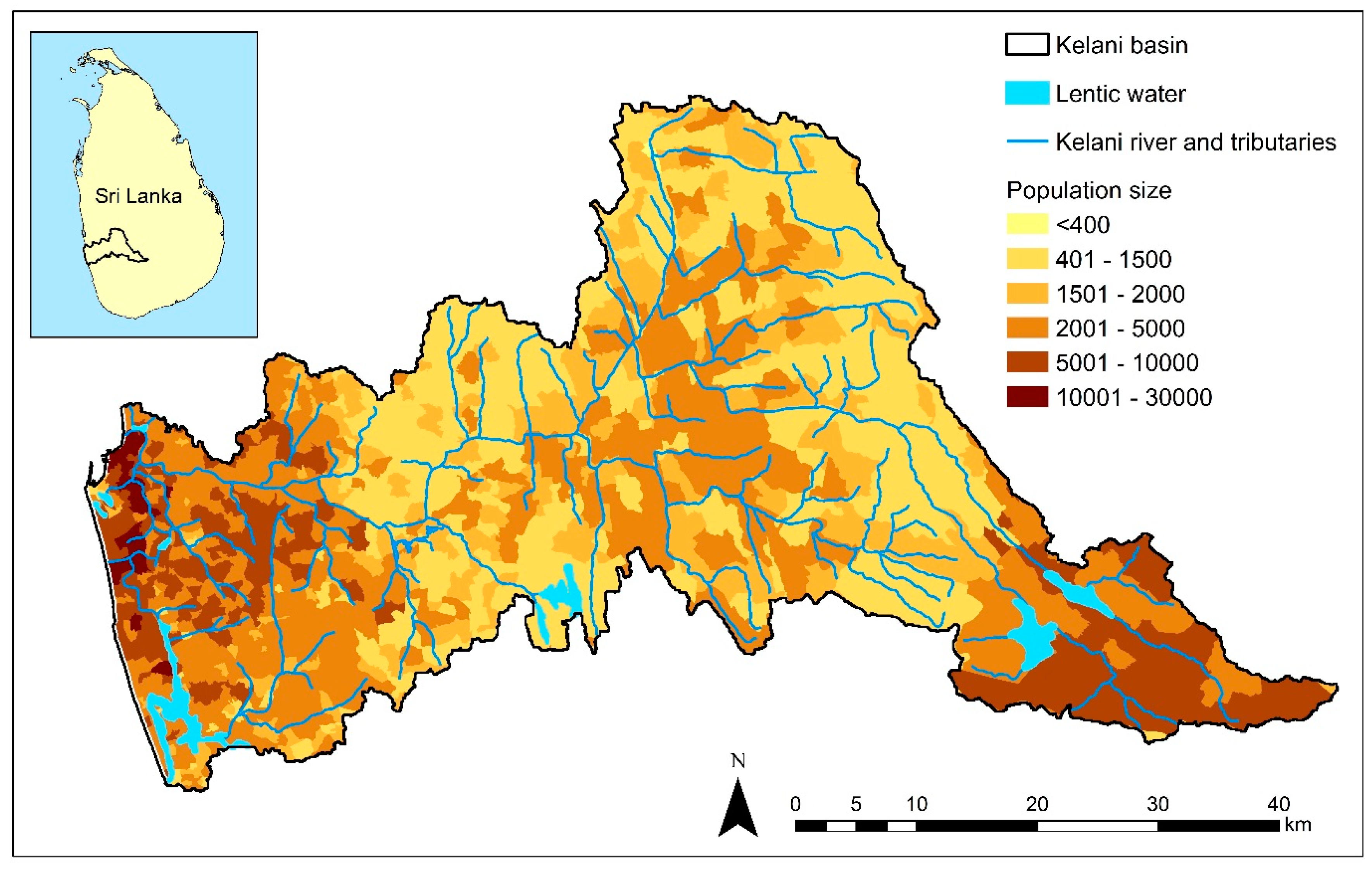
2.1. Loss of Riparian Vegetation and Land-Cover Modifications in the River Basin
2.1.1. Urbanization
2.1.2. Agriculture
2.1.3. Wetland Loss
2.2. Water Pollution
2.2.1. Point-Source Pollution
2.2.2. Non-Point Source Pollution
2.3. Hydrological Changes and Overexploitation
2.4. Policy Issues
3. Freshwater Biodiversity in Kelani River Basin
Freshwater Fish Diversity in Kelani River Basin and Threats Encountered by Fish Communities
4. Future Directions for Conservation and Management of Kelani River Basin
4.1. Aquatic Biodiversity Conservation
4.2. Future Research
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Malmqvist, B.; Rundle, S. Threats to the running water ecosystems of the world. Environ. Conserv. 2002, 29, 134–153. [Google Scholar] [CrossRef]
- Dudgeon, D. The ecology of tropical Asian rivers and streams in relation to biodiversity conservation. Annu. Rev. Ecol. Syst. 2000, 31, 239–263. [Google Scholar] [CrossRef] [Green Version]
- Dudgeon, D. Conservation of freshwater biodiversity in Oriental Asia: Constraints, conflicts, and challenges to science and sustainability. Limnology 2000, 1, 237–243. [Google Scholar] [CrossRef]
- Dudgeon, D. Riverine biodiversity in Asia: A challenge for conservation biology. Hydrobiologia 2000, 418, 1–13. [Google Scholar] [CrossRef]
- Dudgeon, D. Large-Scale Hydrological Changes in Tropical Asia: Prospects for Riverine Biodiversity the construction of large dams will have an impact on the biodiversity of tropical Asian rivers and their associated wetlands. Bioscience 2000, 50, 793–806. [Google Scholar] [CrossRef]
- Sodhi, N.S.; Koh, L.P.; Brook, B.W.; Ng, P.K.L. Southeast Asian biodiversity: An impending disaster. Trends Ecol. Evol. 2004, 19, 654–660. [Google Scholar] [CrossRef]
- Jayakody, P.; Raschid-Sally, L.; Abayawardana, S.; Najim, M. Urban growth and wastewater agriculture: A study from Sri Lanka. In Proceedings of the 32nd WEDC International Conference: Sustainable Development of Water Resources, Water Supply and Environmental Sanitation, Colombo, Sri Lanka, 13–17 September 2006; pp. 105–111. [Google Scholar]
- Subramanian, V. Water quality in south Asia. Asian J. Water Environ. Pollut. 2004, 1, 41–54. [Google Scholar]
- Mallawatantri, A.; Rodrigo, A.; De Silva, K. Medium to Long-Term Multi-Stakeholder Strategy and Action Plan for Management and Conservation of Kelani River Basin; Central Environment Authority and International Union for the Conservation of Nature Sri Lanka Country Office: Colombo, Sri Lanka, 2016; p. 153. [Google Scholar]
- Nguyen, T.T.; De Silva, S.S. Freshwater finfish biodiversity and conservation: An Asian perspective. Biodivers Conserv. 2006, 15, 175–200. [Google Scholar] [CrossRef]
- Luby, S. Water Quality in South Asia. J. Health Popul. Nutr. 2008, 26, 123–124. [Google Scholar]
- Dudgeon, D. The contribution of scientific information to the conservation and management of freshwater biodiversity in tropical Asia. Hydrobiologia 2003, 500, 295–314. [Google Scholar] [CrossRef]
- Imbulana, K.A.U.S.; Wijesekara, N.T.S.; Neupane, B.R. Sri Lanka National Water Development Report; MAI & MD, UN-WWAP, UNESCO: Paris, France; University of Moratuwa: Moratuwa, Sri Lanka, 2006; p. 221. [Google Scholar]
- Oki, T.; Kanae, S. Global hydrological cycles and world water resources. Science 2006, 313, 1068–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Beenaerts, N.; Pethiyagoda, R.; Ng, P.K.; Yeo, D.C.; Bex, G.J.; Bahir, M.M.; Artois, T. Phylogenetic diversity of Sri Lankan freshwater crabs and its implications for conservation. Mol. Ecol. 2010, 19, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Environment. The National Red List 2012 of Sri Lanka; Conservation Status of the Fauna and Flora; Biodiversity Secretariat, Ministry of Environment: Colombo, Sri Lanka, 2012; p. 476.
- Meegaskumbura, M.; Senevirathne, G.; Manamendra-Arachchi, K.; Pethiyagoda, R.; Hanken, J.; Schneider, C.J. Diversification of shrub frogs (Rhacophoridae, Pseudophilautus) in Sri Lanka–Timing and geographic context. Mol. Phylogenet. Evol. 2019, 132, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Weerakoon, D.; Goonatilake, W.d.A. Taxonomic status of the mammals of Sri Lanka. In Fauna of Sri Lanka: Status of Taxonomy, Research and Conservation; Bambaradeniya, C.N.B., Ed.; World Conservation Union and Government of Sri Lanka: Colombo, Sri Lanka, 2006; pp. 216–231. [Google Scholar]
- Pethiyagoda, R. Conservation of Sri Lankan freshwater fishes. In Fauna of Sri Lanka: Status of Taxonomy, Research and Conservation; Bambaradeniya, C.N.B., Ed.; World Conservation Union and Government of Sri Lanka: Colombo, Sri Lanka, 2006; pp. 103–112. [Google Scholar]
- Bahir, M.M.; Ng, P.K.; Crandall, K.; Pethiyagoda, R. A conservation assessment of the freshwater crabs of Sri Lanka. Raffles Bull. Zool. 2005, 12, 121–126. [Google Scholar]
- Pethiyagoda, R.; Manamendra-Arachchi, K.; Bahir, M.M.; Meegaskumbura, M. Sri Lankan amphibians: Diversity, uniqueness and conservation. In Fauna of Sri Lanka. Status of Taxonomy, Research and Conservation; Bambaradeniya, C.N.B., Ed.; World Conservation Union and Government of Sri Lanka: Colombo, Sri Lanka, 2006; pp. 125–133. [Google Scholar]
- Kotagama, S.W.; De Silva, R.I.; Wijayasinha, A.S.; Abeygunawardane, V. Avifaunal list of Sri Lanka. In Fauna of Sri Lanka: Status of Taxonomy, Research and Conservation; Bambaradeniya, C.N.B., Ed.; The World Conservation Union and Government of Sri Lanka: Colombo, Sri Lanka, 2006; pp. 164–203. [Google Scholar]
- Somaweera, R.; Somaweera, N. Lizards of Sri Lanka, a Colour Guide with Field Keys; Edition Chimaira/Serpent’s Tale NHBD: Frankfurt am Main, Germany, 2009; p. 304. [Google Scholar]
- De Zoysa, M.; Inoue, M. Forest governance and community based forest management in Sri Lanka: Past, present and future perspectives. Int. J. Soc. For. 2008, 1, 27–49. [Google Scholar]
- Geekiyanage, N.; Vithanage, M.; Wijesekara, H.; Pushpakumara, G. State of the environment, environmental challenges and governance in Sri Lanka. In Environmental Challenges and Governance: Diverse Perspectives from Asia; Mukherjee, S., Chakraborty, D., Eds.; Routledge: Abingdon, Oxon, UK, 2015; pp. 92–108. [Google Scholar]
- Ileperuma, O. Environmental pollution in Sri Lanka: A review. J. Natl. Sci. Found. Sri. Lanka 2000, 28, 301–325. [Google Scholar] [CrossRef]
- Amarasinghe, P.B.; Vijverberg, J.; Boersma, M. Production biology of copepods and cladocerans in three south-east Sri Lankan low-land reservoirs and its comparison to other tropical freshwater bodies. Hydrobiologia 1997, 350, 145–162. [Google Scholar] [CrossRef]
- Amarasinghe, U.; De Silva, S. Sri Lankan reservoir fishery: A case for introduction of a co-management strategy. Fish. Manag. Ecol. 1999, 6, 387–400. [Google Scholar] [CrossRef]
- Fernando, C. Impact of Sri Lankan reservoirs, their fisheries, management and conservation. In Proceedings of the Ecology and Landscape Management in Sri Lanka, the International and Interdisciplinary Symposium, Colombo, Sri Lanka, 12–16 March 1990; pp. 351–374. [Google Scholar]
- Centre for Environmental Justice. The Industrial Water Pollution of Kelani River with Emphasis on Chemical Pollutants with Recommendations on Pollution Control.; United States Development Project Sri Lanka: Colombo, Sri Lanka, 2015; p. 260.
- de Alwis Goonatilake, S. The Taxonomy and Conservation Status of the Freshwater Fishes in Sri Lanka. In The National Red List 2012 of Sri Lanka, Conservation Status of the Fauna and Flora; Weerakoon, D.K., Wijesundara, S., Eds.; Ministry of Environment: Colombo, Sri Lanka, 2012; pp. 77–87. [Google Scholar]
- Amarasinghe, U.S.; Shirantha, R.; Wijeyaratne, M. Some aspects of ecology of endemic freshwater fishes of Sri Lanka. In Fauna of Sri Lanka: Status of Taxonomy, Research and Conservation.; Bambaradeniya, C.N.B., Ed.; The World Conservation Union and Government of Sri Lanka: Colombo, Sri Lanka, 2006; pp. 113–124. [Google Scholar]
- Zubair, L. Sensitivity of Kelani streamflow in Sri Lanka to ENSO. Hydrol. Process. 2003, 17, 2439–2448. [Google Scholar] [CrossRef]
- Ohgaki, S.; Takizawa, S.; Herath, G.; Kataoka, Y.; Hara, K.; Kathiwada, N.R.; Moon, H.J. Tainable Groundwater Management in Asian Cities: A Final Report of Research on Sustainable Water Management in Asia; Institute for Global Environmental Strategies: Hayama, Japan, 2007; p. 159. [Google Scholar]
- Central Environment Authority. Industrial Pollution in the Kelani River: Preliminary Survey and Interim Report Volum II; Central Environmental Authority: Colombo, Sri Lanka, 1985; p. 205. [Google Scholar]
- Liyanage, C.; Yamada, K. Impact of population growth on the water quality of natural water bodies. Sustainability 2017, 9, 1405. [Google Scholar] [CrossRef] [Green Version]
- Dissanayaka, K.; Rajapakse, R. Long-term precipitation trends and climate extremes in the Kelani River basin, Sri Lanka, and their impact on streamflow variability under climate change. Paddy Water Environ. 2019, 17, 281–289. [Google Scholar] [CrossRef]
- Mahagamage, M.; Pathmalal, M. Water Quality Index (CCME-WQI) Based Assessment Study of Water Quality in Kelani River Basin, Sri Lanka. In Proceedings of the 1st Environment and Natural Resources International Conference, Bangkok, Thailand, 6–7 November 2014; pp. 199–204. [Google Scholar]
- Ministry of Irrigation and Water Resource Management. Strategic Environmental Assessment of Development of River Basin Level Flood and Drought Mitigation Investment Plans-Kelani River Basin; Consulting Engineering & Architects Associated: Kotte, Sri Lanka, 2018; p. 298. [Google Scholar]
- Dammalage, T.; Jayasinghe, N. Land-Use Change and Its Impact on Urban Flooding: A Case Study on Colombo District Flood on May 2016. Eng. Technol. Appl. Sci. 2019, 9, 3887–3891. [Google Scholar]
- Senanayake, F.R.; Moyle, P.B. Conservation of freshwater fishes of Sri Lanka. Biol. Conserv. 1982, 22, 181–195. [Google Scholar] [CrossRef]
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, C.E. River Continuum Concept. Can. J. Fish. Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- McDearman, B.; Clark, G.; Parilla, J. The 10 Traits of Globally Fluent Metro Areas; The Brookings Institution, Metropolitan Policy Program: Washington, DC, USA, 2013. Available online: https://www.brookings.edu/research/the-10-traits-of-globally-fluent-metro-areas (accessed on 4 December 2019).
- Department of Census and Statistics. Census of Population and Housing: Population Atlas of Sri Lanka; Department of Census and Statistics, Ministry of Finance and Planning: Colombo, Sri Lanka, 2012; p. 170.
- Survey Department of Sri Lanka. The National Atlas of Sri Lanka, 2nd ed.; Survey Department of Sri Lanka: Colombo, Sri Lanka, 2007; p. 234.
- Saparamadu, S.; Yi, Z.; Zongping, Z. Temporal Changes of Land Use Land Cover and Environmental Impacts: A Case Study in Colombo, Sri Lanka. Int. J. Earth Environ. Sci. 2018, 2018. [Google Scholar] [CrossRef]
- Department of Census and Statistics. Lanka Stat Map, Sri Lankan Statistical and information mapping. Available online: http://map.statistics.gov.lk:8080/LankaStatMap/apps/wb/index.html (accessed on 10 June 2019).
- Pethiyagoda, R. Threats to the Indigenous Fresh-Water Fishes of Sri-Lanka and Remarks on Their Conservation. Hydrobiologia 1994, 285, 189–201. [Google Scholar] [CrossRef]
- Moss, B. Biodiversity in fresh waters–an issue of species preservation or system functioning? Environ. Conserv. 2000, 27, 1–4. [Google Scholar] [CrossRef]
- Wickramasinghe, A. People and the Forest: Management of the Adam’s Peak Wilderness.; Forest Department of Sri Lanka, Forestry Information Services: Colombo, Sri Lanka, 1995.
- McDonald, R.I.; Green, P.; Balk, D.; Fekete, B.M.; Revenga, C.; Todd, M.; Montgomery, M. Urban growth, climate change, and freshwater availability. Proc. Natl. Acad. Sci. USA 2011, 108, 6312–6317. [Google Scholar] [CrossRef] [Green Version]
- Wagenaar, D.; Dahm, R.; Diermanse, F.; Dias, W.; Dissanayake, D.; Vajja, H.; Gehrels, J.; Bouwer, L. Evaluating adaptation measures for reducing flood risk: A case study in the city of Colombo, Sri Lanka. Int. J. Disast. Risk. Reduct. 2019, 37, 101162. [Google Scholar] [CrossRef]
- Bandara, N. Water and wastewater related issues in Sri Lanka. Water Sci. Technol. 2003, 47, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Environmental Foundation Limited. Industrial Responsibilities towards the Environment; Environmental Foundation Limited: Colombo, Sri Lanka, 2015. [Google Scholar]
- Bambaradeniya, C.; Edirisinghe, J.; De Silva, D.; Gunatilleke, C.; Ranawana, K.; Wijekoon, S. Biodiversity associated with an irrigated rice agro-ecosystem in Sri Lanka. Biodivers Conserv. 2004, 13, 1715. [Google Scholar] [CrossRef]
- Herath, G.; Amaresekera, T. Assessment of urban and industrial pollution on water quality: Kelani River Sri Lanka. Southeast. Asian Water Environ. 2007, 2, 91–98. [Google Scholar]
- Vaughn, C.C. Biodiversity losses and ecosystem function in freshwaters: Emerging conclusions and research directions. Bioscience 2010, 60, 25–35. [Google Scholar] [CrossRef]
- Gunawardena, A.; Wijeratne, E.; White, B.; Hailu, A.; Pandit, R. Industrial pollution and the management of river water quality: A model of Kelani River, Sri Lanka. Environ. Monit. Assess. 2017, 189, 457. [Google Scholar] [CrossRef] [PubMed]
- Hettige, N.; Weerasekara, K.; Azmy, S.; Jinadasa, K. Water Pollution in Selected Coastal Areas in Western Province, Sri Lanka: A Baseline Survey. J. Environ. Prof. 2014, 3. [Google Scholar] [CrossRef]
- Dissanayake, C.; Weerasooriya, S.; Senaratne, A.; Rupasinghe, M. The heavy metal pollution of the Kelani River in Sri Lanka. Aqua 1985, 2, 79–88. [Google Scholar]
- Kotalawala, A. Impact of weirs on fish fauna of Wak-Oya, a tributory of the Kelani River. J. Natl. Sci. Found. Sri Lanka 1994, 22, 65–86. [Google Scholar] [CrossRef] [Green Version]
- Guruge, K.; Tanabe, S. Contamination by persistent organochlorines and butyltin compounds in the west coast of Sri Lanka. Mar. Pollut Bull. 2001, 42, 179–186. [Google Scholar] [CrossRef]
- Athukorala, S.; Weerasinghe, L.; Jayasooria, M.; Rajapakshe, D.; Fernando, L.; Raffeeze, M.; Miguntanna, N. Ananlysis of Water Quality Variation in Kelani River, Sri Lanka Using Principal Componet Analysis. In Proceedings of the SAITM Research Symposium on Engineering Advancements, South Asian Institute of Technology and Medicine, Malabe, Sri Lanka, 27 April 2012–28 April 2017; pp. 129–135. [Google Scholar]
- Fayas, C.M.; Abeysingha, N.S.; Nirmanee, K.G.S.; Samaratunga, D.; Mallawatantri, A. Soil loss estimation using rusle model to prioritize erosion control in KELANI river basin in Sri Lanka. Int. Soil. Water Conserv. Res. 2019, 7, 130–137. [Google Scholar] [CrossRef]
- Silva, E.I.L.; Jayawardhana, R.A.S.N.; Liyanage, N.P.P.; Silva, E.N.S. Effects of construction and operation of mini hydropower plants on fish fauna endemic to Sri Lanka-A case study on Kelani River basin. In Proceedings of the Water Professionals’ Day Symposium in Sri Lanka, Peradeniya, Sri Lanka, 1 October 2015. [Google Scholar]
- Silva, E.I.L.; Jayawardhana, R.A.S.N.; Silva, E.N.S.; Liyanage, N.P.P. The Effects of Escalating Small Hydropower Development on Hill Stream Fish Fauna Endemic to Sri Lanka. In Proceedings of the ASIA 2016: Sixth International Conference and Exhibition on Water Resources and Hydropower Development in Asia, At Vientiane, Lao PRD, 29 February–3 March 2016. [Google Scholar]
- Kondolf, G.M. Hungry water: Effects of dams and gravel mining on river channels. Environ. Manag. N. Y. 1997, 21, 533–552. [Google Scholar] [CrossRef] [PubMed]
- Ailapperuma, W.; Wijayadasa, K. Survey of Environmental Legislation and Institutions in the SACEP Countries Sri Lanka; Ministry of Local Government, Housing and Construction, Colombo, Sri Lanka, and Central Environmental Authority: Colombo, Sri Lanka, 2014.
- South Asia Cooperation For Environment Programme; Sri Lanka. Environmental Legislation and Institutions in Sri Lanka: Handbook on National Environmental Legislation and Institutions in South Asia; Environment Division, Ministry of Environment & Natural Resources, Sampathpaya, Battaramulla, Sri Lanka: Colombo, Sri Lanka, 2002. [Google Scholar]
- Chandrasekara, S.; Gunawardena, E. Effectiveness of existing laws and regulations to prevent encroachments of stream reservations. Trop. Agric. Res. 2011, 22, 134–143. [Google Scholar] [CrossRef] [Green Version]
- South Asia Cooperation For Environment Programme. Environmental Legislation and Institutions in Sri Lanka: Handbook on National Environmental Legislation and Institutions in South Asia; United Nations Environment Programme (UNEP), SACEP and the Norwegian Agency for Development (NORAD): Colombo, Sri Lanka, 2009. [Google Scholar]
- Agarwal, A.; Angeles, M.S.D.; Bhatia, R.; Chéret, I.; Davila-Poblete, S.; Falkenmark, M.; Villarreal, F.G.; Jønch-Clausen, T.; Kadi, M.A.; Kindler, J.; et al. Integrated Water Resources Management, Technical Advisory Committee Background Papers, 4; Global Water Partnership Technical Advisory Committee, Sida: Stockholm, Sweden, 2000. [Google Scholar]
- Ministry of Land and Land Development. National Policy on Protection and Conservation of Water Sources, their Catchments and Reservations in Sri Lanka; Land Secretariat: Battaramulla, Sri Lanka, 2014. [Google Scholar]
- Jayasuriya, A.; Kitchener, D.; Biradar, C. Portfolio of Strategic Conservation Sites/Protected Area Gap Analysis in Sri Lanka; EML Consultants: Colombo, Sri Lanka, 2006; p. 340. [Google Scholar]
- Goonatilake, S.d.A.; Perera, N.; Silva, G.D.; Weerakoon, D.; Mallawatantri, A. Natural Resource Profile of the Kelani River Basin. International Union for Conservation of Nature Sri Lanka; Country Office and Central Environment Authority: Colombo, Sri Lanka, 2016; p. 38. [Google Scholar]
- Sudasinghe, H.; Pethiyagoda, R.; Maduwage, K.; Meegaskumbura, M. Mystus nanus, a new striped catfish from Sri Lanka (Teleostei: Bagridae). Ichthyol. Explor. Freshw. 2016, 27, 163–172. [Google Scholar]
- Sudasinghe, H.; Meegaskumbura, M. Ompok argestes, a new species of silurid catfish endemic to Sri Lanka (Teleostei: Siluridae). Zootaxa 2016, 4158, 261–271. [Google Scholar] [CrossRef]
- Pethiyagoda, R. Freshwater fishes of Sri Lanka; Wildlife Heritage Trust of Sri Lanka: Colombo, Sri Lanka, 1991. [Google Scholar]
- Sudasinghe, B.S.A.T.H.; Nilupul, P.T.; Bandara, S.P. Freshwater ichthyofauna in the Mullegama-Habarakada area, Colombo District, Sri Lanka. J. Threat. Taxa 2014, 6, 5731–5737. [Google Scholar] [CrossRef] [Green Version]
- Keith, P.; Lord, C.; Maeda, K. Indo-Pacific Sicydiine Gobies: Biodiversity, Life Traits and Conservation; Société Française d’Ichtyologie: Paris, France, 2015. [Google Scholar]
- Sudasinghe, H. The problem of aquarium escapes: A new record of the tinfoil barb from Sri Lanka. Widlanka 2016, 4, 17–25. [Google Scholar]
- Marambe, B.; Silva, P.; Ranwala, S.; Gunawardena, J.; Weerakoon, D.; Wijesundara, S.; Manawadu, L.; Atapattu, N.; Kurukulasuriya, M. Invasive alien fauna in Sri Lanka: National list, impacts and regulatory framework. In Island Invasives: Eradication and Management; Veitch, V.R., Clout, M.N., Towns, D.R., Eds.; IUCN: Gland, Switzerland, 2011; pp. 445–450. [Google Scholar]
- Bambaradeniya, C.N. The status and implications of alien invasive species in Sri Lanka. Zoos’ Print J. 2002, 17, 930–935. [Google Scholar] [CrossRef]
- Marambe, B.; Bambaradeniya, C.; Pushpa Kumara, D.; Pallewatta, N. Human dimensions of invasive alien species in Sri Lanka. In The Great Reshuffling; Human Dimensions of Invasive Alien Species, IUCN, Gland, Switzerland and Cambridge, UK; McNeely, J.A., Ed.; IUCN: Gland, Switzerland, 2001; pp. 135–142. [Google Scholar]
- Nico, L.G.; Butt, P.L.; Johnston, G.R.; Jelks, H.L.; Kail, M.; Walsh, S.J. Discovery of South American suckermouth armored catfishes (Loricariidae, Pterygoplichthys spp.) in the Santa Fe River drainage, Suwannee River basin, USA. Bioinvasions Rec. 2012, 1, 179–200. [Google Scholar] [CrossRef]
- Willey, A. Notes on the freshwater fisheries of Ceylon. Spolia Zeylan 1910, 7, 88–105. [Google Scholar]
- IUCN; UNEP-WCMC. The World Database on Protected Areas (WDPA). Cambridge (UK): United Nations Environmnetal Program, World Conservation Monitoring Centre; UNEP and World Conservation Monitoring Centre: Cambridge, UK, 2017. [Google Scholar]
- Fu, C.; Wu, J.; Chen, J.; Wu, Q.; Lei, G. Freshwater fish biodiversity in the Yangtze River basin of China: Patterns, threats and conservation. Biodivers Conserv. 2003, 12, 1649–1685. [Google Scholar] [CrossRef]
- Abell, R. Conservation biology for the biodiversity crisis: A freshwater follow-up. Conserv. Biol. 2002, 16, 1435–1437. [Google Scholar] [CrossRef] [Green Version]
- Abell, R.; Thieme, M.L.; Revenga, C.; Bryer, M.; Kottelat, M.; Bogutskaya, N.; Coad, B.; Mandrak, N.; Balderas, S.C.; Bussing, W. Freshwater ecoregions of the world: A new map of biogeographic units for freshwater biodiversity conservation. Bioscience 2008, 58, 403–414. [Google Scholar] [CrossRef] [Green Version]
- Olson, D.H.; Anderson, P.D.; Frissell, C.A.; Welsh, H.H.; Bradford, D.F. Biodiversity management approaches for stream–riparian areas: Perspectives for Pacific Northwest headwater forests, microclimates, and amphibians. For. Ecol. Manag. 2007, 246, 81–107. [Google Scholar] [CrossRef]
- Olson, D.H.; Burnett, K.M. Design and management of linkage areas across headwater drainages to conserve biodiversity in forest ecosystems. For. Ecol. Manag. 2009, 258, S117–S126. [Google Scholar] [CrossRef]
- Semlitsch, R.D.; Jensen, J.B. Core habitat, not buffer zone. Nat. Wetl. Newsl. 2001, 23, 5–6. [Google Scholar]
- Semlitsch, R.D.; Bodie, J.R. Biological criteria for buffer zones around wetlands and riparian habitats for amphibians and reptiles. Conserv. Biol. 2003, 17, 1219–1228. [Google Scholar] [CrossRef] [Green Version]
- Semlitsch, R.D. Critical Elements for Biologically Based Recovery Plans of Aquatic-Breeding Amphibians. Conserv. Biol. 2002, 16, 619–629. [Google Scholar] [CrossRef] [Green Version]
- The Canadian Heritage Rivers Board. Canadian Heritage Rivers System Strategic Plan 2008-2018; Canadian Heritage Rivers System, Indigenous Affairs and Cultural Heritage Directorate Parks Canada Agency: Gatineau, QC, USA, 2008. [Google Scholar]
- Tarlock, A.D.; Tippy, R. Wild and Scenic Rivers Act of 1968. Cornell Law Rev. 1969, 55, 707–739. [Google Scholar]
- International Water Management Institute. Water Information System for Sri Lanka; Water Information System for Sri Lanka: Battaramulla, Sri Lanka, 2017. [Google Scholar]
- Long, A.J.; Nair, P.R. Trees outside forests: Agro-, community, and urban forestry. New For. 1999, 17, 145–174. [Google Scholar] [CrossRef]
- Saunders, D.; Meeuwig, J.; Vincent, A. Freshwater protected areas: Strategies for conservation. Conserv. Biol. 2002, 16, 30–41. [Google Scholar] [CrossRef] [Green Version]
- Kingsford, R.T.; Nevill, J. Scientists urge expansion of freshwater protected areas. Ecol. Manag. Restor. 2005, 6, 161–162. [Google Scholar] [CrossRef]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.I.; Knowler, D.J.; Leveque, C.; Naiman, R.J.; Prieur-Richard, A.H.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Kotagama, S.; Bambaradeniya, C. An overview of the wetlands of Sri Lanka and their conservation significance. Natl. Wetl. Dir. Sri Lanka 2006, 7–16. [Google Scholar]
- Pethiyagoda, R.; Meegaskumbura, M.; Maduwage, K. A synopsis of the South Asian fishes referred to Puntius (Pisces: Cyprinidae). Ichthyol. Explor. Freshw. 2012, 23, 69. [Google Scholar]
- Silva, A.; Maduwage, K.; Pethiyagoda, R. A review of the genus Rasbora in Sri Lanka, with description of two new species (Teleostei: Cyprinidae). Ichthyol. Explor. Freshw. 2010, 21, 27. [Google Scholar]
- Sudasinghe, H.; Ranasinghe, R.T.; Goonatilake, S.; Meegaskumbura, M. A review of the genus Labeo (Teleostei: Cyprinidae) in Sri Lanka. Zootaxa 2018, 4486, 201–235. [Google Scholar] [CrossRef]
- Sudasinghe, H.; Pethiyagoda, R. A commentary on the taxonomic review of Sri Lankan Devario by Batuwita et al. 2017 (Teleostei: Danionidae). Zootaxa 2019, 4543, 421–430. [Google Scholar] [CrossRef]
- Sudasinghe, H.; Pethiyagoda, R.; Maduwage, K.; Meegaskumbura, M. The identity of the Sri Lankan Amblypharyngodon (Teleostei, Cyprinidae). ZooKeys 2019, 820, 25–49. [Google Scholar] [CrossRef]
- Costanza, R.; Chichakly, K.; Dale, V.; Farber, S.; Finnigan, D.; Grigg, K.; Heckbert, S.; Kubiszewski, I.; Lee, H.; Liu, S. Simulation games that integrate research, entertainment, and learning around ecosystem services. Ecosyst. Serv. 2012, 10, 61–78. [Google Scholar] [CrossRef] [Green Version]
- Daily, G.C.; Söderqvist, T.; Aniyar, S.; Arrow, K.; Dasgupta, P.; Ehrlich, P.R.; Folke, C.; Jansson, A.; Jansson, B.-O.; Kautsky, N. The value of nature and the nature of value. Science 2000, 289, 395–396. [Google Scholar] [CrossRef] [Green Version]
- Roth, N.E.; Allan, J.D.; Erickson, D.L. Landscape influences on stream biotic integrity assessed at multiple spatial scales. Landsc. Ecol. 1996, 11, 141–156. [Google Scholar] [CrossRef]
- Wang, L.Z.; Lyons, J.; Kanehl, P. Impacts of urbanization on stream habitat and fish across multiple spatial scales. Environ. Manag. 2001, 28, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Pease, A.A.; González-Díaz, A.A.; Rodiles-Hernández, R.O.C.Í.O.; Winemiller, K.O. Functional diversity and trait–environment relationships of stream fish assemblages in a large tropical catchment. Freshw. Biol. 2012, 57, 1060–1075. [Google Scholar] [CrossRef]
- Ekaratne, S.U.K. A Review of the Status and Trends of Exported Ornamental Fish Resources and Their Habitats in Sri Lanka; Bay of Bengal Programme, Abhiramapuram: Chennai, India, 2000. [Google Scholar]
- Gunasekara, S. Export Trade of Indigenous Freshwater Fishes of Sri Lanka; Samantha Gunasekara: Colombo, Sri Lanka, 2011. [Google Scholar]




| Pollutant | Source of the Pollutant |
|---|---|
| Sewage and other organic compounds | Municipalities, slums, hotels, hospitals, restaurants, water and sewage treatment plants, breweries, leather tanneries, plywood factories, rubber and latex factories, beverage and food processing plants |
| Heavy metals and other inorganic compounds | Tanneries, petroleum refineries, metal processing and manufacturing plants, battery manufacturing |
| Detergents | Hotels, households, breweries |
| Fabric dye | Export processing zones, textile and garment factories |
| Synthetic organic compounds | Brewery, wood processing plants, beverage factories, rubber and plastic factories, wood processing industries |
| Petroleum wastes and oil | Petroleum and oil refineries, transportation businesses, motor vehicle service stations |
| Synthetic inorganic compounds | Fertilizer manufactures, beverage factories, lather tanning, rubber and plastic factories, tire manufactures |
| Medical and pharmaceutical waste | Open waste disposal sites, hospitals and medical centers |
| Solid waste | Households, businesses and commercial enterprises, construction sites, garbage disposal sites |
| Statute Name | Issues Addressed Related to Inland Aquatic Resources | Enforcing Agency | Enactment Year (Last Amendment) |
|---|---|---|---|
| Overarching Environmental Statues | |||
| National environmental Act | Provisions on environmental management, protection, and monitoring. Approval of projects via overseeing environmental impact assessment methods. | Central Environmental Authority | 1980 (2000) |
| National Environmental Policy and Strategies | Provides directions to conserve and manage environment in all aspects, including forestry and wildlife conservation, fisheries and other aquatic resources, particularly with respect to agricultural sustainability. | Ministry of Mahaweli Development and Environment | 2003 |
| Cleaner Product Policy | Improve efficiency of water and energy consumption by minimizing wastage and over exploitation. | Ministry of Mahaweli Development and Environment | 2005 |
| National Forest Policy Forest Conservation Ordinance Amendment Act | Conserve forests for their biodiversity, soils, water and historical, cultural, religious, and aesthetic values. Increase the tree cover and forest productivity. Enhance contribution of sustainable forestry to rural development and national economy. | Forest Conservation Department | 1995 1907 (2009) |
| National Wildlife Policy Fauna and Flora Protection Ordinance Amendment Act | Conserve aquatic biodiversity and habitats to prevent misuses. Maintain ecological processes and genetic diversity. Delineate conservation lands, inclusive of embed aquatic systems. Engage local communities in protected-area management. | Department of Wildlife Conservation | 2000 1937 (2009) |
| Watershed or Basin-Scale Statues | |||
| National Watershed Management Policy | Conserve, restore, and manage watersheds while managing their critical environmental dynamics. | Ministry of Mahaweli Development and Environment | 2004 |
| National Land-use Policy National Policy on Protection and Conservation of Water Sources, their Catchments and Reservations in Sri Lanka | Design land-use policies at watershed scale. Conserve and restore all water sources, including watersheds as a reservation. Promote sustainable water consumption through participatory management of responsible governmental institutions and communities. Identify needs for regulation reforms. Remedy shortcoming of existing policies and promote watershed-wide management of inland aquatic resources. | Ministry of Land and Land Development | 2007 2014 |
| Inland Aquatic Resources Conservation and Management Statues | |||
| National Policy on Wetlands | Conserve wetland ecosystems, to prevent illegal utilization. Restore and maintain the biological diversity and productivity. Enhance wetland ecosystem services. Assure sustainable use of wetlands by local communities. Achieve national commitments to the Ramsar Convention. | Ministry of Mahaweli Development and Environment | 2005 |
| Irrigation ordinance | Construct, maintain, and protect irrigation works, water conservation | Department of Irrigation | 1856 |
| National Drinking Water Policy | Provide a framework for sustainable and efficient supply of safe water in adequate quantity at an affordable cost. | Ministry of Water Supply and Drainage | 2002 |
| National Water Supply and Drainage Board Act | Provide safe drinking water and facilitate sanitation. | National Water Supply and Drainage Board | 1974 (1992) |
| Water Resource Board Act | Conserve and sustainably utilize aquatic resources by harnessing new technologies and management tools to meet the growing demands. | Water Resource Board | 1984 (1999) |
| Fisheries and Aquatic Resources Act | Manage, regulate, conserve, and develop the fisheries and aquatic resources, including any native aquatic organisms in any stage of their life cycle and non-living substances found in aquatic media | Department of Fisheries and Aquatic Resources | 1996 (2016) |
| Land and Infrastructure Development Statues | |||
| Land Reclamation and Development Cooperation Act | Mitigate flood damage by restoring and maintaining pollution-free inland water bodies. Design basin-scale land development plans. Establish wetland reclamation standards and provisions against wetland filling. | Land | Land Reclamation and Development Cooperation Act |
| Urban Development Act | Promote integrated planning and implementation for environmental, economic, social, and physical development of urban areas | Urban Development Authority | 1978 |
| Flood Protection Ordinance | Protection, land-use regulation, and management of flood-prone areas, such as floodplains | Department of Irrigation | 1924 (1955) |
| Soil Conservation Act | Introduce provisions to conserve soil resources, prevent or mitigate soil erosion, and protect land against flood and drought related damage. Identify and regulate erodible areas. | Soil Conservation Board | 1951 (1996) |
| Land Development Ordinance Crown Lands Ordinance State Lands (recovery of possession) Act | Establish provisions for riparian forest encroachment by stipulating minimum buffer-width reservations. Regulation of aquatic resources in lakes and streams. | Ministry of Lands and Land Development | 1935 1947 1979 |
| Family | Species | Common Name | National Conservation Status | Information Source |
|---|---|---|---|---|
| Cyprinidae | Amblypharyngodon grandisquamis(SLE) | Silver Carplet | LC | FOA/MUR |
| Cyprinidae | Dawkinsia singhala(SLE) | Sri Lankan Filamented Barb | LC | FOA/MUR |
| Cyprinidae | Devario malabaricus(NTV) | Giant Danio | LC | FOA/MUR |
| Cyprinidae | Devario micronema(SLE) | - | FOA/MUR | |
| Cyprinidae | Esomus thermoicos(NTV) | Sri Lankan Flying Barb | LC | FOA/MUR |
| Cyprinidae | Garra ceylonensis(SLE) | Sri Lankan Stone Sucker | VU | FOA/MUR |
| Cyprinidae | Horadandia atukorali(SLE) | Horadandia | VU | FOA |
| Cyprinidae | Labeo heladiva(SLE) | Common Labeo | LC | FOA |
| Cyprinidae | Laubuka varuna(SLE) | Varuna Laubuka | CR | FOA/MUR |
| Cyprinidae | Pethia bandula(SLE) | Bandula Barb | CR | FOA/MUR |
| Cyprinidae | Pethia nigrofasciata(SLE) | Black Ruby Barb | EN | FOA/MUR |
| Cyprinidae | Pethia reval(SLE) | Red-finned Barb | EN | FOA/MUR |
| Cyprinidae | Puntius bimaculatus(NTV) | Red Side Barb | LC | FOA/MUR |
| Cyprinidae | Puntius dorsalis(NTV) | Long Snouted Barb | LC | FOA/MUR |
| Cyprinidae | Puntius kamalika(SLE) | Kamalika’s Barb | EN | FOA/MUR |
| Cyprinidae | Puntius kelumi(SLE) | Kelum’s Barb | EN | FOA/MUR |
| Cyprinidae | Puntius chola(NTV) | Swamp Barb | LC | FOA/MUR |
| Cyprinidae | Puntius titteya(SLE) | Cherry Barb | EN | FOA/MUR |
| Cyprinidae | Puntius vittatus(NTV) | Silver Barb | LC | FOA/MUR |
| Cyprinidae | Rasbora dandia(NTV) | Broad-lined Striped Rasbora | LC | FOA/MUR |
| Rasbora microcephalus(NTV) | Narrow-lined Striped Rasbora | LC | FOA/MUR | |
| Cyprinidae | Rasboroides vaterifloris(SLE) | Fire Rasbora | - | FOA |
| Cyprinidae | Systomus pleurotaenia(SLE) | Black Lined Barb | EN | FOA/MUR |
| Cyprinidae | Systomus asoka(SLE) | Asoka Barb | CR | FOA/MUR |
| Cyprinidae | Systomus sarana(NTV) | Olive Barb | LC | FOA/MUR |
| Cyprinidae | Tor khudree(NTV) | Mahseer | NT | FOA/MUR |
| Cobitidae | Lepidocephalichthys thermalis(NTV) | Common Spiny Loach | LC | FOA/MUR |
| Nemacheilidae | Paracanthocobitis urophthalma(SLE) | Tiger Loach | EN | FOA/MUR |
| Nemacheilidae | Schistura notostigma(SLE) | Banded Mountain Loach | NT | FOA/MUR |
| Bagridae | Mystus gulio(NTV) | Long Whiskered Catfish | LC | FOA |
| Bagridae | Mystus nanus(SLE) | Sri Lankan Dwarfed Striped Catfish | LC | FOA/MUR [77] |
| Bagridae | Mystus ankutta(SLE) | Sri Lankan Dwarf Catfish | EN | FOA/MUR |
| Bagridae | Mystus zeylanicus(SLE) | Sri Lankan Yellow Catfish | LC | FOA/MUR |
| Siluridae | Ompok argestes(SLE) | Sri Lankan Mottled Butter Catfish | DD | FOA/MUR [78] |
| Siluridae | Wallago attu(NTV) | Silver Shark | EN | FOA |
| Clariidae | Clarias brachysoma(SLE) | Walking Catfish | NT | FOA/MUR |
| Heteropneustidae | Heteropneustes fossilis(NTV) | Stinging Catfish | LC | FOA |
| Aplocheilidae | Aplocheilus dayi(SLE) | Day’s Killifish | EN | FOA/MUR |
| Aplocheilidae | Aplocheilus parvus(NTV) | Dwarf Killifish | LC | FOA |
| Cichlidae | Etroplus suratensis(NTV) | Green Chromide | LC | FOA |
| Cichlidae | Pseudetroplus maculatus(NTV) | Yellow Chromide | LC | FOA |
| Gobiidae | Awaous melanocephalus(NTV) | Scribbled Goby | LC | FOA |
| Gobiidae | Glossogobius giuris(NTV) | Bar Eyed Goby | LC | FOA |
| Gobiidae | Schismatogobius deraniyagalai(NTV) | Red Neck Goby | EN | FOA |
| Gobiidae | Sicyopterus griseus(NTV) | Stone Goby | CR | FOA/MUR |
| Gobiidae | Sicyopus jonklaasi(SLE) | Lipstick Goby | EN | FOA |
| Anguillidae | Anguilla bicolor(NTV) | Level Finned Eel | LC | FOA |
| Anguillidae | Anguilla nebulosa(NTV) | Long Finned Eel | LC | FOA |
| Anabantidae | Anabas testudineus(NTV) | Climbing Perch | LC | FOA |
| Synbranchidae | Monopterus desilvai(SLE) | Sri Lankan Lesser Swamp Eel | CR | FOA |
| Synbranchidae | Ophisternon bengalense(NTV) | Asian Swamp Eel | CR | MUR |
| Osphronemidae | Belontia signata(SLE) | Combtail | NT | FOA/MUR |
| Osphronemidae | Pseudosphromenus cupanus(NTV) | Spike Tailed Paradisfish | LC | FOA/MUR |
| Channidae | Channa ara(SLE) | Sri Lankan Giant Snakehead | EN | FOA |
| Channidae | Channa gachua(SLE) | Brown Snakehead | LC | FOA |
| Channidae | Channa orientalis(SLE) | Smooth Breasted Snakehead | VU | FOA |
| Channidae | Channa punctata(NTV) | Spotted Snakehead | LC | FOA |
| Channidae | Channa striata(NTV) | Murrel | LC | FOA |
| Mastacembelidae | Mastacembelus armatus(NTV) | Marbled Spiny Eel | LC | FOA/MUR |
| Belonidae | Xenentodon cancila(NTV) | Freshwater Garfish | NT | FOA |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surasinghe, T.; Kariyawasam, R.; Sudasinghe, H.; Karunarathna, S. Challenges in Biodiversity Conservation in a Highly Modified Tropical River Basin in Sri Lanka. Water 2020, 12, 26. https://doi.org/10.3390/w12010026
Surasinghe T, Kariyawasam R, Sudasinghe H, Karunarathna S. Challenges in Biodiversity Conservation in a Highly Modified Tropical River Basin in Sri Lanka. Water. 2020; 12(1):26. https://doi.org/10.3390/w12010026
Chicago/Turabian StyleSurasinghe, Thilina, Ravindra Kariyawasam, Hiranya Sudasinghe, and Suranjan Karunarathna. 2020. "Challenges in Biodiversity Conservation in a Highly Modified Tropical River Basin in Sri Lanka" Water 12, no. 1: 26. https://doi.org/10.3390/w12010026
APA StyleSurasinghe, T., Kariyawasam, R., Sudasinghe, H., & Karunarathna, S. (2020). Challenges in Biodiversity Conservation in a Highly Modified Tropical River Basin in Sri Lanka. Water, 12(1), 26. https://doi.org/10.3390/w12010026





