Desorption of Organic Micropollutants from Loaded Granular Activated Carbon
Abstract
1. Introduction
2. Materials and Methods
2.1. Activated Carbon
2.2. Analyzed Substances and Analytics
2.3. Pilot-Scale GAC Filter
2.4. Laboratory Adsorption Batch Tests
2.5. Desorption Experiments
2.5.1. Drying Methods Applied
2.5.2. Setup for Particle-Size Experiments
2.5.3. Solvent and Extraction Time
2.5.4. Batch Desorption Experiments
3. Results
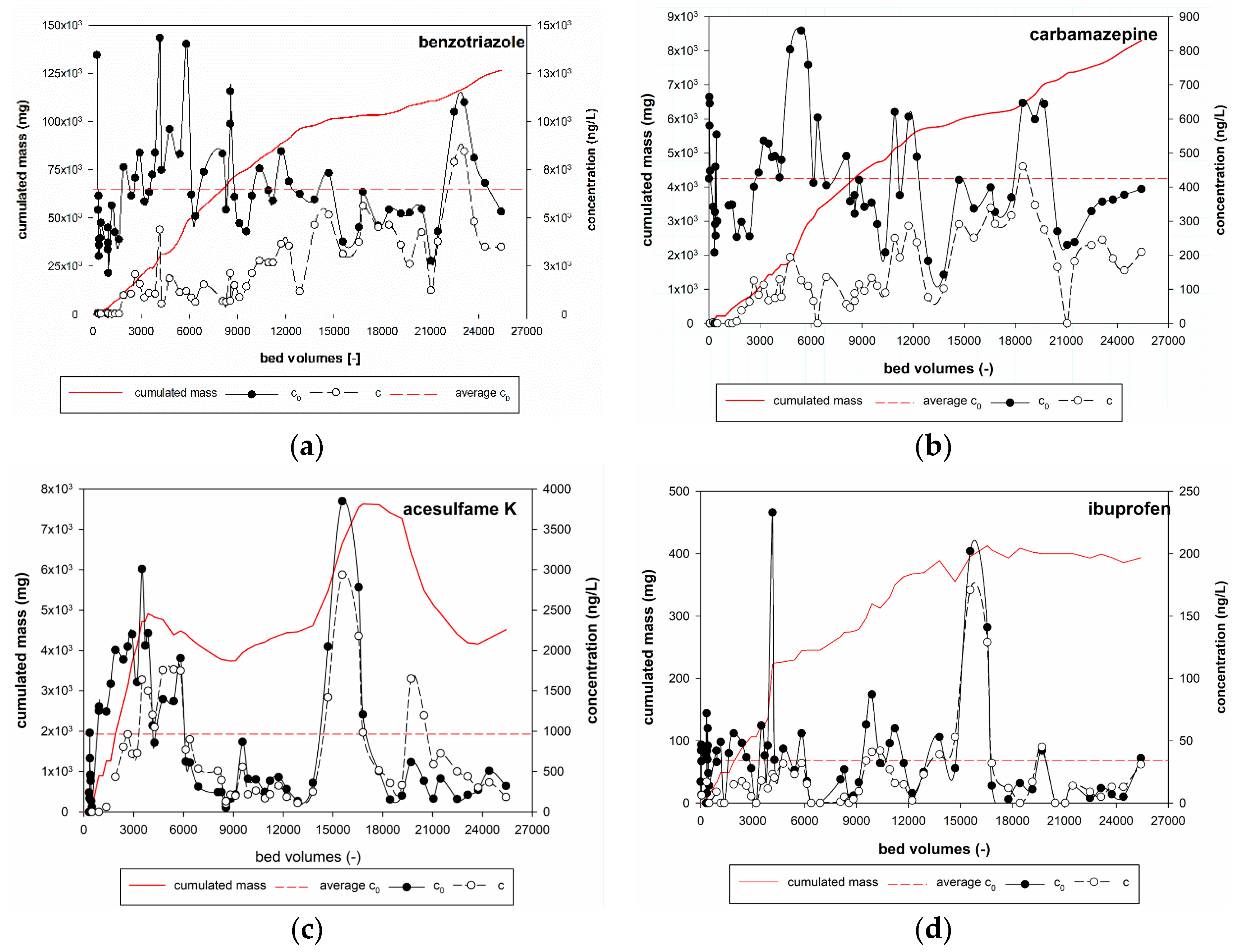
3.1. Results from the Pilot-Scale GAC Filter
- Phase I (<1500 BV)
- Phase II (1500–12,000 BV)
- Phase III (>12,000 BV)
3.2. Adsorption Experiments
3.3. Optimization of Desorption Batch Experiments
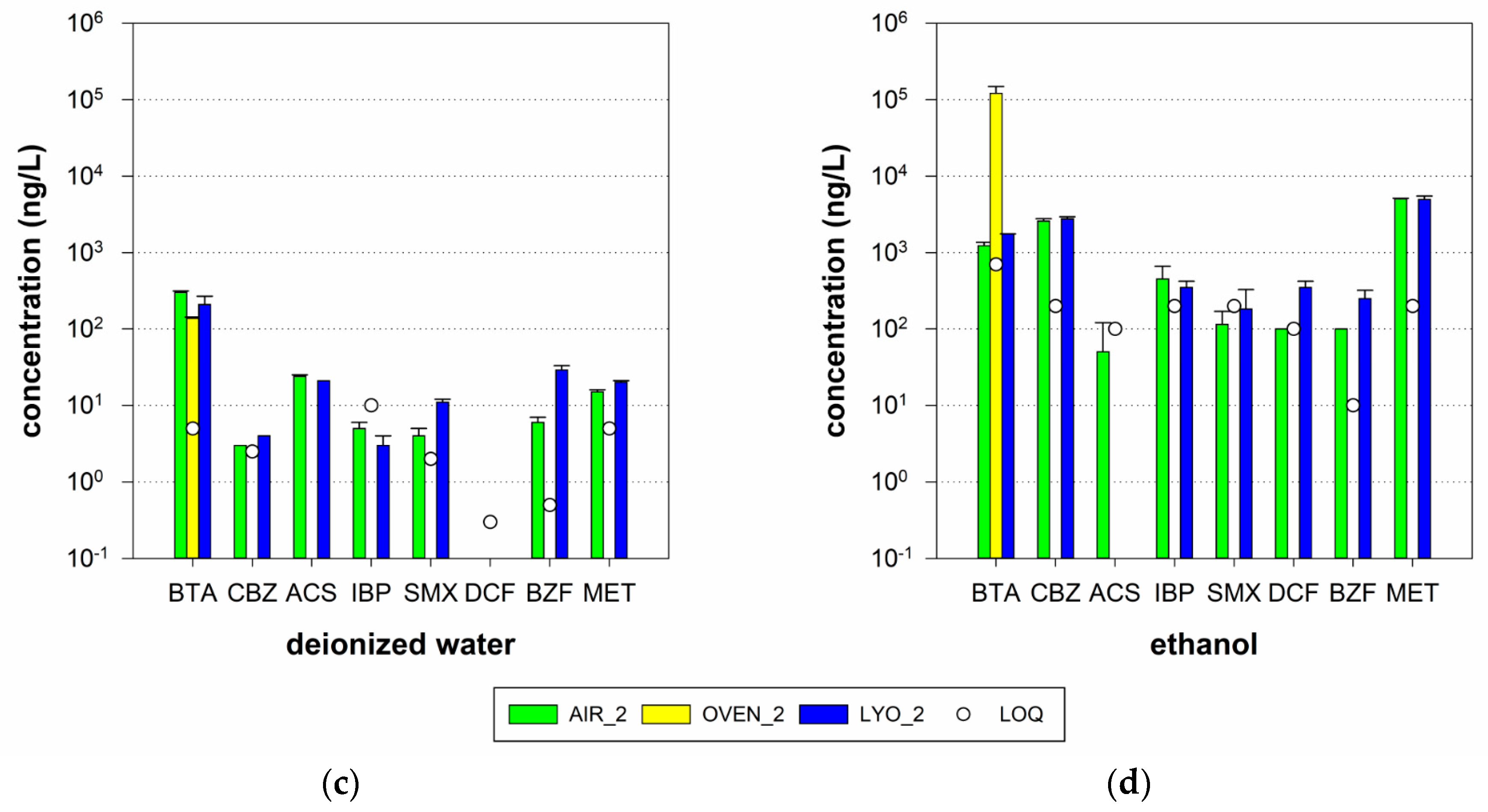
3.3.1. Impact of Drying Method
3.3.2. Impact of Extraction Time
3.3.3. Impact of AC Particle Size and Applied Dose
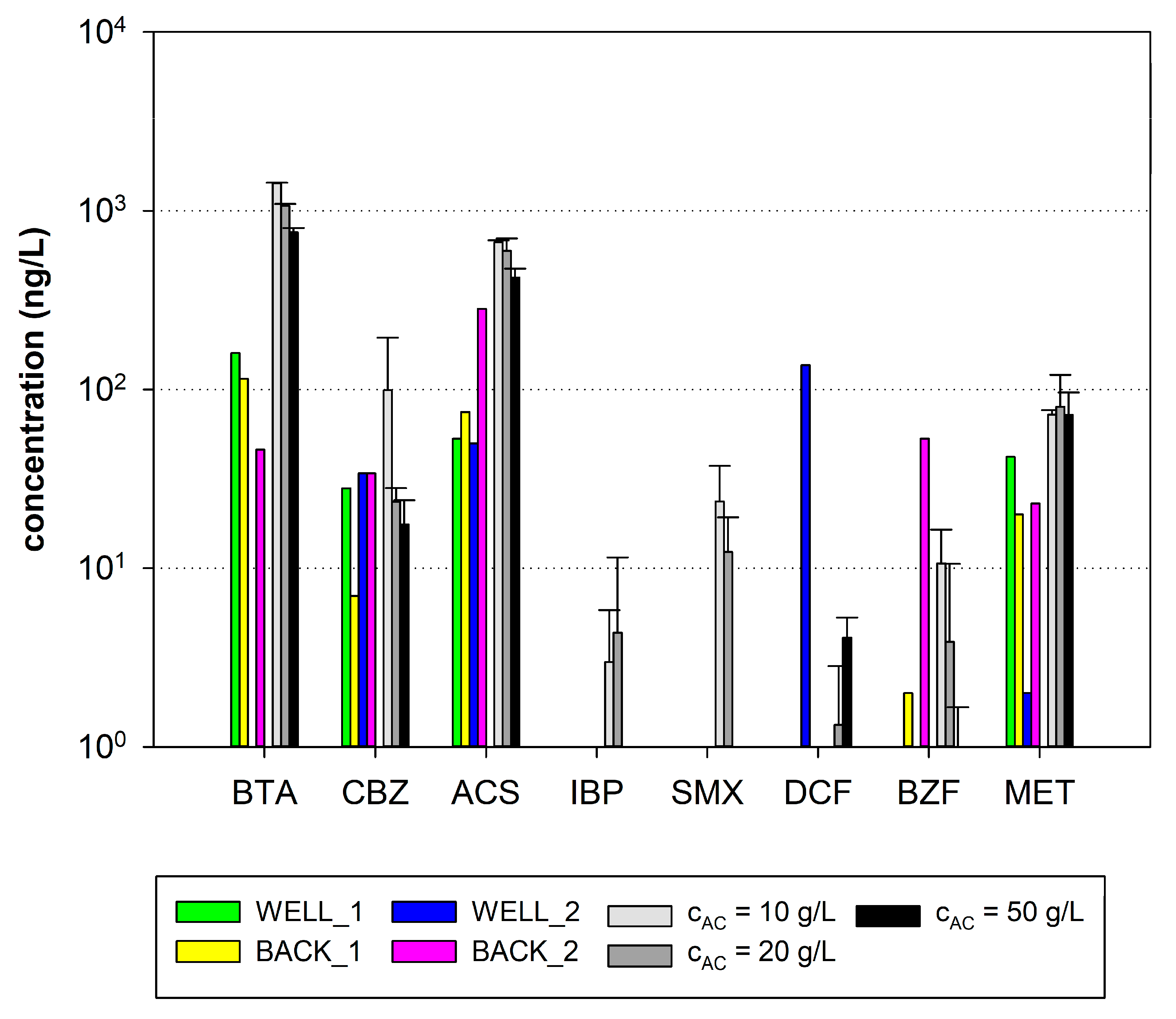
3.4. Comparing Desorption Experiments with Results from Backwashing of Pilot-Scale Filter
4. Discussion
4.1. Optimization of Desorption Batch Experiments
- Air-drying at room temperature: Drying at room temperature in the exicator is the easiest method but takes the longest because the water must completely evaporate. Room temperatures may vary, and the method might therefore be less reproducible than thermal drying in an oven or lyophilization.
- Oven drying at 105 °C: The equilibrium between activated carbon and organic micropollutants depends on the adsorption capacity, temperature applied, and concentration of the substances in the liquid phase [9,25]. Therefore, an increase in temperature might influence the desorption behavior of some substances, leading to a change in the adsorbent load. Another aspect to consider is the thermal stability of different organic micropollutants, although the boiling point of the targeted substances always was well above 105 °C in our case.
- Lyophilization: During the desorption experiments, we observed the physical destruction of the grain structure of the tested GAC samples, which resulted in a reduction in the AC particle size. Since adsorption and desorption are influenced by the diffusive transport of organic micropollutants in the GAC structure, a reduction in grain size strongly influences the obtained adsorbed or desorbed masses [26]. In particular, for desorption experiments focusing on organic micropollutant desorption in water due to a reversal of the concentration gradient, drying with lyophilization might not be the best choice.
4.2. Pilot-Scale Results
4.3. Maximum Desorption of Organic Micropollutants in Water
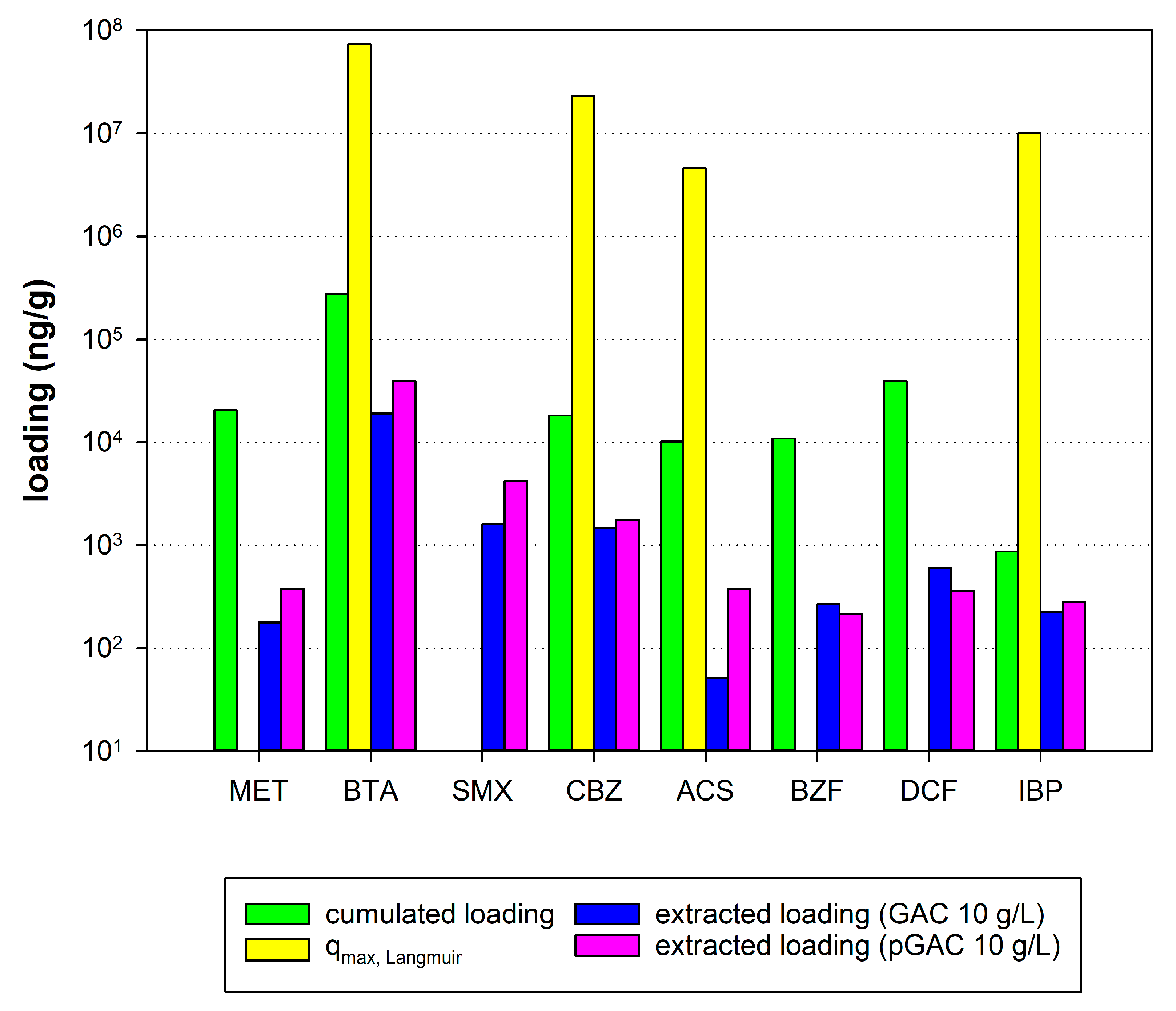
4.4. Comparison of Maximum Adsorption Capacities and Extracted and Cumulated Masses
4.5. Recommendations and Final Findings
- The extraction time for the loaded GAC and solvent has a major impact on the distribution of the organic compounds between liquid and solid phases. We observed that well-adsorbable compounds, such as benzotriazole or metoprolol, desorb first but then replace other moderate adsorbates that show slower desorption kinetics, as they re-adsorb on the then-available sites previously occupied by the moderate adsorbates. For further experiments, we recommend an extensive study of the desorption process over an even longer time period. Comparable studies conducted for GAC samples from drinking-water treatment plants for water purifiers used extraction times between 2 and 120 h. Since the amount of dissolved organic matter in WWTP effluent is generally many times higher than that in drinking water, resulting in a higher number of blocked pores, even 120 h might not be sufficient to reach equilibrium. Additionally, the kinetics of organic micropollutant adsorption are strongly influenced by the properties of the applied GAC and are therefore not directly transferable.
- A change in particle size might provide free adsorption sites that were initially not accessible and may lead to uncontrollable bias in the extraction result. We therefore recommend carrying out future experiments with original-sized activated carbon particles only.
- A lower GAC/solvent ratio leads to higher extraction efficiencies and is therefore recommended. Additionally, replacement of some of the organic micropollutant-enriched solvent may increase the concentration gradient and extraction efficiency, and it would provide several points of the desorption isotherm of the very same sample.
- Although desorption in ethanol was observed to be about 10 times higher than that in water, and the application is easy, harmless and environmentally friendly, other organic solvents might be more suitable for the complete desorption of organic micropollutants from GAC. Kwon et al. (2017) reported the better extraction of organic micropollutants from GAC in acetonitrile, a solvent typically used in liquid chromatography for the analytical determination of the very same compounds, than in water or methanol [21]. The application of acetonitrile was considered at an early stage of our experiments but decided against due to toxicity and handling issues in the experimental setup. Studies focusing on the in situ regeneration of GAC filters have reported the high removal of adsorbed pesticides by the application of sodium ethoxide as a solvent [32].
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Benstoem, F.; Nahrstedt, A.; Boehler, M.; Knopp, G.; Montag, D.; Siegrist, H.; Pinnekamp, J. Performance of granular activated carbon to remove micropollutants from municipal wastewater—A meta-analysis of pilot-and large-scale studies. Chemosphere 2017, 185, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Bourgin, M.; Beck, B.; Boehler, M.; Borowska, E.; Fleiner, J.; Salhi, E.; Teichler, R.; Von Gunten, U.; Siegrist, H.; McArdell, C.S. Evaluation of a full-scale wastewater treatment plant upgraded with ozonation and biological post-treatments: Abatement of micropollutants, formation of transformation products and oxidation by-products. Water Res. 2018, 129, 486–498. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.; Malato, S.; Antakyali, D.; Beretsou, V.G.; Đolić, M.B.; Gernjak, W.; Heath, E.; Ivancev-Tumbas, I.; Karaolia, P.; Ribeiro, A.R.L.J.S.O.T.T.E. Consolidated vs new advanced treatment methods for the removal of contaminants of emerging concern from urban wastewater. Sci. Total Environ. 2019, 655, 986–1008. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C.J.S.O.T.T.E. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Michael, I.; Rizzo, L.; McArdell, C.; Manaia, C.; Merlin, C.; Schwartz, T.; Dagot, C.; Fatta-Kassinos, D.J.W.R. Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: A review. Water Res. 2013, 47, 957–995. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.; Manaia, C.; Merlin, C.; Schwartz, T.; Dagot, C.; Ploy, M.; Michael, I.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: A review. Sci. Total Environ. 2013, 447, 345–360. [Google Scholar] [CrossRef]
- Snyder, S.A.; Adham, S.; Redding, A.M.; Cannon, F.S.; DeCarolis, J.; Oppenheimer, J.; Wert, E.C.; Yoon, Y. Role of membranes and activated carbon in the removal of endocrine disruptors and pharmaceuticals. Desalination 2007, 202, 156–181. [Google Scholar] [CrossRef]
- Rossner, A.; Snyder, S.A.; Knappe, D.R. Removal of emerging contaminants of concern by alternative adsorbents. Water Res. 2009, 43, 3787–3796. [Google Scholar] [CrossRef]
- Worch, E. Adsorption Technology in Water Treatment: Fundamentals, Processes, and Modeling; Walter de Gruyter: Berlin, Germany, 2012. [Google Scholar]
- Crittenden, J.; Trusell, R.; Hand, D.; Howe, K.; Techobanoglous, G. Water Treatment Principle and Design; John Wiley & Sons: New Jersey, NJ, USA, 2005. [Google Scholar]
- Aschermann, G.; Neubert, L.; Zietzschmann, F.; Jekel, M.J.W.R. Impact of different DOM size fractions on the desorption of organic micropollutants from activated carbon. Water Res. 2019, 161, 161–170. [Google Scholar] [CrossRef]
- Zietzschmann, F.; Worch, E.; Altmann, J.; Ruhl, A.S.; Sperlich, A.; Meinel, F.; Jekel, M.J.W.R. Impact of EfOM size on competition in activated carbon adsorption of organic micro-pollutants from treated wastewater. Water Res. 2014, 65, 297–306. [Google Scholar] [CrossRef]
- De Ridder, D.J.; Verliefde, A.R.D.; Heijman, S.G.J.; Verberk, J.Q.J.C.; Rietveld, L.C.; Van Der Aa, L.T.J.; Amy, G.L.; Van Dijk, J.C. Influence of natural organic matter on equilibrium adsorption of neutral and charged pharmaceuticals onto activated carbon. Water Sci. Technol. 2011, 63, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Shang, R.; Heijman, B.; Rietveld, L.J.C. Influence of activated carbon preloading by EfOM fractions from treated wastewater on adsorption of pharmaceutically active compounds. Chemosphere 2016, 150, 49–56. [Google Scholar] [CrossRef]
- Aschermann, G.; Zietzschmann, F.; Jekel, M. Influence of dissolved organic matter and activated carbon pore characteristics on organic micropollutant desorption. Water Res. 2018, 133, 123–131. [Google Scholar] [CrossRef]
- Corwin, C.J.; Summers, R.S. Adsorption and desorption of trace organic contaminants from granular activated carbon adsorbers after intermittent loading and throughout backwash cycles. Water Res. 2011, 45, 417–426. [Google Scholar] [CrossRef] [PubMed]
- To, P.C.; Mariñas, B.J.; Snoeyink, V.L.; Ng, W.J. Effect of pore-blocking background compounds on the kinetics of trace organic contaminant desorption from activated carbon. Environ. Sci. Technol. 2008, 42, 4825–4830. [Google Scholar] [CrossRef] [PubMed]
- Aschermann, G.; Schröder, C.; Zietzschmann, F.; Jekel, M. Organic micropollutant desorption in various water matrices-Activated carbon pore characteristics determine the reversibility of adsorption. Chemosphere 2019, 237, 124415. [Google Scholar] [CrossRef]
- Rattier, M.; Reungoat, J.; Keller, J.; Gernjak, W. Removal of micropollutants during tertiary wastewater treatment by biofiltration: Role of nitrifiers and removal mechanisms. Water Res. 2014, 54, 89–99. [Google Scholar] [CrossRef]
- Sbardella, L.; Comas, J.; Fenu, A.; Rodriguez-Roda, I.; Weemaes, M. Advanced biological activated carbon filter for removing pharmaceutically active compounds from treated wastewater. Sci. Total Environ. 2018, 636, 519–529. [Google Scholar] [CrossRef]
- Kwon, D.-S.; Tak, S.-Y.; Lee, J.-E.; Kim, M.-K.; Lee, Y.H.; Han, D.W.; Kang, S.; Zoh, K.-D. Desorption of micropollutant from spent carbon filters used for water purifier. Environ. Sci. Pollut. Res. 2017, 24, 17606–17615. [Google Scholar] [CrossRef]
- Jekel, M.; Dott, W. Leitfaden Polare organische Spurenstoffe als Indikatoren im anthropogen beeinflussten Wasserkreislauf, Ergebnisse des Querschnittsthemas “Indikatorsubstanzen”. Vom Wasser 2013, 111, 67–114. [Google Scholar]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2019 update: Improved access to chemical data. Nucleic Acids Res. 2018, 47, D1102–D1109. [Google Scholar] [CrossRef] [PubMed]
- Schaar, H.; Clara, M.; Gans, O.; Kreuzinger, N. Micropollutant removal during biological wastewater treatment and a subsequent ozonation step. Environ. Pollut. 2010, 158, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Aumeier, B.M.; Dang, A.H.; Ohs, B.; Yüce, S.l.; Wessling, M. Aqueous-phase temperature swing adsorption for pesticide removal. Environ. Sci. Technol. 2018, 53, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Freihardt, J.; Jekel, M.; Ruhl, A.S. Comparing test methods for granular activated carbon for organic micropollutant elimination. J. Environ. Chem. Eng. 2017, 5, 2542–2551. [Google Scholar] [CrossRef]
- Sontheimer, H.; Crittenden, J.C.; Summers, R.S. Activated Carbon for Water Treatment; DVGW-Forschungsstelle, Engler-Bunte-Institut, Universitat Karlsruhe TH: Karlsruhe, Germany, 1988; Volume 90. [Google Scholar]
- Piai, L.; Dykstra, J.E.; Adishakti, M.G.; Blokland, M.; Langenhoff, A.A.; van der Wal, A. Diffusion of hydrophilic organic micropollutants in granular activated carbon with different pore sizes. Water Res. 2019, 162, 518–527. [Google Scholar] [CrossRef]
- Nowotny, N.; Epp, B.; von Sonntag, C.; Fahlenkamp, H. Quantification and modeling of the elimination behavior of ecologically problematic wastewater micropollutants by adsorption on powdered and granulated activated carbon. Environ. Sci. Technol. 2007, 41, 2050–2055. [Google Scholar] [CrossRef]
- Zietzschmann, F.; Stützer, C.; Jekel, M. Granular activated carbon adsorption of organic micro-pollutants in drinking water and treated wastewater–aligning breakthrough curves and capacities. Water Res. 2016, 92, 180–187. [Google Scholar] [CrossRef]
- Bunmahotama, W.; Hung, W.-N.; Lin, T.-F. Prediction of the adsorption capacities for four typical organic pollutants on activated carbons in natural waters. Water Res. 2017, 111, 28–40. [Google Scholar] [CrossRef]
- Larasati, A.; Fowler, G.D.; Graham, N.J. Chemical regeneration of granular activated carbon: Preliminary evaluation of alternative regenerant solutions. Environ. Sci. Water Res. Technol. 2020, 6, 2043–2056. [Google Scholar] [CrossRef]




| Substance | Abbreviation | CAS Number | Compound Class | Adsorptive Removal | Molecular Weight (g/mol) | Log KOW (-) |
|---|---|---|---|---|---|---|
| benzotriazole | BTA | 95-14-7 | complexing agent | high (1) | 119.13 (2) | 1.44 (2) |
| carbamazepine | CBZ | 298-46-4 | antiepileptic | high (1) | 236.27 (2) | 2.77 (2) |
| acesulfame K | ACS | 55589-62-3 | sweetener | medium (1) | 201.24 (2) | −1.33 (3) |
| ibuprofen | IBP | 15687-27-1 | analgesic | medium (1) | 206.29 (2) | 3.97 (2) |
| sulfamethoxazole | SMX | 723-46-6 | antibiotic | medium (1) | 253.28 (2) | 0.89 (2) |
| diclofenac | DCF | 15307-79-6 | analgesic | high (1) | 296.15 (2) | 0.70 (2) |
| bezafibrate | BZF | 41859-67-0 | lipid-lowering agent | high (1) | 361.82 (2) | 4.25 (4) |
| metoprolol | MET | 37350-58-6 | beta blocker | high (1) | 267.36 (2) | 1.88 (2) |
| Drying Method | Temperature (°C) | Time |
|---|---|---|
| Air-drying | 25 | 7 days |
| Oven-drying | 105 | 48 h |
| Lyophilization | −80/−30 | 24 h/24 h |
| ACS | MET | BTA | SMX | CBZ | BZF | DCF | IBP | |
|---|---|---|---|---|---|---|---|---|
| ng/L | ng/L | ng/L | ng/L | ng/L | ng/L | ng/L | ng/L | |
| Number of measurements | 65 | 66 | 67 | 71 | 68 | 72 | 72 | 71 |
| Median | 509 | 441 | 6.141 | 20 | 400 | 250 | 1.330 | 28 |
| Mean ± standard deviation | 965± | 476± | 6.468± | 23± | 425± | 336± | 1.250± | 34± |
| 897 | 270 | 2.529 | 22 | 149 | 249 | 563 | 40 |
| Freundlich Parameters | Langmuir Parameters | |||||
|---|---|---|---|---|---|---|
| KF | n | R2 | qmax | b | R2 | |
| (ng1–n × Ln)/g | - | - | ng/g | L/ng | - | |
| BTA | 1.80 ± 3.28 × 106 | 0.46 ± 0.26 × 106 | 0.88 | 73.46 ± 136.79 × 106 | 1.82 ± 2.79 × 103 | 0.88 |
| CBZ | 0.77 ± 1.24 × 106 | 0.39 ± 0.24 × 106 | 0.91 | 27.50 ± 51.40 × 106 | 2.76 ± 4.22 × 103 | 0.90 |
| ACS | 0.61 ± 0.66 × 106 | 0.27 ± 0.16 × 106 | 0.83 | 4.58 ± 5.43 × 106 | 11.96 ± 10.61 × 103 | 0.90 |
| IBP | 1.20 ± 1.40 × 106 | 0.31 ± 0.18 × 106 | 0.86 | 10.07 ± 15.83 × 106 | 20.61 ± 24.07 × 103 | 0.93 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reif, D.; Saracevic, E.; Šabić Runjavec, M.; Haslinger, J.; Schaar, H.; Kreuzinger, N. Desorption of Organic Micropollutants from Loaded Granular Activated Carbon. Water 2020, 12, 2754. https://doi.org/10.3390/w12102754
Reif D, Saracevic E, Šabić Runjavec M, Haslinger J, Schaar H, Kreuzinger N. Desorption of Organic Micropollutants from Loaded Granular Activated Carbon. Water. 2020; 12(10):2754. https://doi.org/10.3390/w12102754
Chicago/Turabian StyleReif, Daniela, Ernis Saracevic, Monika Šabić Runjavec, Julia Haslinger, Heidemarie Schaar, and Norbert Kreuzinger. 2020. "Desorption of Organic Micropollutants from Loaded Granular Activated Carbon" Water 12, no. 10: 2754. https://doi.org/10.3390/w12102754
APA StyleReif, D., Saracevic, E., Šabić Runjavec, M., Haslinger, J., Schaar, H., & Kreuzinger, N. (2020). Desorption of Organic Micropollutants from Loaded Granular Activated Carbon. Water, 12(10), 2754. https://doi.org/10.3390/w12102754





