Algal Bloom Occurrence and Effects in Russia
Abstract
:1. Introduction
2. Materials and Methods
3. Results
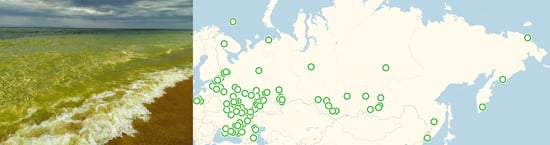
3.1. Geographical Distribution of Algal Blooms in Russia
3.2. Description of Outbreaks in Mass-Media
3.3. Reported Effects of Algal Blooms
3.4. Description of Outbreaks in Scientific Articles
4. Discussion
4.1. Algal Bloom Occurrence in Russia
4.2. Causes and Effects of Algal Blooms
4.3. Public and Governmental Reaction to Algal Blooms in Russia
4.4. Mass Media Representation of Algal Blooms in Russia
4.5. Comparison of the Mass-Media and Scientific Reports
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Directive, E.U.W. Council Directive of 21 May 1991 concerning urban waste water treatment (91/271/EEC). J. Eur. Commun. 1991, 34, 40. [Google Scholar]
- Graham, J.L.; Jones, J.R.; Jones, S.B.; Downing, J.A.; Clevenger, T.E. Environmental factors influencing microcystin distribution and concentration in the Midwestern United States. Water Res. 2004, 38, 4395–4404. [Google Scholar] [CrossRef] [PubMed]
- Schindler, D.W.; Carpenter, S.R.; Chapra, S.C.; Hecky, R.E.; Orihel, D.M. Reducing phosphorus to curb lake eutrophication is a success. Environ. Sci. Technol. 2016, 50, 8923–8929. [Google Scholar] [CrossRef] [PubMed]
- Harris, T.D.; Smith, V.H.; Graham, J.L.; Waa, D.B.V.; Tedesco, L.P.; Clercin, N. Combined effects of nitrogen to phosphorus and nitrate to ammonia ratios on cyanobacterial metabolite concentrations in eutrophic Midwestern USA reservoirs. Inland Waters 2016, 6, 199–210. [Google Scholar] [CrossRef] [Green Version]
- ILEC/Lake Biwa Research Institute. n.d. 1988–1993 Survey of the State of the World’s Lakes; Volumes IIV; International Lake Environment Committee, Otsu and United Nations Environment Programme: Nairobi, Kenya, 1993. [Google Scholar]
- Ho, J.C.; Michalak, A.M.; Pahlevan, N. Widespread global increase in intense lake phytoplankton blooms since the 1980s. Nature 2019, 574, 667–670. [Google Scholar] [CrossRef]
- Stoddard, J.L.; Van Sickle, J.; Herlihy, A.T.; Brahney, J.; Paulsen, S.; Peck, D.V.; Mitchell, R.; Pollard, A.I. Continental-scale increase in lake and stream phosphorus: Are oligotrophic systems disappearing in the United States? Environ. Sci. Technol. 2016, 50, 3409–3415. [Google Scholar] [CrossRef] [Green Version]
- Pouria, S.; de Andrade, Al.; Barbosa, J.; Cavalcanti, R.L.; Barreto, V.T.S.; Ward, C.J.; Preiser, W.; Poon, G.K.; Neild, G.H.; Codd, G.A. Fatal microcystin intoxication in haemodialysis unit in Caruaru, Brazil. Lancet 1998, 352, 21–26. [Google Scholar] [CrossRef]
- Le Moal, M.; Gascuel-Odoux, C.; Ménesguen, A.; Souchon, Y.; Étrillard, C.; Levain, A.; Moatar, F.; Pannard, A.; Souchi, P.; Lefebvre, A.; et al. Eutrophication: A new wine in an old bottle? Sci. Total Environ. 2019, 651, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Dodds, W.; Bouska, W.W.; Eitzmann, J.L.; Pilger, T.J.; Pitts, K.L.; Riley, A.J.; Schloesser, J.T.; Thornbrugh, D.J. Policy analysis eutrophication of U.S. Freshwaters: Damages. Environ. Sci. Technol. 2009, 19, 43. [Google Scholar] [CrossRef] [Green Version]
- Kudela, R.M.; Berdalet, E.; Bernard, S.; Bur-ford, M.; Fernand, L.; Lu, S.; Roy, S.; Tester, P.; Usup, G.; Magnien, R.; et al. Harmful Algal Blooms. A Scientific Summary for Policy Makers; IOC/INF-1320; IOC/UNESCO: Paris, France, 2015. [Google Scholar]
- Beaulieu, J.J.; DelSontro, T.; Downing, J.A. Eutrophication will increase methane emissions from lakes and impoundments during the 21st century. Nat. Commun. 2019, 10, 1375. [Google Scholar] [CrossRef] [Green Version]
- Shiklomanov, I.A. Appraisal and assessment of world water resources. Water Int. 2000, 25, 11–32. [Google Scholar] [CrossRef]
- Gromov, В.V.; Vepritsky, A.A.; Mamkaeva, K.A.; Voloshko, L.N. A survey of cyanobacterial blooms in Lake Ladoga and adjacent water bodies. Hydrobiologia 1996, 322, 129–136. [Google Scholar] [CrossRef]
- Drabkova, V.; Rumyantsev, V.; Sergeeva, L.V.; Slepukhina, T.D. Ecological problems of Lake Ladoga, causes and solutions. Hydrobiologia 1996, 322, 1–7. [Google Scholar] [CrossRef]
- Vershinin, A.O.; Orlova, T.Yu. Toxic and harmful algae in the coastal waters of Russia. Oceanology 2008, 48, 524–537. [Google Scholar] [CrossRef]
- Mineeva, N.M. Plankton Primary Production in the Volga River Reservoirs; Print House: Yaroslavl, Russia, 2009; p. 279. (In Russian) [Google Scholar]
- Korneva, L.G. Phytoplankton of Volga River Basin Reservoirs; Print House: Yaroslavl, Russia, 2015; p. 284. (In Russian) [Google Scholar]
- Voloshko, L.N.; Pinevich, A.V.; Kopeckiy, I.; Titova, N.N.; Hrouseck, P.; Zelik, P. Water blooms and toxins produced by cyanobacteria in the Lower Suzdalskoe lake (Saint-Petersburg, Russia). Algology 2008, 20, 210–223. [Google Scholar]
- Sidelev, S.I.; Golokolenovab, T.B.; Chernovac, E.N.; Russkikh, I.V. Analysis of Phytoplankton in Tsimlyansk Reservoir (RUSSIA) for the Presence of Cyanobacterial Hepato- and Neurotoxins. Microbiology 2015, 84, 732–742. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Available online: https://www.epa.gov/national-aquatic-resource-surveys/nla (accessed on 11 December 2019).
- State Report “On the State and Use of Water Resources of the Russian Federation in 2017”. Available online: https://water-rf.ru/water/gosdoc/476.html (accessed on 11 December 2019).
- Politi, E.; MacCallum, S.; Cutler, M.E.J.; Merchant, C.J.; Rowan, J.S.; Dawson, T.P. Selection of a network of large lakes and reservoirs suitable for global environmental change analysis using Earth Observation. Int. J. Remote Sens. 2016, 37, 3042–3060. [Google Scholar] [CrossRef] [Green Version]
- Monitoring of Water Bodies in Russia—Сyanohab.ru. Available online: https://cyanohab.ru/ (accessed on 11 December 2019). (In Russian).
- News Website—Yandex News. Available online: https://news.yandex.ru (accessed on 11 December 2019). (In Russian).
- Bibliographic Database—Google Scholar. Available online: https://scholar.google.com (accessed on 11 December 2019).
- Scientific Electronic Library—eLIBRARY.RU. Available online: https://elibrary.ru (accessed on 11 December 2019). (In Russian).
- Anne-Wil Harzing’s Website. Available online: https://harzing.com/resources/publish-or-perish (accessed on 11 December 2019).
- Federal State Statistic Service. Available online: https://gks.ru/ (accessed on 11 December 2019). (In Russian).
- Unified Interdepartmental Information and Statistical System. State Statistics. Available online: https://fedstat.ru/ (accessed on 11 December 2019). (In Russian).
- Federal State Statistic Service. Demographics Section. Available online: https://gks.ru/folder/12781 (accessed on 11 December 2019). (In Russian).
- Federal State Statistic Service. Enterprise Economy Section. Available online: https://gks.ru/enterprise_economy (accessed on 11 December 2019). (In Russian).
- Unified Interdepartmental Information and Statistical System. State Statistics. Enterprise Economy Section. Available online: https://fedstat.ru/indicator/31325 (accessed on 11 December 2019). (In Russian).
- Shashlova, N. Natural resources and environmental protection. In Russian Statistical Yearbook; Surinov, A., Ed.; Rosstat: Moscow, Russia, 2017; pp. 68–81. (In Russian) [Google Scholar]
- Shashlova, N. Natural resources and environmental protection. In Russian Statistical Yearbook; Surinov, A., Ed.; Rosstat: Moscow, Russia, 2018; pp. 69–82. (In Russian) [Google Scholar]
- Federal State Statistic Service. Environment Section. Available online: https://gks.ru/folder/11194 (accessed on 11 December 2019). (In Russian).
- WORLD MAP OF THE KÖPPEN-GEIGER CLIMATE CLASSIFICATION UPDATED. Available online: http://koeppen-geiger.vu-wien.ac.at/present.htm (accessed on 11 December 2019).
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Google Earth. Available online: http://www.google.ru/intl/ru/earth/ (accessed on 11 December 2019).
- Pandas. Available online: https://pandas.pydata.org/ (accessed on 11 December 2019).
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0 Contributors. SciPy 1.0 Fundamental Algorithms for Scientific Computing in Python. 2019. Available online: https://arxiv.org/abs/1907.10121 (accessed on 11 December 2019).
- The Television Company KTV-RAY. Available online: http://ktv-ray.ru/novost/vlasti-obyasnili-otkuda-v-samarskoy-oblasti-vzyalis-rozovye-ozera/36283/ (accessed on 11 December 2019).
- Gorbunov, M.Yu.; (Institute of Ecology of the Volga River Basin, Tolyatti, Samara region, Russia). Personal communication, 2018.
- Lepskaya, E.; Efimova, K.; Shubkin, S.; Kolomeitsev, V. Toxic “bloom” and Pacific Salmon (Catch, Spawning Migrations, Production) in the Far Eastern Seas of Russia—Are There New Risks? North Pac. Anadromous Fish Comm. Tech. Rep. 2018, 11, 80–86. (In Russian) [Google Scholar]
- Kostryukova, A.M.; Mashkova, I.V.; Trofimenko, V.V.; Vasilieva, E.I. Taxonomic structure of phytoplankton in Shershnevskoe Reservoir (Chelyabinsk, Russia), an artificial lake. Iop Conf. Ser. Earth Environ. Sci. 2019, 351, 012001. (In Russian) [Google Scholar] [CrossRef]
- Stepanova, N.; Nikitin, O.; Latypova, V.; Kondratyeva, T. Cyanotoxins as a possible cause of fish and waterfowl death in the Kazanka river (Russia). In Proceedings of the 18th International Multidisciplinary Scientific GeoConference SGEM, Albena, Bulgaria, 2–8 July 2018; Volume 18, pp. 229–236. (In Russian). [Google Scholar] [CrossRef]
- Grachev, M.; Zubkov, I.; Tikhonova, I.; Ivacheva, M.; Kuzmin, A.; Sukhanova, E.; Sorokovikova, E.; Fedorova, G.; Galkin, A.; Suslova, M.; et al. Extensive contamination of water with saxitoxin near the dam of the Irkutsk hydropower station reservoir (East Siberia, Russia). Toxins 2018, 10, 402. (In Russian) [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melnik, A.S.; Ezhova, E.E. Blossom cyanobacteria in territorial waters and economic zone of the Russian Federation in southeast Baltic sea in July 2018. In Integrated researches of the world ocean. In Proceedings of the Materials of the IV All-Russian Scientific Conference of Young Scientists, Moscow, Russia, 22–26 April 2019; Marine Hydrophysical Institute of the Russian Academy of Sciences: Sevastopol, Russia, 2019; pp. 230–231. (In Russian). [Google Scholar]
- Vasilenko, N.V.; Medvedeva, A.V. Abnormal flowering of blue-green algae in the Sea of Azov. In Complex studies of the World Ocean. In Proceedings of the Materials of the IV All-Russian Scientific Conference of Young Scientists, Sevastopol, Russia, 22–26 April 2019; pp. 247–248. (In Russian). [Google Scholar]
- Lepskaya, E.V. Cyanoprokariota (blue-green algae) «bloom» in lake Khalaktyrskoye (Eastern Kamchatka). In Conservation of biodiversity of Kamchatka and adjacent seas. In Proceedings of the Materials of the XVIII International Scientific Conference Dedicated to the 70th Birthday of Doctor of Biological Sciences, P.A. Khomentovsky, Petropavlovsk-Kamchatsky, Russia, 15–16 November 2017; pp. 239–241. (In Russian). [Google Scholar]
- Selezneva, A.V.; Bespalova, K.V.; Seleznev, V.A. Estimation of seasonal variability of water quality in a surface source of drinking water supply. Urban Plan. Archit. 2018, 2, 20–26. [Google Scholar] [CrossRef]
- Trifonova, I.S.; Afanas’eva, A.L.; Rodionova, N.V. Seasonal dynamics of phytoand zooplankton of an eutrophicated lake in years with different water content and temperature conditions. Reg. Ecol. 2018, 3, 29–38. [Google Scholar] [CrossRef]
- Shekhmatova, E.I.; Gaponenko, A.V. Influence algae and cyanobacteria on the ecological state of reservoirs (studies Kosinski lakes). In Integrated aquaculture technologies in farms. In Proceedings of the Materials of the International Scientific-Practical Conference, Moscow, Russia, 9 December 2016; pp. 172–177. [Google Scholar]
- Namsaraev, Z.; Melnikova, A.; Ivanov, V.; Komova, A.; Teslyuk, A. Cyanobacterial bloom in the world largest freshwater lake Baikal. IOP Conf. Ser. Earth Environ. Sci. 2018, 3, 032039. [Google Scholar] [CrossRef]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef]
- Mantzouki, E.; Lürling, M.; Fastner, J.; De Senerpont Domis, L.; Wilk-Woźniak, E.; Koreivienė, J.; Seelen, L.; Teurlincx, S.; Verstijnen, Y.; Krztoń, W.; et al. Temperature Effects Explain Continental Scale Distribution of Cyanobacterial Toxins. Toxins 2018, 10, 156. [Google Scholar] [CrossRef] [Green Version]
- Chernova, E.; Sidelev, S.; Russkikh, I.; Voyakina, E.; Babanazarova, O.; Romanov, R.; Kotovshchikov, A.; Mazur-Marzec, H. Dolichospermum and Aphanizomenon as neurotoxins producers in some Russian freshwaters. Toxicon 2017, 130, 47–55. [Google Scholar] [CrossRef]
- Tekanova, E.; Lozovik, P.; Kalinkina, N.; Kulikova, T.; Polyakova, T.; Ryabinkin, A.; Slastina, Y.; Timakova, T.; Chekryzheva, T. Recent state and transformation of the northern part of Vygozerskoe reservoir. Work. Karel Sci. Cent. Russ. Acad. Sci. 2011, 4, 50–56. (In Russian) [Google Scholar]
- Shaikhutdinova, A.A.; Kuksanov, V.F.; Knyazeva, A.N. Studies on the effectiveness of household sewage disposal in the city of Orenburg. Lett. Orenbg. State Agric. Univ. 2017, 4, 240–243. (In Russian) [Google Scholar]
- Martysheva, N.A.; Khoruzhaya, T.A. Features of the “Bloom” of the water of the Tsimlyansk reservoir with blue-green microalgae. News High. Educ. Institutions. North Cauc. Region. Nat. Sci. 2017, 2, 136–141. (In Russian) [Google Scholar]
- The Charity Fund “Rescue and Preservation of Tsimlyansk Reservoir”. Available online: https://www.fon-don.com/single-post/2016/05/25/Как-Волгодонск-травит-сам-себя-и-выращивает-ядовитые-синезеленые-водоросли (accessed on 11 December 2019). (In Russian).
- The Civic Chamber of the Russian. Available online: https://www.oprf.ru/press/news/2015/newsitem/29532 (accessed on 11 December 2019).
- The May Decree Interregional Public Organization. Passport of the Federal Project “Preservation of unique water bodies”. Available online: http://xn--80aavcebfcm6cza.xn--p1ai/biblioteka/federalnye-proekty/%D1%84%D0%B5%D0%B4%D0%B5%D1%80%D0%B0%D0%BB%D1%8C%D0%BD%D1%8B%D0%B9-%D0%BF%D1%80%D0%BE%D0%B5%D0%BA%D1%82-%D1%81%D0%BE%D1%85%D1%80%D0%B0%D0%BD%D0%B5%D0%BD%D0%B8%D0%B5-%D1%83%D0%BD%D0%B8%D0%BA%D0%B0/ (accessed on 11 December 2019).
- Hyder, K.; Wright, S.; Kirby, M.; Brant, J. The role of citizen science in monitoring small-scale pollution events. Mar. Pollut. Bull. 2017, 120, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Lyytimäki, J.M. Temporalities and environmental reporting: Press news on eutrophication in Finland. Environ. Sci. 2007, 4, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Jönsson, A.M. Framing environmental risks in the Baltic Sea: A news media analysis. Ambio 2011, 40, 121–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bera, R.; Hrybyk, A. iWitness pollution map: Crowdsourcing petrochemical accident research. New Solut. A J. Environ. Occup. Health Policy 2013, 23, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Loiselle, S.A.; Frost, P.C.; Turak, E.; Thornhill, I. Citizen scientists supporting environmental research priorities. Sci. Total Environ. 2017, 598, 937. [Google Scholar] [CrossRef] [PubMed] [Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Namsaraev, Z.; Melnikova, A.; Komova, A.; Ivanov, V.; Rudenko, A.; Ivanov, E. Algal Bloom Occurrence and Effects in Russia. Water 2020, 12, 285. https://doi.org/10.3390/w12010285
Namsaraev Z, Melnikova A, Komova A, Ivanov V, Rudenko A, Ivanov E. Algal Bloom Occurrence and Effects in Russia. Water. 2020; 12(1):285. https://doi.org/10.3390/w12010285
Chicago/Turabian StyleNamsaraev, Zorigto, Anna Melnikova, Anastasia Komova, Vasily Ivanov, Anastasia Rudenko, and Evgenii Ivanov. 2020. "Algal Bloom Occurrence and Effects in Russia" Water 12, no. 1: 285. https://doi.org/10.3390/w12010285
APA StyleNamsaraev, Z., Melnikova, A., Komova, A., Ivanov, V., Rudenko, A., & Ivanov, E. (2020). Algal Bloom Occurrence and Effects in Russia. Water, 12(1), 285. https://doi.org/10.3390/w12010285