Behavior of PBTC, HEDP, and Aminophosphonates in the Process of Wastewater Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Concept
2.1.1. Overview
2.1.2. Liquid Samples
2.1.3. Solids Extraction from WWTP Influent Samples
2.2. Analytical Methods
2.2.1. pH Determination
2.2.2. Solids Concentration
2.2.3. Phosphorus (Total P, Dissolved P, o-PO4-P)
2.2.4. Sample Preparation for Phosphonate Analysis
2.2.5. Phosphonate Quantification via IC-ESI-MS/MS
3. Results and Discussion
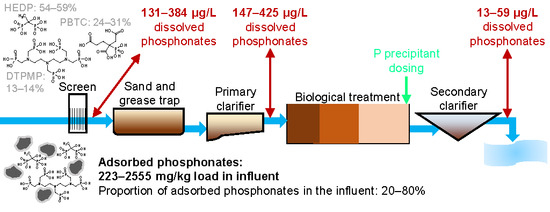
3.1. Dissolved and Adsorbed Phosphonates in WWTP Influents
3.2. Phosphonates in Individual Treatment Stages
3.2.1. Primary Clarification
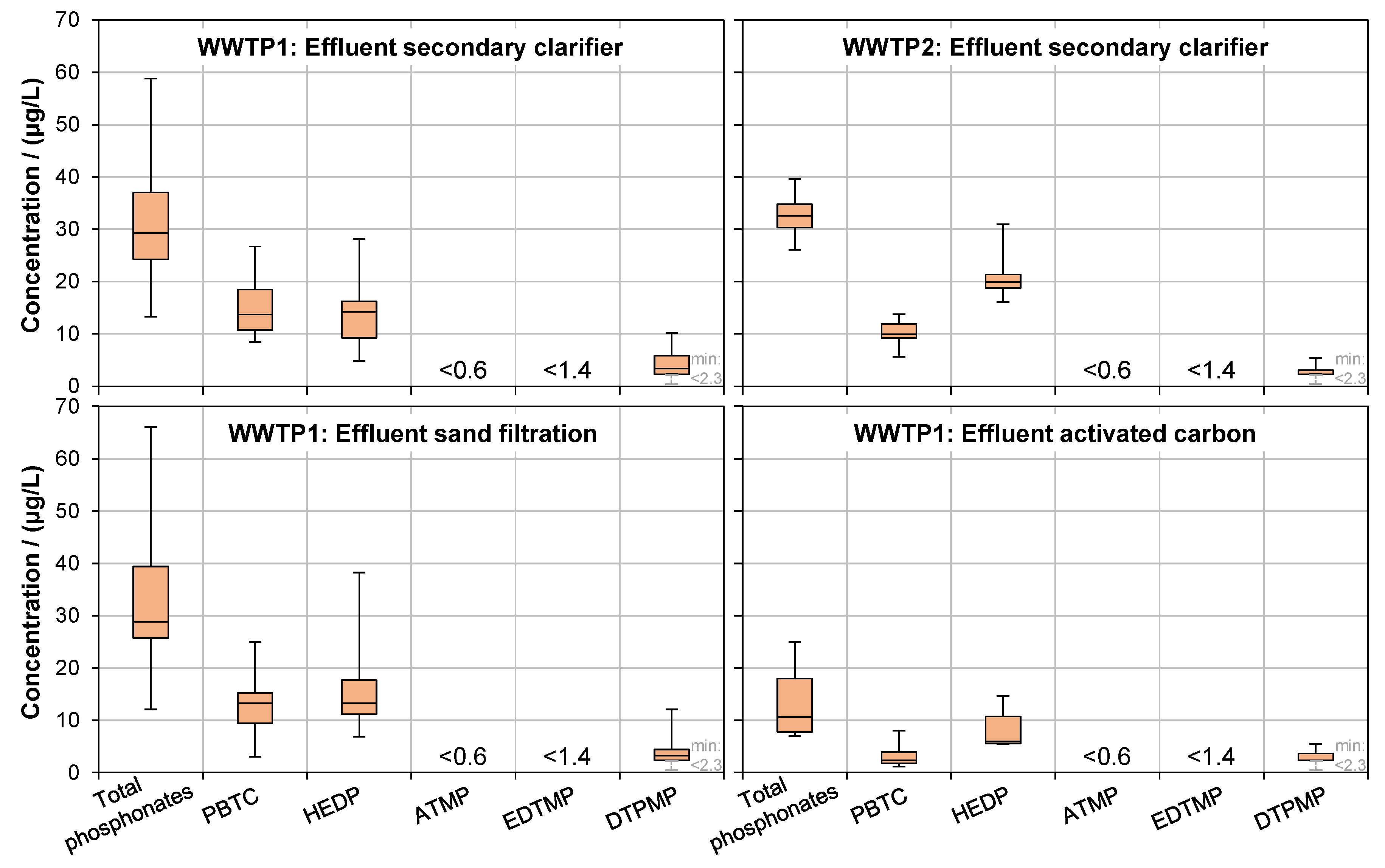
3.2.2. Secondary Clarification
3.2.3. Tertiary Treatment
3.3. Contribution of Phosphonate-P to the DUP Fraction
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A



References
- EPA. Phosphonates in Detergents. European Phosphonate Association Detergent Phosphonates Dossier; EPA: Brussels, Belgium, 2013. [Google Scholar]
- Industrieverband Körperpflege- und Waschmittel e.V. Report Sustainability in the Detergent, Care and Cleaning Products Sector in Germany 2015–2016 (Bericht Nachhaltigkeit in der Wasch-, Pflege- und Reinigungsmittelbranche in Deutschland 2015–2016); Industrieverband Körperpflege- und Waschmittel e.V.: Frankfurt am Main, Germany, 2017. [Google Scholar]
- Industrieverband Körperpflege- und Waschmittel e.V. Report Sustainability in the Detergent, Care and Cleaning Products Sector in Germany Edition 2019 (Bericht Nachhaltigkeit in der Wasch-, Pflege- und Reinigungsmittelbranche in Deutschland Ausgabe 2019); Industrieverband Körperpflege- und Waschmittel e.V.: Frankfurt am Main, Germany, 2019. [Google Scholar]
- Davenport, B.; DeBoo, A.; Dubois, F.; Kishi, A. CEH Report: Chelating Agents; Cited by Nowack (2003); IHS Markit: London, UK, 2000. [Google Scholar]
- Nowack, B. Environmental chemistry of phosphonates. Water Res. 2003, 37, 2533–2546. [Google Scholar] [CrossRef]
- Lucci, G.M.; McDowell, R.W.; Condron, L.M. Phosphorus source areas in a dairy catchment in Otago, New Zealand. Soil Res. 2012, 50, 145. [Google Scholar] [CrossRef]
- Reichert, J.K. Organophosphonic Acids Evaluation of the Performance of Biological Sewage Treatment Plants for Their Elimination and Assessment of Their Environmental Risk Potential (Organophosphonsäuren Bewertung der Leistungsfähigkeit biologischer Abwasserbehandlungsanlagen zu ihrer Eliminierung und Beurteilung ihres Umweltgefährdungspotentials); Final report to Deutsche Bundesstiftung Umwelt: Osnabrück, Germany, 1996. [Google Scholar]
- Fischer, K. Sorption of chelating agents (HEDP and NTA) onto mineral phases and sediments in aquatic model systems: Part I: Sorption onto clay minerals. Chemosphere 1991, 22, 15–27. [Google Scholar] [CrossRef]
- Fischer, K. Sorption of chelating agents (HEDP and NTA) onto mineral phases and sediments in aquatic model systems: Part II: Sorption onto sediments and sewage sludges. Chemosphere 1992, 24, 51–62. [Google Scholar] [CrossRef]
- Nowack, B.; Stone, A.T. Adsorption of Phosphonates onto the Goethite-Water Interface. J. Colloid Interface Sci. 1999, 214, 20–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoelger, C.; Held-Beller, S.; Palmtag, J. Phosphonates as an ecologically sensible alternative to DTPA - results of a study at MD Albbruck (Phosphonate als ökologisch sinnvolle Alternative zur DTPA - Ergebnisse einer Studie bei MD Albbruck). Wochenblatt für Papierfabrikation, Chemische Technologie 2008, 11–12, 666–669. [Google Scholar]
- Metzner, G. Behaviour of organic complexing agents based on phosphonic acid in sewage treatment plants (Verhalten von organischen Komplexbildnern auf Phosphonsäurebasis in Kläranlagen). In Umweltverträglichkeit von Wasch- und Reinigungsmitteln: Münchener Beiträge zur Abwasser-, Fischerei- und Flußbiologie; Bayerische Landesanstalt für Wasserforschung, Ed.; R. Oldenbourg Verlag GmbH: Munich, Germany, 1990; pp. 323–336. ISBN 3-486-26198-3. [Google Scholar]
- Müller, G.; Steber, J.; Waldhoff, H. On the influence of hydroxyethanediphosphonic acid on phosphate elimination with FeCl3 and the remobilization of heavy metals: Results of laboratory and field tests (Zum Einfluß von Hydroxyethandiphosphonsäure auf die Phosphatelimination mit FeCl3 und die Remobilisierung von Schwermetallen: Ergebnisse von Labor- und Feldversuchen). Vom Wasser 1984, 63, 63–78. [Google Scholar]
- Nowack, B. Aminopolyphosphonate removal during wastewater treatment. Water Res. 2002, 36, 4636–4642. [Google Scholar] [CrossRef]
- Fürhacker, M.; Lesueur, C.; Pfeffer, M.; Köllensperger, G.; Popp, M.; Mentler, A. Phosphonates - AMPA (aminomethylphosphonic acid). Assessing Origin, Environmental Concentrations and Photolysis Degradation (Phosphonate – AMPA (Aminomethylphosphonsäure). Herkunftsabschätzung, Umweltkonzentrationen und Photolyseabbau); Final report (project no. 1378); Institute of Sanitary Engineering and Water Pollution Control, University of Natural Resources and Life Sciences: Vienna, Austria, 2005. [Google Scholar]
- Nowack, B. The behavior of phosphonates in wastewater treatment plants of Switzerland. Water Res. 1998, 32, 1271–1279. [Google Scholar] [CrossRef]
- Wang, S.; Sun, S.; Shan, C.; Pan, B. Analysis of trace phosphonates in authentic water samples by pre-methylation and LC-Orbitrap MS/MS. Water Res. 2019, 161, 78–88. [Google Scholar] [CrossRef]
- Armbruster, D.; Rott, E.; Minke, R.; Happel, O. Trace-level determination of phosphonates in liquid and solid phase of wastewater and environmental samples by IC-ESI-MS/MS. Anal. Bioanal. Chem. 2019. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization. ISO 6878:2004, Water Quality – Determination of Phosphorus – Ammonium Molybdate Spectrometric Method; International Organization for Standardization: Geneva, Switzerland, 2004. [Google Scholar]
- Rott, E.; Reinhardt, T.; Wasielewski, S.; Raith-Bausch, E.; Minke, R. Optimized Procedure for Determining the Adsorption of Phosphonates onto Granular Ferric Hydroxide using a Miniaturized Phosphorus Determination Method. J. Vis. Exp. 2018, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groß, R.; Leisewitz, A.; Moch, K. Investigation of the application quantities of hardly degradable organic ingredients in detergents and cleaning agents in comparison to the use of these substances in other industries with regard to the benefit of a substitution (Untersuchung der Einsatzmengen von schwer abbaubaren organischen Inhaltsstoffen in Wasch- und Reinigungsmitteln im Vergleich zum Einsatz dieser Stoffe in anderen Branchen im Hinblick auf den Nutzen einer Substitution); UFOPLAN FKZ 3709 65 430; Öko-Institut e.V., Institute for Applied Ecology: Freiburg, Germany, 2012. [Google Scholar]
- Batarseh, M.I. Polynuclear Aromatic Hydrocarbons (PAH) and Heavy Metals in Dry and Wet Sludge from As-Samra Wastewater Treatment Plant, Jordan. Soil Sediment Contam. 2011, 20, 535–549. [Google Scholar] [CrossRef]
- Álvarez, E.A.; Mochón, M.C.; Sánchez, J.C.J.; Rodríguez, M.T. Heavy metal extractable forms in sludge from wastewater treatment plants. Chemosphere 2002, 47, 765–775. [Google Scholar] [CrossRef]
- Gianico, A.; Braguglia, C.M.; Mascolo, G.; Mininni, G. Partitioning of nutrients and micropollutants along the sludge treatment line: A case study. Environ. Sci. Pollut. Res. Int. 2013, 20, 6256–6265. [Google Scholar] [CrossRef]
- Frigge, E.; Jackwerth, E. Preconcentration and determination of organophosphonic acids: Application to natural waters. Anal. Chim. Acta 1991, 254, 65–73. [Google Scholar] [CrossRef]
- Zenobi, M.C.; Rueda, E.H. Adsorption of Me(II), HEDP, and Me(II)−HEDP onto Boehmite at Nonstoichiometric Me(II)−HEDP Concentrations. Environ. Sci. Technol. 2006, 40, 3254–3259. [Google Scholar] [CrossRef]
- Dange, C.; Phan, T.N.T.; André, V.; Rieger, J.; Persello, J.; Foissy, A. Adsorption mechanism and dispersion efficiency of three anionic additives poly(acrylic acid), poly(styrene sulfonate) and HEDP on zinc oxide. J. Colloid Interface Sci. 2007, 315, 107–115. [Google Scholar] [CrossRef]
- Jung, A.; Bisaz, S.; Fleisch, H. The binding of pyrophosphate and two diphosphonates by hydroxyapatite crystals. Calc. Tis. Res. 1973, 11, 269–280. [Google Scholar] [CrossRef]
- Rott, E.; Minke, R.; Steinmetz, H. Removal of phosphorus from phosphonate-loaded industrial wastewaters via precipitation/flocculation. J. Wat. Proc. Eng. 2017, 17, 188–196. [Google Scholar] [CrossRef]
- Steber, J.; Wierich, P. Properties of hydroxyethane diphosphonate affecting its environmental fate: Degradability, sludge adsorption, mobility in soils, and bioconcentration. Chemosphere 1986, 15, 929–945. [Google Scholar] [CrossRef]
- Steber, J.; Wierich, P. Properties of aminotris (methylenephosphonate) affecting its environmental fate: Degradability, sludge adsorption, mobility in soils, and bioconcentration. Chemosphere 1987, 16, 1323–1337. [Google Scholar] [CrossRef]
- Tran, N.H.; Reinhard, M.; Gin, K.Y.-H. Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions-a review. Water Res. 2018, 133, 182–207. [Google Scholar] [CrossRef] [PubMed]
- Rott, E.; Nouri, M.; Meyer, C.; Minke, R.; Schneider, M.; Mandel, K.; Drenkova-Tuhtan, A. Removal of phosphonates from synthetic and industrial wastewater with reusable magnetic adsorbent particles. Water Res. 2018, 145, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Metzner, G.; Nägerl, H.-D. Environmental behavior of two water conditioning agents based on phosphonate and polyacrylate (Umweltverhalten zweier Wasserkonditionierungsmittel auf Phosphonat- und Polyacrylatbasis). Tenside Deterg. 1982, 19, 23–29. [Google Scholar]
- Rott, E.; Steinmetz, H.; Metzger, J.W. Organophosphonates: A review on environmental relevance, biodegradability and removal in wastewater treatment plants. Sci. Total Environ. 2018, 615, 1176–1191. [Google Scholar] [CrossRef] [PubMed]
- Schowanek, D.; Verstraete, W. Phosphonate utilization by bacterial cultures and enrichments from environmental samples. Appl. Environ. Microbiol. 1990, 56, 895–903. [Google Scholar] [PubMed]
- Raschke, H.; Rast, H.-G.; Kleinstück, R.; Sicius, H.; Wischer, D. Utilization of 2-phosphonobutane-1,2,4-tricarboxylic acid as source of phosphorus by environmental bacterial isolates. Chemosphere 1994, 29, 81–88. [Google Scholar] [CrossRef]
- Schöberl, P.; Huber, L. Ecologically relevant data from non-surfactant ingredients in detergents and cleaners (Ökologisch relevante Daten von nichttensidischen Inhaltsstoffen in Wasch- und Reinigungsmitteln). Tenside Surfactant Deterg. 1988, 25, 99–107. [Google Scholar]
- Klinger, J.; Sacher, F.; Brauch, H.-J.; Maier, D.; Worch, E. Behaviour of Phosphonic Acids During Drinking Water Treatment. Vom Wasser 1998, 91, 15–27. [Google Scholar]
- Chen, Y.; Baygents, J.C.; Farrell, J. Removing phosphonate antiscalants from membrane concentrate solutions using granular ferric hydroxide. J. Wat. Proc. Eng. 2017, 19, 18–25. [Google Scholar] [CrossRef] [Green Version]
- Armbruster, D.; Müller, U.; Happel, O. Characterization of phosphonate-based antiscalants used in drinking water treatment plants by anion-exchange chromatography coupled to electrospray ionization time-of-flight mass spectrometry and inductively coupled plasma mass spectrometry. J. Chromatogr. A 2019, 1601, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Industrieverband Körperpflege und Waschmittel e.V. Available online: http://web.archive.org/web/20040404120236/http://umweltbundesamt.de/uba-info-daten/daten/wasch/trends.htm (accessed on 18 December 2019).
- Industrieverband Körperpflege- und Waschmittel e.V. Sustainability Report for the 2006 Reporting Year for the Detergents and Cleaning Agents Sector (Nachhaltigkeitsbericht für das Berichtsjahr 2006 für die Wasch- und Reinigungsmittelbranche); Industrieverband Körperpflege- und Waschmittel e.V.: Frankfurt am Main, Germany, 2006. [Google Scholar]
- Industrieverband Körperpflege- und Waschmittel e.V. Sustainability Report Laundry & Detergent Industry in Germany Reporting Years 2007 and 2008 (Nachhaltigkeitsbericht Wasch- und Reinigungsmittelbranche in Deutschland Berichtsjahre 2007 und 2008); Industrieverband Körperpflege- und Waschmittel e.V.: Frankfurt am Main, Germany, 2009. [Google Scholar]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rott, E.; Happel, O.; Armbruster, D.; Minke, R. Behavior of PBTC, HEDP, and Aminophosphonates in the Process of Wastewater Treatment. Water 2020, 12, 53. https://doi.org/10.3390/w12010053
Rott E, Happel O, Armbruster D, Minke R. Behavior of PBTC, HEDP, and Aminophosphonates in the Process of Wastewater Treatment. Water. 2020; 12(1):53. https://doi.org/10.3390/w12010053
Chicago/Turabian StyleRott, Eduard, Oliver Happel, Dominic Armbruster, and Ralf Minke. 2020. "Behavior of PBTC, HEDP, and Aminophosphonates in the Process of Wastewater Treatment" Water 12, no. 1: 53. https://doi.org/10.3390/w12010053
APA StyleRott, E., Happel, O., Armbruster, D., & Minke, R. (2020). Behavior of PBTC, HEDP, and Aminophosphonates in the Process of Wastewater Treatment. Water, 12(1), 53. https://doi.org/10.3390/w12010053