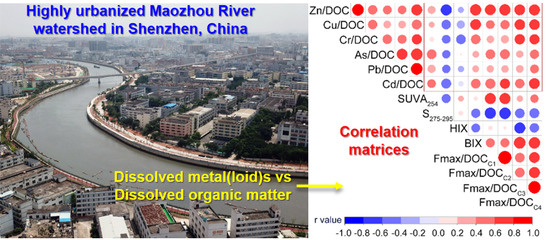
Dissolved Metal(loid) Concentrations and Their Relations with Chromophoric and Fluorescent Dissolved Organic Matter in an Urban River in Shenzhen, South China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Sample Collection
2.3. Chemical Analyses
2.4. Data Analysis
3. Results
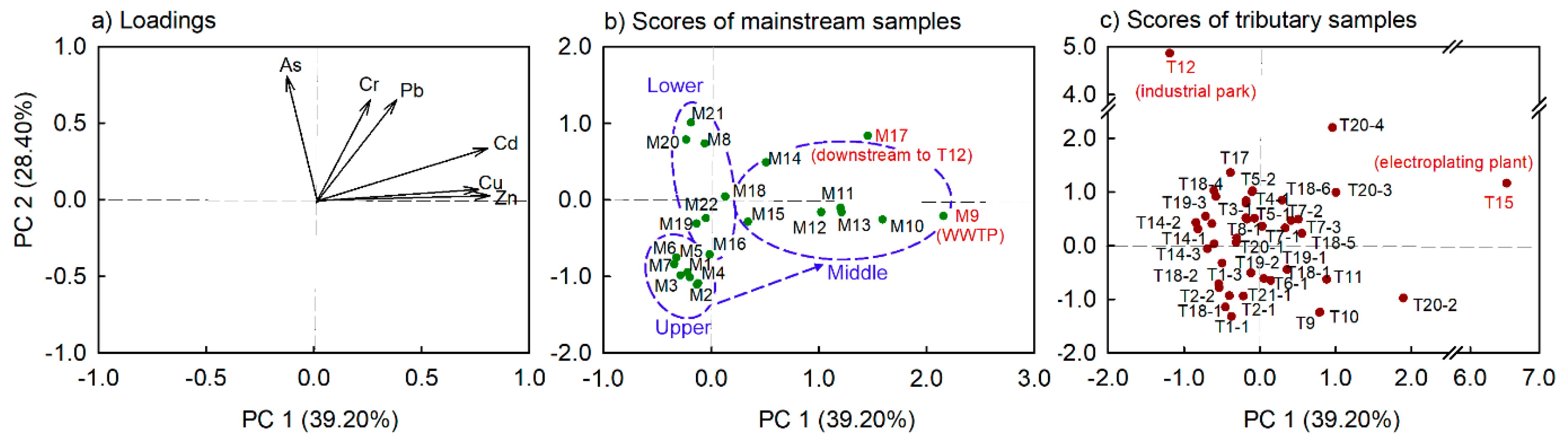
3.1. Spatial Variations in Water Quality and Dissolved Metal(loid) Concentrations
3.2. Relationships between Dissolved Metal(loid) Concentrations and DOM Parameters
4. Discussion
4.1. Concentrations and Distribution of Dissolved Metal(loid)s
4.2. Relationships between Dissolved Metal(loid)s and DOM Abundances
4.3. Relationships between Me/DOC and DOM Characteristics
5. Conclusions
- (a)
- The mainstream and tributary water of the Maozhou River watershed was mainly contaminated by dissolved Cu and Zn. Greater variations in the metal(loid) concentrations and their maximum concentrations were more often noted in tributaries than in the mainstream.
- (b)
- Urbanization may weaken the correlations between dissolved metal(loid)s and DOM concentrations. Dissolved metal(loid) concentrations were more likely to be significantly correlated with the abundances of protein-like components of DOM than with the abundances of bulk DOM (DOC concentration) or CDOM (UV254) in this black and odorous river.
- (c)
- The significant correlations between the Me/DOC for Zn or Cu and the SUVA254 found in a previous study were not found here. Rather, the Me/DOC for Zn or Cu had more significant correlations with fluorescence-based parameters such as the BIX and Fmax/DOC ratios.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liang, B.; Han, G.L.; Liu, M.; Yang, K.H.; Li, X.Q.; Liu, J.K. Distribution, sources, and water quality assessment of dissolved heavy metals in the Jiulongjiang River water, southeast China. Int. J. Environ. Res. Public Health 2018, 15, 2752. [Google Scholar] [CrossRef] [Green Version]
- Lourino-Cabana, B.; Lesven, L.; Billon, G.; Proix, N.; Recourt, P.; Ouddane, B.; Fischer, J.C.; Boughriet, A. Impacts of metal contamination in Calcareous Waters of Deûle River (France): Water quality and thermodynamic studies on metallic mobility. Water Air Soil Pollut. 2010, 206, 187–201. [Google Scholar] [CrossRef]
- Sekabira, K.; Origa, H.O.; Basamba, T.A.; Mutumba, G.; Kakudidi, E. Assessment of heavy metal pollution in the urban stream sediments and its tributaries. Int. J. Environ. Sci. Technol. 2010, 7, 435–446. [Google Scholar] [CrossRef] [Green Version]
- Mansoor, S.Z.; Louie, S.; Lima, A.T.; Van Cappellen, P.; MacVicar, B. The spatial and temporal distribution of metals in an urban stream: A case study of the Don River in Toronto, Canada. J. Gt. Lakes Res. 2018, 44, 1314–1326. [Google Scholar] [CrossRef]
- Sun, X.; Crittenden, J.C.; Li, F.; Lu, Z.M.; Dou, X.L. Urban expansion simulation and the spatio-temporal changes of ecosystem services, a case study in Atlanta Metropolitan area, USA. Sci. Total Environ. 2018, 622, 974–987. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.L.; Chen, L.; Deng, X.W.; Liang, G.D.; Giesy, J.P.; Rao, Q.Y.; Wen, Z.H.; Wu, Y.; Chen, J.; Xie, P. Spatial and interspecies differences in concentrations of eight trace elements in wild freshwater fishes at different trophic levels from middle and eastern China. Sci. Total Environ. 2019, 672, 883–892. [Google Scholar] [CrossRef] [PubMed]
- De Lemos, C.T.; Iranco, F.D.; de Oliveira, N.C.D.; de Souza, G.D.; Fachel, J.M.G. Biomonitoring of genotoxicity using micronuclei assay in native population of Astyanax jacuhiensis (Characiformes: Characidae) at sites under petrochemical influence. Sci. Total Environ. 2008, 406, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, T.Y.; Ni, K.; Liu, S.J.; Wang, P.; Xie, S.W.; Meng, J.; Zheng, X.Q.; Lu, Y.L. Metals contamination along the watershed and estuarine areas of southern Bohai Sea, China. Mar. Pollut. Bull. 2013, 74, 453–463. [Google Scholar] [CrossRef]
- Patel, P.; Raju, N.J.; Reddy, B.; Suresh, U.; Sankar, D.B.; Reddy, T.V.K. Heavy metal contamination in river water and sediments of the Swarnamukhi River Basin, India: Risk assessment and environmental implications. Environ. Geochem. Health 2018, 40, 609–623. [Google Scholar] [CrossRef]
- Yan, M.Q.; Ma, J.; Zhang, C.Y.; Zhou, Y.X.; Liu, F.; Han, X.Z.; Li, M.Y.; Ni, J.R. Optical property of dissolved organic matters (DOMs) and its link to the presence of metal ions in surface freshwaters in China. Chemosphere 2017, 188, 502–509. [Google Scholar] [CrossRef]
- Adusei-Gyamfi, J.; Ouddane, B.; Rietveld, L.; Cornard, J.P.; Criquet, J. Natural organic matter-cations complexation and its impact on water treatment: A critical review. Water Res. 2019, 160, 130–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, B.; Wang, P.F.; Wang, C.; Qian, J.; Hou, J.; Cui, X.A.; Zhang, N.N. The effect of anthropogenic impoundment on dissolved organic matter characteristics and copper binding affinity: Insights from fluorescence spectroscopy. Chemosphere 2017, 188, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.; Osterholz, H.; Koschinsky, A.; Dittmar, T. Riverine mixing at the molecular scale—An ultrahigh-resolution mass spectrometry study on dissolved organic matter and selected metals in the Amazon confluence zone (Manaus, Brazil). Org. Geochem. 2019, 129, 45–62. [Google Scholar] [CrossRef] [Green Version]
- Berto, S.; De Laurentiis, E.; Tota, T.; Chiavazza, E.; Daniele, P.G.; Minella, M.; Isaia, M.; Brigante, M.; Vione, D. Properties of the humic-like material arising from the photo-transformation of L-tyrosine. Sci. Total Environ. 2016, 545, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Baken, S.; Degryse, F.; Verheyen, L.; Merckx, R.; Smolders, E. Metal complexation properties of freshwater dissolved organic matter are explained by its aromaticity and by anthropogenic ligands. Environ. Sci. Technol. 2011, 45, 2584–2590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.J.; He, X.S.; Li, C.W.; Li, N.X. The binding properties of copper and lead onto compost-derived DOM using Fourier-transform infrared, UV-vis and fluorescence spectra combined with two-dimensional correlation analysis. J. Hazard. Mater. 2019, 365, 457–466. [Google Scholar] [CrossRef]
- Huang, M.; Li, Z.W.; Luo, N.L.; Yang, R.; Wen, J.J.; Huang, B.; Zeng, G.M. Application potential of biochar in environment: Insight from degradation of biochar-derived DOM and complexation of DOM with heavy metals. Sci. Total Environ. 2019, 646, 220–228. [Google Scholar] [CrossRef]
- Kikuchi, T.; Fujii, M.; Terao, K.; Jiwei, R.; Lee, Y.P.; Yoshimura, C. Correlations between aromaticity of dissolved organic matter and trace metal concentrations in natural and effluent waters: A case study in the Sagami River Basin, Japan. Sci. Total Environ. 2017, 576, 36–45. [Google Scholar] [CrossRef] [Green Version]
- Lavoie, R.A.; Amyot, M.; Lapierre, J.F. Global meta-analysis on the relationship between mercury and dissolved organic carbon in freshwater environments. J. Geophys. Res. Biogeosci. 2019, 124, 1508–1523. [Google Scholar] [CrossRef] [Green Version]
- Wong, K.H.; Obata, H.; Kim, T.; Wakuta, Y.; Takeda, S. Distribution and speciation of copper and its relationship with FDOM in the East China Sea. Mar. Chem. 2019, 212, 96–107. [Google Scholar] [CrossRef]
- Fujii, M.; Imaoka, A.; Yoshimura, C.; Waite, T.D. Effects of Molecular Composition of Natural Organic Matter on Ferric Iron Complexation at Circumneutral pH. Environ. Sci. Technol. 2014, 48, 4414–4424. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.C.; Guan, D.X.; Zou, L.; Lin, H.; Guo, L.D. Contrasting effects of photochemical and microbial degradation on Cu(II) binding with fluorescent DOM from different origins. Environ. Pollut. 2018, 239, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.B.; Smith, D.S.; Gueguen, C. Influence of water chemistry and dissolved organic matter (DOM) molecular size on copper and mercury binding determined by multiresponse fluorescence quenching. Chemosphere 2013, 92, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Kinniburgh, D.G.; van Riemsdijk, W.H.; Koopal, L.K.; Borkovec, M.; Benedetti, M.F.; Avena, M.J. Ion binding to natural organic matter: Competition, heterogeneity, stoichiometry and thermodynamic consistency. Colloid Surf. A Physicochem. Eng. Asp. 1999, 151, 147–166. [Google Scholar] [CrossRef]
- Cabaniss, S.E. Forward Modeling of Metal Complexation by NOM: II. Prediction of Binding Site Properties. Environ. Sci. Technol. 2011, 45, 3202–3209. [Google Scholar] [CrossRef] [PubMed]
- Shenzhen Water Goup 2016. Disclosure of Environmental Information. Available online: https://www.sz-water.com.cn/environmentpolicy (accessed on 10 September 2019).
- Redman, A.D.; Macalady, D.L.; Ahmann, D. Natural organic matter affects arsenic speciation and sorption onto hematite. Environ. Sci. Technol. 2002, 36, 2889–2896. [Google Scholar] [CrossRef]
- Ye, Q.H.; Zhang, Z.T.; Liu, Y.C.; Wang, Y.H.; Zhang, S.; He, C.; Shi, Q.; Zeng, H.X.; Wang, J.J. Spectroscopic and molecular-level characteristics of dissolved organic matter in a highly polluted urban river in south China. ACS Earth Space Chem. 2019, 3, 2033–2044. [Google Scholar] [CrossRef]
- He, D.; He, C.; Li, P.H.; Zhang, X.W.; Shi, Q.; Sun, Y.G. Optical and molecular signatures of dissolved organic matter reflect anthropogenic influence in a Coastal River, northeast China. J. Environ. Qual. 2019, 48, 603–613. [Google Scholar] [CrossRef]
- Chaminda, G.G.T.; Nakajima, F.; Furumai, H.; Kasuga, I.; Kurisu, F. Comparison of metal (Zn and Cu) complexation characteristics of DOM in urban runoff, domestic wastewater and secondary effluent. Water Sci. Technol. 2010, 62, 2044–2050. [Google Scholar] [CrossRef]
- Wang, N.; Wang, X.C.C.; Liu, H.L.; Zheng, Y.C.; Zhang, Y.; Xiong, J.Q.; Pan, P.; Liu, Y.Z. Speciation, distribution and risk assessment of metals in sediments from a water body replenished by effluent from a wastewater treatment plant. Bull. Environ. Contam. Toxicol. 2019, 102, 525–530. [Google Scholar] [CrossRef]
- Wu, F.C.; Evans, D.; Dillon, P.; Schiff, S. Molecular size distribution characteristics of the metal-DOM complexes in stream waters by high-performance size-exclusion chromatography (HPSEC) and high-resolution inductively coupled plasma mass spectrometry (ICP-MS). J. Anal. At. Spectrom. 2004, 19, 979–983. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, Y.; Tian, Y.L.; Hou, Z.J.; He, K.J.; Fu, L.; Xu, H. Impact of land use on the DOM composition in different seasons in a subtropical river flowing through a region undergoing rapid urbanization. J. Clean Prod. 2019, 212, 1224–1231. [Google Scholar] [CrossRef] [Green Version]
- Liang, M.-Q.; Shao, M.-L.; Cao, C.-L.; Zong, Y.-N.; Tang, J.-F. Characteristics of dissolved organic matter (DOM) and relationship with dissolved heavy metals in a peri-urban and an urban river. Huan jing ke xue= Huanjing kexue 2018, 39, 2095–2103. [Google Scholar] [PubMed]
- Zhang, D.; Wang, J.J.; Ni, H.G.; Zeng, H. Spatial-temporal and multi-media variations of polycyclic aromatic hydrocarbons in a highly urbanized river from South China. Sci. Total Environ. 2017, 581, 621–628. [Google Scholar] [CrossRef]
- Qiu, W.H.; Sun, J.; Fang, M.J.; Luo, S.S.; Tian, Y.Q.; Dong, P.Y.; Xu, B.T.; Zheng, C.M. Occurrence of antibiotics in the main rivers of Shenzhen, China: Association with antibiotic resistance genes and microbial community. Sci. Total Environ. 2019, 653, 334–341. [Google Scholar] [CrossRef]
- Yu, K.; Duan, Y.; Liao, P.; Xie, L.; Li, Q.; Ning, Z.; Liu, C. Watershed-scale distributions of heavy metals in the hyporheic zones of a heavily polluted Maozhou River watershed, southern China. Chemosphere 2019, 239, 124773. [Google Scholar] [CrossRef]
- Liao, H.H.; Yu, K.; Duan, Y.H.; Ning, Z.G.; Li, B.R.; He, L.Y.; Liu, C.X. Profiling microbial communities in a watershed undergoing intensive anthropogenic activities. Sci. Total Environ. 2019, 647, 1137–1147. [Google Scholar] [CrossRef]
- Cao, C.; Chen, X.P.; Ma, Z.B.; Jia, H.H.; Wang, J.J. Greenhouse cultivationmitigates metal-ingestion-associated health risks from vegetables in wastewater-irrigated agroecosystems. Sci. Total Environ. 2016, 560, 204–211. [Google Scholar] [CrossRef]
- Fellman, J.B.; Hood, E.; Spencer, R.G.M. Fluorescence spectroscopy opens new windows into dissolved organic matter dynamics in freshwater ecosystems: A review. Limnol. Oceanogr. 2010, 55, 2452–2462. [Google Scholar] [CrossRef]
- Korak, J.A.; Wert, E.C.; Rosario-Ortiz, F.L. Evaluating fluorescence spectroscopy as a tool to characterize cyanobacteria intracellular organic matter upon simulated release and oxidation in natural water. Water Res. 2015, 68, 432–443. [Google Scholar] [CrossRef]
- Birdwell, J.E.; Engel, A.S. Characterization of dissolved organic matter in cave and spring waters using UV-Vis absorbance and fluorescence spectroscopy. Org. Geochem. 2010, 41, 270–280. [Google Scholar] [CrossRef]
- Tank, S.E.; Lesack, L.F.W.; Gareis, J.A.L.; Osburn, C.L.; Hesslein, R.H. Multiple tracers demonstrate distinct sources of dissolved organic matter to lakes of the Mackenzie Delta, western Canadian Arctic. Limnol. Oceanogr. 2011, 56, 1297–1309. [Google Scholar] [CrossRef] [Green Version]
- Weishaar, J.L.; Aiken, G.R.; Bergamaschi, B.A.; Fram, M.S.; Fujii, R.; Mopper, K. Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ. Sci. Technol. 2003, 37, 4702–4708. [Google Scholar] [CrossRef] [PubMed]
- Helms, J.R.; Stubbins, A.; Ritchie, J.D.; Minor, E.C.; Kieber, D.J.; Mopper, K. Absorption spectral slopes and slope ratios as indicators of molecular weight, source, and photobleaching of chromophoric dissolved organic matter. Limnol. Oceanogr. 2008, 53, 955–969. [Google Scholar] [CrossRef] [Green Version]
- Ohno, T. Fluorescence inner-filtering correction for determining the humification index of dissolved organic matter. Environ. Sci. Technol. 2002, 36, 742–746. [Google Scholar] [CrossRef]
- Wilson, H.F.; Xenopoulos, M.A. Effects of agricultural land use on the composition of fluvial dissolved organic matter. Nat. Geosci. 2009, 2, 37–41. [Google Scholar] [CrossRef]
- State Environmental Protection Administration. Environmental Quality Standard of Surface Water GB 3838-2002; State Environmental Protection Administration: Beijing, China, 2002. [Google Scholar]
- Harguinteguy, C.A.; Gudino, G.L.; Aran, D.S.; Pignata, M.L.; Fernandez-Cirelli, A. Comparison between two submerged macrophytes as biomonitors of trace elements related to anthropogenic activities in the ctalamochita river, argentina. Bull. Environ. Contam. Toxicol. 2019, 102, 105–114. [Google Scholar] [CrossRef]
- Leventeli, Y.; Yalcin, F.; Kilic, M. An investigation about heavy metal pollution of duden and goksu streams (Antalya, Turkey). Appl. Ecol. Environ. Res. 2019, 17, 2423–2436. [Google Scholar] [CrossRef]
- Islam, M.S.; Ahmed, M.K.; Raknuzzaman, M.; Habibullah-Al-Mamun, M.; Islam, M.K. Heavy metal pollution in surface water and sediment: A preliminary assessment of an urban river in a developing country. Ecol. Indic. 2015, 48, 282–291. [Google Scholar] [CrossRef]
- Le Pape, P.; Ayrault, S.; Quantin, C. Trace element behavior and partition versus urbanization gradient in an urban river (Orge River, France). J. Hydrol. 2012, 472, 99–110. [Google Scholar] [CrossRef]
- Li, S.Y.; Zhang, Q.F. Spatial characterization of dissolved trace elements and heavy metals in the upper Han River (China) using multivariate statistical techniques. J. Hazard. Mater. 2010, 176, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Elbaz-Poulichet, F.; Seidel, J.L.; Casiot, C.; Tusseau-Vuillemin, M.H. Short-term variability of dissolved trace element concentrations in the Marne and Seine Rivers near Paris. Sci. Total Environ. 2006, 367, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.T.; Nguyen, H.M.; Truong, C.K.; Ahmed, M.B.; Huang, Y.; Zhou, J.L. Chemical and microbiological risk assessment of urban river water quality in Vietnam. Environ. Geochem. Health 2019, 41, 2559–2575. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, E.; Verma, K.; Pandey, U.; Pandey, J. Metal Contamination in Seven Tributaries of the Ganga River and Assessment of Human Health Risk from Fish Consumption. Arch. Environ. Contam. Toxicol. 2019, 77, 263–278. [Google Scholar] [CrossRef]
- Franklin, N.M.; Stauber, J.L.; Lim, R.P.; Petocz, P. Toxicity of metal mixtures to a tropical freshwater alga (Chlorella sp): The effect of interactions between copper, cadmium, and zinc on metal cell binding and uptake. Environ. Toxicol. Chem. 2002, 21, 2412–2422. [Google Scholar] [CrossRef]
- Woody, C.A.; O’Neal, S. Effects of Copper on Fish and Aquatic Resources; Fisheries Research and Consulting: Alaska, AK, USA, 2012; Available online: https://www.conservationgateway.org/ConservationByGeography/NorthAmerica/UnitedStates/alaska/sw/cpa/Documents/W2013ECopperF062012.pdf (accessed on 10 September 2019).
- Gan, Y.D.; Huang, X.M.; Li, S.S.; Liu, N.; Li, Y.C.C.; Freidenreich, A.; Wang, W.X.; Wang, R.Q.; Dai, J.L. Source quantification and potential risk of mercury, cadmium, arsenic, lead, and chromium in farmland soils of Yellow River Delta. J. Clean Prod. 2019, 221, 98–107. [Google Scholar] [CrossRef]
- Feng, J.J.; Chen, X.L.; Jia, L.; Liu, Q.Z.; Chen, X.J.; Han, D.M.; Cheng, J.P. Effluent concentration and removal efficiency of nine heavy metals in secondary treatment plants in Shanghai, China. Environ. Sci. Pollut. Res. 2018, 25, 17058–17065. [Google Scholar] [CrossRef]
- Islam, F.S.; Gault, A.G.; Boothman, C.; Polya, D.A.; Charnock, J.M.; Chatterjee, D.; Lloyd, J.R. Role of metal-reducing bacteria in arsenic release from Bengal delta sediments. Nature 2004, 430, 68–71. [Google Scholar] [CrossRef]
- Mladenov, N.; Zheng, Y.; Simone, B.; Bilinski, T.M.; McKnight, D.M.; Nemergut, D.; Radloff, K.A.; Rahman, M.M.; Ahmed, K.M. Dissolved organic matter quality in a shallow aquifer of bangladesh: Implications for arsenic mobility. Environ. Sci. Technol. 2015, 49, 10815–10824. [Google Scholar] [CrossRef]
- Kozyatnyk, I.; Bouchet, S.; Bjorn, E.; Haglund, P. Fractionation and size-distribution of metal and metalloid contaminants in a polluted groundwater rich in dissolved organic matter. J. Hazard. Mater. 2016, 318, 194–202. [Google Scholar] [CrossRef]
- Warren, L.A.; Haack, E.A. Biogeochemical controls on metal behaviour in freshwater environments. Earth-Sci. Rev. 2001, 54, 261–320. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, H.; Shao, L.M.; He, P.J. Fluorescent characteristics and metal binding properties of individual molecular weight fractions in municipal solid waste leachate. Environ. Pollut. 2012, 162, 63–71. [Google Scholar] [CrossRef] [PubMed]




| Water Type | n | pH | EC (mS·m−1) | Zn (µg·L−1) | Cu (µg·L−1) | Cr (µg·L−1) | As (µg·L−1) | Pb (µg·L−1) | Cd (µg·L−1) | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Maozhou River, Shenzhen (China) | Mainstream | 22 | 7.35 ± 0.21 | 2.00 ± 3.38 | 64.01 ± 43.31 | 6.33 ± 3.94 | 1.48 ± 0.97 | 1.24 ± 0.37 | 0.32 ± 0.28 | 0.14 ± 0.15 | this study |
| Tributary | 43 | 7.69 ± 0.50 | 3.06 ± 4.97 | 41.56 ± 53.12 | 13.49 ± 38.68 | 2.96 ± 4.87 | 1.47 ± 0.62 | 0.38 ± 0.32 | 0.11 ± 0.13 | ||
| Pond | 11 | 8.25 ± 0.30 | 0.76 ± 0.50 | 9.58 ± 6.75 | 0.74 ± 0.57 | 0.29 ± 0.28 | 1.87 ± 0.81 | 0.19 ± 0.17 | 0.01 ± 0.01 | ||
| Reservoir | 6 | 7.33 ± 0.14 | 0.09 ± 0.03 | 8.38 ± 3.03 | 0.53 ± 0.20 | 0.10 ± 0.02 | 0.69 ± 0.23 | 0.02 ± 0.02 | <0.01 | ||
| Ctalamochita River (Argentina) | River water | 30 | 8.5 | 3.74 | 32.3 | 12.7 | 12.7 | 45.5 | 5.6 | - | [49] |
| Goksu Stream (Turkey) | River water | 18 | - | - | - | 4.69 | 0.80 | 6.86 | - | - | [50] |
| Sagami River (Japan) | River water | 92 | 6.4–9.4 | - | 1.27 | 0.81 | - | - | - | - | [18] |
| Korotoa River (Bangladesh) | River water | 10 | - | - | 73 | - | 83 | 46 | 11 | 35 | [51] |
| Orge River (France) | River water | 32 | - | - | 1.6 | 62 | 0.68 | - | - | 0.17 | [52] |
| Han River (China) | River water | 42 | - | - | - | 13.35 | 8.14 | 14.2 | 9.26 | 2.31 | [53] |
| National standards (China) a | 50 | 10 | 10 | 50 | 10 | 1 |
| Water Type | Single Factor Index Method (Pi) | Synthetic Pollution Index (Psyn) | ||||||
|---|---|---|---|---|---|---|---|---|
| Zn | Cu | Cr | As | Pb | Cd | |||
| Mainstream | upper | 0.658 | 0.173 | 0.063 | 0.018 | 0.004 | 0.014 | 0.54 |
| middle | 1.962 | 0.828 | 0.142 | 0.024 | 0.055 | 0.212 | 1.67 | |
| lower | 0.982 | 0.877 | 0.254 | 0.034 | 0.032 | 0.182 | 0.97 | |
| overall | 1.201 | 0.626 | 0.153 | 0.025 | 0.030 | 0.136 | 0.63 | |
| Tributary | 0.831 | 1.350 | 0.300 | 0.030 | 0.038 | 0.112 | 1.22 | |
| Pond | 0.192 | 0.074 | 0.029 | 0.037 | 0.019 | 0.014 | 0.17 | |
| Reservoir | 0.168 | 0.052 | 0.010 | 0.014 | 0.002 | 0.004 | 0.14 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Chen, X.-W.; Ye, Q.; Zhang, Z.-T.; Kong, S.-F.; Cao, C.; Wang, J.-J. Dissolved Metal(loid) Concentrations and Their Relations with Chromophoric and Fluorescent Dissolved Organic Matter in an Urban River in Shenzhen, South China. Water 2020, 12, 281. https://doi.org/10.3390/w12010281
Zhang S, Chen X-W, Ye Q, Zhang Z-T, Kong S-F, Cao C, Wang J-J. Dissolved Metal(loid) Concentrations and Their Relations with Chromophoric and Fluorescent Dissolved Organic Matter in an Urban River in Shenzhen, South China. Water. 2020; 12(1):281. https://doi.org/10.3390/w12010281
Chicago/Turabian StyleZhang, Song, Xun-Wen Chen, Quanhui Ye, Zi-Ting Zhang, Si-Fang Kong, Chun Cao, and Jun-Jian Wang. 2020. "Dissolved Metal(loid) Concentrations and Their Relations with Chromophoric and Fluorescent Dissolved Organic Matter in an Urban River in Shenzhen, South China" Water 12, no. 1: 281. https://doi.org/10.3390/w12010281
APA StyleZhang, S., Chen, X.-W., Ye, Q., Zhang, Z.-T., Kong, S.-F., Cao, C., & Wang, J.-J. (2020). Dissolved Metal(loid) Concentrations and Their Relations with Chromophoric and Fluorescent Dissolved Organic Matter in an Urban River in Shenzhen, South China. Water, 12(1), 281. https://doi.org/10.3390/w12010281