Measurement of Biochemical Methane Potential of Heterogeneous Solid Substrates: Results of a Two-Phase French Inter-Laboratory Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. BMP Measurement
2.2. Organization and Methodology of the Inter-Laboratory Study
2.2.1. Generalities
2.2.2. Building of the Inter-Laboratory Study
2.2.3. Experimental Data Collection
2.2.4. Experimental Data Calculation
- Test Carried Out in a Closed System
- By pressure measurement using a differential manometer;
- By volumetric measurement using a suitable device (flowmeter, “inverted specimen”, syringe, etc.)
- Monitoring by pressure measurement
- The differential pressure ΔP(i);
- The methane content;
- Atmospheric pressure on the day of analysis;
- The pressure in the reaction chamber:
- The saturating vapor pressure of water at the working temperature T, e.g., according to the Rankine formula:
- The volume of methane (NCTP) produced since the last sampling:
- -
- P(i)—the pressure in the reaction chamber at the time of analysis (Pa);
- -
- ΔP(i)—the differential pressure read on the pressure gauge (Pa);
- -
- Patm(i−1)—the atmospheric pressure on the time i−1 of the previous analysis (Pa);
- -
- Patm(i)—the atmospheric pressure on the time i of analysis (Pa);
- -
- Psat(T)—the saturating vapor pressure of water at temperature T (Pa);
- -
- Y(i−1)—the CH4 content in the dry biogas on the time i−1 of previous sampling (%);
- -
- y(i)—the CH4 content in the dry biogas on the time i of sampling (%);
- -
- ΔN(i)—the number of moles of methane produced since the last sample was taken (mol);
- -
- ΔV(i)—the volume of NCTP methane produced since the last sampling (Nm3);
- -
- Vh—the volume of the gas headspace (including the measuring device) (L);
- -
- T—temperature of the reaction chamber during measurement (K);
- -
- T0—standard temperature (273.15 K);
- -
- P0—standard pressure (1 × 105 Pa).
- Monitoring by volumetric measurement
- Volume of biogas produced since the last collection;
- Methane content of the biogas;
- The atmospheric pressure on the day of the previous sampling.
- The pressure in the reaction chamber is balanced to the atmosphere after each measurement.
- Each time a sample was taken, ΔP(i), Patm(i) and y(i) were measured.
- Calculations:
- The saturating vapor pressure of the water at the working temperature T, by using the Rankine formula as shown above.
- The volume of methane (NCTP) produced since the last sampling:
- -
- Vb(i)—the volume of biogas measured on the time i of the analysis (m3);
- -
- Patm(i−1)—the atmospheric pressure on the time i−1 of the previous analysis (Pa);
- -
- Patm(i)—the atmospheric pressure on the time i of analysis (Pa);
- -
- Psat(T)—the saturating vapor pressure of water at temperature T (Pa);
- -
- Y(i−1)—the CH4 content in the dry biogas on the time i−1 of previous sampling (%);
- -
- y(i)—the CH4 content in the dry biogas on the time i of sampling (%);
- -
- ΔN(i)—the number of moles of methane produced since the last sample was taken (mol);
- -
- ΔV(i)—the volume of NCTP methane produced since the last sampling (Nm3);
- -
- Vh—the volume of the gas headspace (including the measuring device) (L);
- -
- T—temperature of the reaction chamber during measurement (K);
- -
- T0—standard temperature (273.15 K);
- -
- P0—standard pressure (1 × 105 Pa).
- Test Carried Out in an Open System
- The CO2 solubilization device (trap) must be separated from the reaction chamber, preventing the return of the CO2-free biogas into the headspace, in contact with the reaction medium;
- Knowledge of the methane content of the biogas at the beginning and end of the test was necessary.
- The variation of the methane content of the headspace in the reaction chamber;
- The variation in the methane content of the biogas produced between two analyses.
- With the saturating vapor pressure of water at the working temperature T by using the Rankine formula as shown above.
- -
- Vb(i)—the volume of biogas produced since the last analysis (m3);
- -
- P—the pressure in the reaction vessel (Pa);
- -
- Psat(T)—the saturating vapor pressure of water at temperature T (Pa);
- -
- y(i−1)—the CH4 content in the dry biogas on the time i−1 of previous sampling (%);
- -
- y(i)—the CH4 content in the dry biogas on the time i of sampling (%);
- -
- ΔV(i)—the volume of NCTP methane produced since the last analysis (Nm3);
- -
- Vh—the volume of headspace (including the of the gas phase measuring device) (m3);
- -
- T—reaction chamber temperature (K).
2.2.5. Results Analysis
- -
- 1 test was eliminated from a series of 3 replicates if RSD was higher than 10% and the two other tests had close values;
- -
- 1 whole series (the 3 replicates) was eliminated if RSD was higher than 10% and the three replicates were all sufficiently different from each other (RSD higher than 10% leaving 2 replicates);
- -
- 1 test or 1 series was eliminated if technical justification (gas leakage, broken bottles, electrical shutdown, failure of devices or sensors, problem of heating system, etc.).
2.3. Choice, Composition and Preparation of Substrates
2.3.1. Choice of the Different Substrates
2.3.2. Composition of the Substrates
2.3.3. Preparation of Substrates
2.4. Physicochemical Parameters
3. Results
3.1. Substrate Characterization
3.2. First Phase (Free Protocols)
3.3. Second Phase: Harmonized Protocol
3.3.1. Harmonization of the BMP Protocol
- BMP protocol
- Systematic addition (20 mL in the cultivation medium) of a mineral nutritive solution;
- Systematic use of carbonate buffer (3 g/L NaHCO3 added in the cultivation medium);
- Fixed I/S Ratio equal to two (except for highly biodegradable substrates where the I/S ratio could be equal to 5 gVS/gVS);
- Implementation of one blank and one positive control substrate both in triplicate, as described before in Material and Methods section, carried out in the same conditions as the substrates.
- Validation criteria
- The validation criteria were also defined according to the targeted consensus obtained among all participants. To validate the BMP test, all these following four criteria had to be reached together:
- The repeatability of triplicates (RSD < 10%);
- The final pH value had to be higher than 6.5;
- The maximal methanogenic activity measured on the positive control during the test must be higher or equal to 35 NmLCH4 gVS−1 day−1, and the value of its methanogenic potential must be defined between 90 to (and) 100% of the stoichiometric methanogenic potential of acetate, i.e., 0.373 NLCH4 per gram of acetate;
- The methane part due to the endogenic activity would not exceed one-third of the whole methane production of the assay.
- If one of these criteria was not fulfilled, the test was considered as invalid.
- End of the BMP criteria
- The measurement was considered as over when the increase of the methanogenic potential did not excess 1% per day.
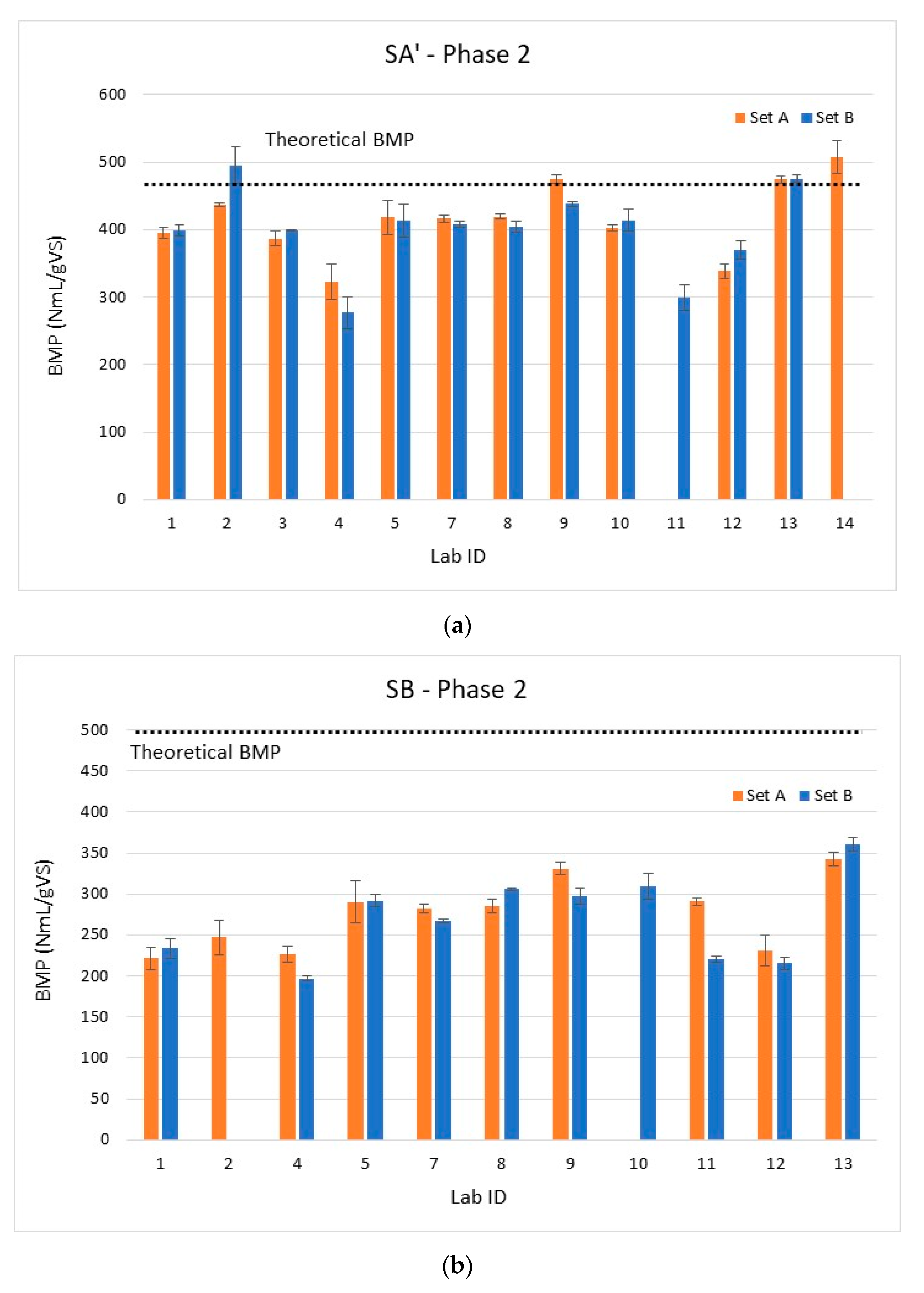
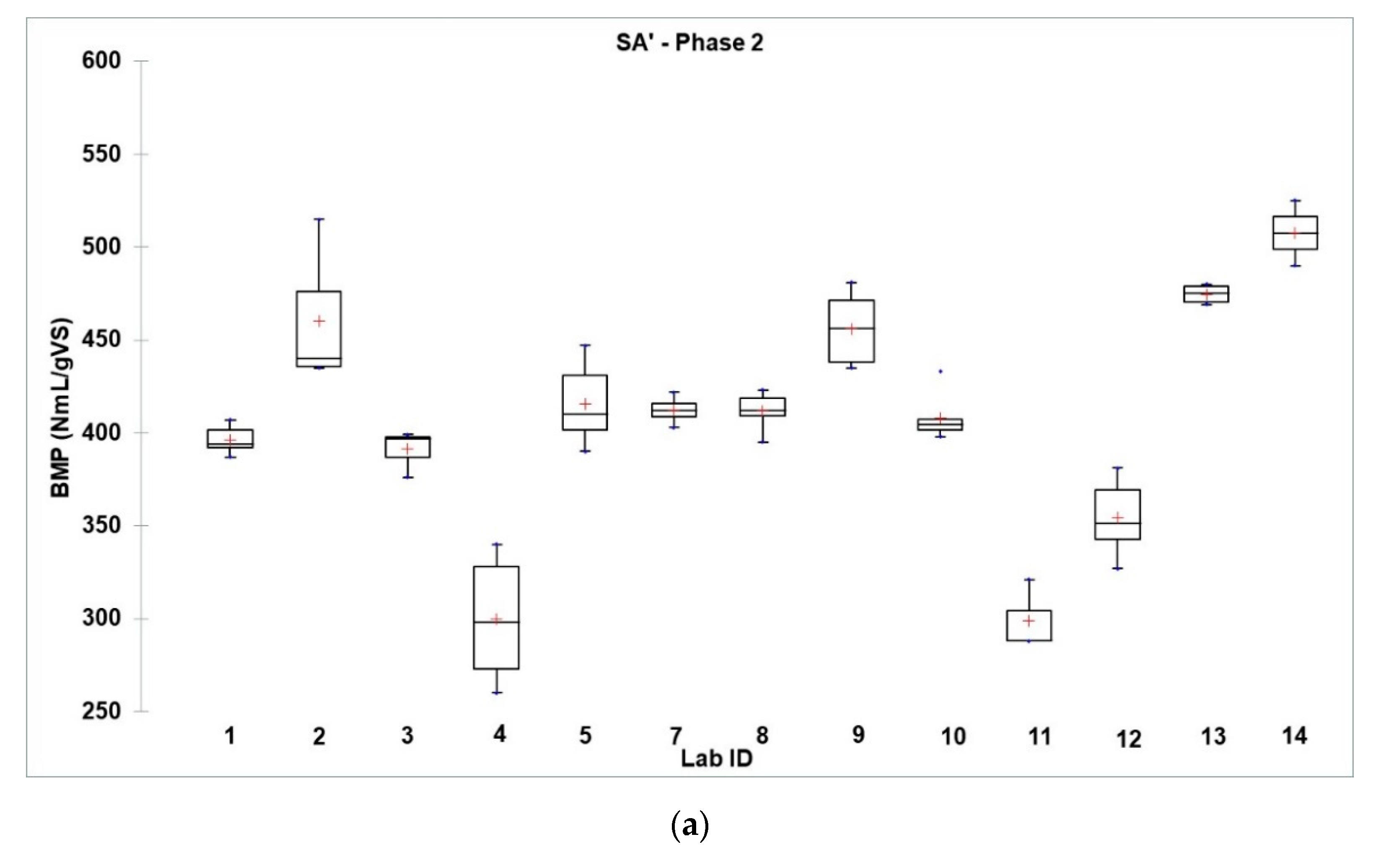
3.3.2. Results of the Second Phase
3.4. Synthesis of the Two Phases
3.4.1. Intra-Laboratory and Inter-Laboratory Reproducibility
3.4.2. Control Positive Limits
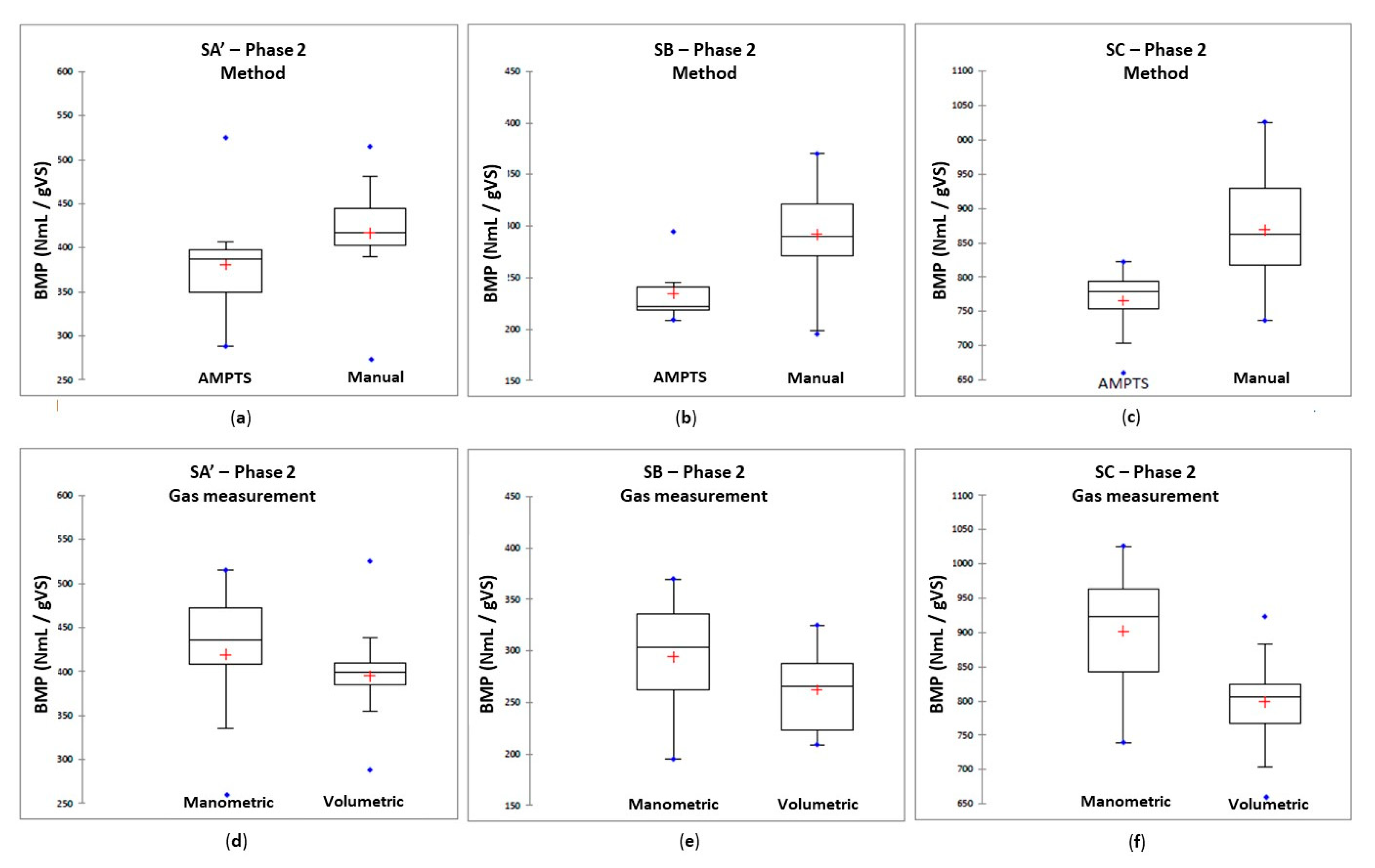
3.4.3. Effect of the Experimental Method on BMP
- -
- The experimental method, which was either “manual” if the monitoring of biogas production and its qualitative analysis were carried out manually by an operator or “automatic” when carried out using an automatic measurement system (AMPTS);
- -
- The biogas measurement method, carried out either using a manometer or using a volumetric flowmeter (the semi-automatic measuring device AMPTS was considered as volumetric measurement);
- -
- The initial substrate concentration [S], expressed in gVS/L;
- -
- The maximum methanogenic activity (SMA) expressed in NmLCH4 gVS−1 day−1;
- -
- The maximum endogenous activity (ENDO) expressed in NmLCH4 gVS−1 day−1.
3.4.4. Potential Effect of Inoculum on BMP
4. Conclusions
- Using the protocols specific to each laboratory, the intra-laboratory variations are acceptable, with an average repeatability of 6%, after removal of about 16% of outliers. For each laboratory, the results are considered credible, but inter-laboratory variations are significant, on average 18%.
- No experimental factor (agitation, method of measurement, data processing) can statistically explain these variations which persist, even with the use of a harmonized protocol.
- The reasons for inter-laboratory variability in BMP remain unclear at the end of these trials; inoculum diversity and variability have been shown not to affect BMP in other recent studies.
- The use of a positive control might have allowed a better sorting and refinement of the results.
- Drying the SA complex substrate (containing proteins, carbohydrates, lipids and fibers) does not change the BMP. This net and interesting result can probably be extrapolated to other substrates but containing no volatile molecules.
- Eleven laboratories participated in this trial, generating approximately 350 BMP. Despite this large number, due to the different measurement methods, the removal of the outliers resulted in variable and sometimes small numbers of results to compare. The mathematically accurate statistical conclusions are therefore to be considered with hindsight. Understanding the variability of BMPs would therefore require a much larger panel, which would not guarantee control of the conditions for obtaining reproducible and comparable BMPs. The inter-laboratory variability remains significant and comparable to those observed in this work, even in the study involving more than 30 laboratories [46].
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Calculation of Theoretical Methane Productivity
| Component | Crude Formula and Weight Percentages of VS | Theoretical Methane Yield (NL/gVS) | |
|---|---|---|---|
| Cellulose, starch, galactose, mannose in macromolecules | C6H10O5 | 0.415 | |
| Proteins | C6H12O3N2 | 0.420 | |
| Lipides (glycerol trioleate) | C57H104O6 | 1.013 | |
| Xyloses | C5H8O4 | 0.424 | |
| Coumarylic acid (in lignin) | C9H10O2 | 0.784 | |
| Coniferylic acid (in lignin) | C10H12O3 | 0.715 | |
| Sinapylic acid (in lignin) | C11H14O4 | 0.666 | |
| Hemicellulose | xylose | 66 % | 0.283 |
| galactose, mannose | 33 % | 0.138 | |
| Total for hemicellulose | 0.421 | ||
| Lignin | Coumarylic alcohol | 30 % | 0.235 |
| Coniferylic alcohol | 40 % | 0.286 | |
| Sinapylic alcohol | 30 % | 0.200 | |
| Total for lignin | 0.721 | ||
| Component | Weight Percentages of VS (%) | Theoretical Methane Yield (NLCH4/gVS) | ||
|---|---|---|---|---|
| Potato | starch | 100 | 27 | 0.112 |
| Maize | glucides | 72 | 18 | 0.054 |
| proteins | 10 | 0.008 | ||
| lipids | 8 | 0.015 | ||
| lignin | 10 | 0.012 | ||
| Meat | proteins | 85 | 26 | 0.093 |
| lipids | 15 | 0.039 | ||
| Straw (see below) | 29 | 0.145 | ||
| Total | 0.476 | |||
| Component | Weight Percentages of VS (%) | Theoretical Methane Yield (NL CH4/gVS) |
|---|---|---|
| Cellulose | 43 | 0.178 |
| Hemicellulose | 29 | 0.122 |
| Lignin | 28 | 0.200 |
| Total | 0.499 | |
| Component | Weight Percentages of VS (%) | Theoretical Methane Yield (NL CH4/gVS) |
|---|---|---|
| Lipids | 100 | 1.013 |
References
- Fedorov, V. Modern technologies of treatment and stabilization for sewage sludge from water treatment plant. Agric. Sci. Procedia 2016, 10, 417–430. [Google Scholar] [CrossRef] [Green Version]
- Jain, S.; Wolf, I.T.; Lee, J.; Tong, Y.W. A comprehensive review on operating parameters and different pretreatment methodologies for anaerobic digestion of municipal solid waste. Renew. Sustain. Energy Rev. 2012, 52, 142–154. [Google Scholar] [CrossRef]
- Tchobanoglous, G.; Burton, F.; Stensel, D. Wastewater Engineering Treatment Reuse, 4th ed.; McGraw-Hill Companies, Inc.: New York, NY, USA, 1991; pp. 1505–1533. [Google Scholar]
- EBA. Annual Statistical Report of the European Biogas Association—European Overview 2019. Available online: https://www.europeanbiogas.eu/eba-statistical-report-2019/ (accessed on 9 October 2020).
- Remigi, E.U.; Buckley, C.A. Co-Digestion of High-Strength/Toxic Organic Effluents in Anaerobic Digesters at Wastewater Treatment Works; Water Research Commission: Pretoria, South Africa, 2006. [Google Scholar]
- Achinas, S.; Euverink, G.J.W. Theoretical analysis of biogas potential prediction from agricultural waste. Resour. Effic. Technol. 2016, 2, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Filer, J.; Huihuang, H.; Ding, H.H.; Chang, S. Biochemical methane potential (BMP) assay method for anaerobic digestion research. Water 2019, 11, 921. [Google Scholar] [CrossRef] [Green Version]
- Jingura, R.M.; Kamusoko, R. Methods for determination of biomethane potential of feedstocks: A review. Biofuel Res. J. 2017, 4, 573–586. [Google Scholar] [CrossRef]
- Ariunbaatar, J.; Esposito, G.; Yeh, D.H.; Lens, P.N.L. Enhanced anaerobic digestion of food waste by supplementing trace elements: Role of selenium (VI) and iron (II). Front. Environ. Sci. 2016, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Prabhudessai, V.; Ganguly, A.; Mutnuri, S. Biochemical methane potential of agro wastes. J. Energy. 2013, 2013, 350731. [Google Scholar] [CrossRef] [Green Version]
- Yeshanew, M.M.; Frunzo, L.; Lens, P.N.L.; Pirozzi, F.; Esposito, G. Mass loss controlled thermal pretreatment system to assess the effects of pretreatment temperature on organic matter solubilization and methane yield from food waste. Front. Environ. Sci. 2016, 4, 62. [Google Scholar] [CrossRef] [Green Version]
- Benn, N.; Zitomer, D. Pretreatment and anaerobic codigestion of selected PHB and PLA bioplastics. Front. Environ. Sci. 2018, 5, 93. [Google Scholar] [CrossRef] [Green Version]
- Hafner, S.D.; Koch, K.; Carrere, H.; Astals, S.; Weinrich, S.; Rennuit, C. Software for biogas research: Tools for measurement and prediction of methane production. SoftwareX 2018, 7, 205–210. [Google Scholar] [CrossRef]
- Raposo, F.; Borja, R.; Ibelli-Bianco, C. Predictive regression models for biochemical methane potential tests of biomass samples: Pitfalls and challenges of laboratory measurements. Renew. Sustain. Energy Rev. 2020, 127, 109890. [Google Scholar] [CrossRef]
- Koch, K.; Plabst, M.; Schmidt, A.; Helmreich, B.; Drewes, J.E. Co-digestion of food waste in a municipal wastewater treatment plant: Comparison of batch tests and full-scale experiences. Waste Manag. 2016, 47, 28–33. [Google Scholar] [CrossRef]
- Gandiglio, M.; Lanzini, A.; Soto, A.; Leone, P.; Santarelli, M. Enhancing the energy efficiency of wastewater treatment plants through co-digestion and fuel cell systems. Front. Environ. Sci. 2017, 5, 70. [Google Scholar] [CrossRef] [Green Version]
- Lippert, T.; Bandelin, J.; Musch, A.; Drewes, J.E.; Koch, K. Energy-positive sewage sludge pre-treatment with a novel ultrasonic flatbed reactor at low energy input. Bioresour. Technol. 2018, 264, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Baird, R.B.; Eaton, A.D.; Rice, E.W. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Owen, W.F.; Stuckey, D.C.; Healy, J.B., Jr.; Young, L.Y.; McCarty, P.L. Bioassay for monitoring biochemical methane potential and anaerobic toxicity. Water Res. 1979, 13, 485–492. [Google Scholar] [CrossRef]
- Angelidaki, I.; Alves, M.; Bolzonella, D.; Borzacconi, L.; Campos, J.L.; Guwy, A.J.; Kalyuzhnyi, S.; Jenicek, P.; Van Lier, J.B. Defining the biomethane potential (BMP) of solid organic wastes and energy crops: A proposed protocol for batch assays. Water Sci. Technol. 2009, 59, 927–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owens, J.M.; Chynoweth, D.P. Biochemical methane potential of municipal solid-waste (MSW) components. Water Sci. Technol. 1993, 27, 1–14. [Google Scholar] [CrossRef]
- Angelidaki, I.; Sanders, W. Assessment of the anaerobic biodegradability of macropollutants. Rev. Environ. Sci. Biotechnol. 2004, 3, 117. [Google Scholar] [CrossRef]
- Hansen, T.L.; Schmidt, J.E.; Angelidaki, I.; Marca, E.; La Cour Jansen, J.; Mosbæk, H.; Christensen, T.H. Method for determination of methane potentials of solid organic waste. Waste Manag. 2004, 24, 393. [Google Scholar]
- Rozzi, A.; Remigi, E. Methods of assessing microbial activity and inhibition under anaerobic conditions: A literature review. Rev. Environ. Sci. Biotechnol. 2004, 3, 93–115. [Google Scholar] [CrossRef]
- Fernandez, B.; Porrier, P.; Chamy, R. Effect of inoculum-substrate ratio on the start-up of solid waste anaerobic digesters. Water Sci. Technol. 2001, 44, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.G.; Ma, Y.S.; Chao, A.C.; Huang, C.L. BMP test on chemically pretreated sludge. Bioresour. Technol. 1999, 68, 187–192. [Google Scholar] [CrossRef]
- Muller, W.; Frommert, I.; Jörg, R. Standardized methods for anaerobic biodegradability testing. Rev. Environ. Sci. Biotechnol. 2004, 3, 141–158. [Google Scholar] [CrossRef]
- Pham, C.H.; Triolo, J.M.; Cu, T.T.T.; Pedersen, L.; Sommer, S.G. Validation and recommendation of methods to measure biogas production potential of animal manure. Asian Australas. J. Anim. Sci. 2013, 26, 864–873. [Google Scholar] [CrossRef] [Green Version]
- Möller, K.; Müller, T. Effects of anaerobic digestion on digestate nutrient availability and crop growth: A review. Eng. Life Sci. 2012, 12, 242–257. [Google Scholar] [CrossRef]
- VDI 4630. Fermentation of Organic Materials—Characterisation of the Substrate, Sampling, Collection of Material Data, Fermentation Tests; Verein Deutscher Ingenieure: Düsseldorf, Germany, 2016. [Google Scholar]
- ISO. ISO/DIS 14853:1999-04: Plastics—Determination of the Ultimate Anaerobic Biodegradability in an Aqueous System—Method by Measurement of Biogas Production; Bestimmmung der Vollständigen Anaeroben Bioabbaubarkeit in Einem Wässrigen System—Verfahren Mittels Messung von Biogasproduktion; ISO: Geneva, Switzerland, 1999. [Google Scholar]
- ISO. ISO 14853:2016: Plastics—Determination of the Ultimate Anaerobic Biodegradation of Plastic Materials in an Aqueous System—Method by Measurement of Biogas Production; ISO: Geneva, Switzerland, 2016; p. 28. [Google Scholar]
- ASTM International. ASTM D5511-94: Standard Test Method for Determining Anaerobic Biodegradation of Plastic Materials Under High-Solids Anaerobic-Digestion Conditions; ASTM International: West Conshohocken, PA, USA, 1994. [Google Scholar]
- ASTM International. ASTM 5210-1992: American Society for Testing and Materials: Test Method for Determining the Anaerobic Biodegradation of Plastic Materials in the Presence of Municipal Sewage Sludge; ASTM International: West Conshohocken, PA, USA, 1992. [Google Scholar]
- ASTM International. ASTM E 2170-2001: Test Method for Determining Anaerobic Biodegradation Potential of Organic Chemicals Under Methanogenic Conditions; ASTM International: West Conshohocken, PA, USA, 2001. [Google Scholar]
- Germany Institute for Normative. DIN 38414-8: Determination of the Amenability to Anaerobic Digestion (S8). German Standard Methods for the Examination of Water, Waste Water and Sludge: Sludge and Sediments (Group S); Deutsches Institut für Normung (Germany Institute for Normative): Koblenz, Germany, 1985. [Google Scholar]
- ISO. ISO 15473:2002: Qualité du Sol—Lignes Directrices Relatives aux Essais en Laboratoire pour la Biodégradation de Produits Chimiques Organiques dans le sol Sous Conditions Anaérobies, 1st ed.; ISO: Geneva, Switzerland, 2002; p. 13. [Google Scholar]
- ISO. ISO 11734:1995: Water Quality—Evaluation of the “Ultimate” Anaerobic Biodegradability of Organic Compounds in Digested Sludge—Method by Measurement of the Biogas Production, 1st ed.; ISO: Geneva, Switzerland, 1995; p. 13. [Google Scholar]
- Ohemeng-Ntiamoah, J.; Datta, T. Perspectives on variabilities in biomethane potential test parameters and outcomes: A review of studies published between 2007 and 2018. Sci. Total Environ. 2019, 664, 1052–1062. [Google Scholar] [CrossRef]
- Raposo, F.; Fernández-Cegrí, V.; De la Rubia, M.A.; Borja, R.; Béline, F.; Cavinato, C.; Demirer, G.; Fernández, B.; Fernández-Polanco, M.; Frigon, J.C.; et al. Biochemical methane potential (BMP) of solid organic substrates: Evaluation of anaerobic biodegradability using data from an international inter-laboratory study. J. Chem. Technol. Biotechnol. 2011, 86, 1088–1098. [Google Scholar] [CrossRef]
- Porqueddu, I.; Ficara, E.; Alibardi, L.; Bona, D.; Brina, A.; Calabrò, P.S.; Casaletta, E.; Cavinato, C.; Daffonchio, D.; De Gioannis, G.; et al. Results of an italian inter-laboratory study on biochemical methane potential. In Proceedings of the 13th World Congress on Anaerobic Digestion—Recovering (bio) Resources for the World, Santiago de Compostela, Spain, 25–28 June 2013. [Google Scholar]
- Bachmann, N.; Deront, M.; Fruteau, H.; Holliger, C.; Membrez, Y.; Wellinger, A. Optimisation des Tests Standardisés de Digestibilité dans des Reacteurs Batch; Office Fédéral de L’énergie OFEN: Ittigen, Switzerland, 2011; p. 79. [Google Scholar]
- Weinrich, S.; Schäfer, F.; Bochmann, G.; Liebetrau, J. Value of Batch Tests for Biogas Potential Analysis; Method Comparison and Challenges of Substrate and Efficiency Evaluation of Biogas Plants; Murphy, J.D., Ed.; IEA Bioenergy Task 37: Cork, Ireland, 2018; p. 10. ISBN 978-1-910154-49-6. (eBook electronic edition). [Google Scholar]
- KTBL/VDLUFA. Report for KTBL/VDLUFA Proficiency Test Biogas 2016; Edt. German Agricultural Analytic and Research Institutes (VDLUFA): Kassel, Germany; German Association for Technology and Structures in Agriculture (KTBL): Darmstadt, Germany, 2017. [Google Scholar]
- Holliger, C.; Alves, M.; Andrade, D.; Angelidaki, I.; Astals, S.; Baier, U.; Bougrier, C.; Buffière, P.; Carballa, M.; De Wilde, V.; et al. Towards a standardization of biomethane potential tests. Water Sci. Technol. 2016, 74, 2515–2522. [Google Scholar] [CrossRef]
- Hafner, S.D.; Fruteau de Laclos, H.; Koch, K.; Holliger, C. Improving inter-laboratory reproducibility in measurement of biochemical methane potential (BMP). Water 2020, 12, 1752. [Google Scholar] [CrossRef]
- VDLUFA. Measurement of Biogas and Methane Yields in Fermentation Tests; Verband Deutscher Landwirtschaftlicher Untersuchungsund Forschungsanstalten (VDLUFA): Speyer, Germany, 2011. [Google Scholar]
- Carrillo-Reyes, J.; Buitrón, G.; Moreno-Andrade, I.; Tapia-Rodríguez, A.C.; Palomo-Briones, R.; Razo-Flores, E.; Aguilar-Juárez, O.; Arreola-Vargas, J.; Bernet, N.; Ferreira Maluf Braga, A.; et al. Standardized protocol for determination of biohydrogen potential. MethodsX 2020, 7, 100754. [Google Scholar] [CrossRef]
- Pereira, C.P.P.; Castañares, G.; Lier, J.B. Van An OxiTop® protocol for screening plant material for its biochemical methane potential (BMP). Water Sci. Technol. 2012, 66, 1416–1423. [Google Scholar] [CrossRef] [PubMed]
- Hafner, S.D.; Rennuit, C.; Triolo, J.M.; Richards, B.K. Validation of a simple gravimetric method for measuring biogas production in laboratory experiments. Biomass Bioenergy 2015, 83, 297–301. [Google Scholar] [CrossRef]
- Justesen, C.G.; Astals, S.; Mortensen, J.R.; Thorsen, R.; Koch, K.; Weinrich, S.; Triolo, J.M.; Hafner, S.D. Development and validation of a low-cost gas density method for measuring biochemical methane potential (BMP). Water 2019, 11, 2431. [Google Scholar] [CrossRef] [Green Version]
- Amodeo, C.; Hafner, S.D.; Teixeira Franco, R.; Benbelkacem, H.; Moretti, P.; Bayard, R.; Buffière, P. How different are manometric, gravimetric, and automated volumetric BMP results? Water 2020, 12, 1839. [Google Scholar] [CrossRef]
- ISO. NF ISO 5725: Application de la Statistique—Exactitude (Justesse et Fidélité) des Résultats et Méthodes de Mesure; ISO: Geneva, Switzerland, 1994. [Google Scholar]
- ISO. ISO 13528:2015: Méthodes Statistiques Utilisées dans les Essais D’aptitude par Comparaison Interlaboratoires; ISO: Geneva, Switzerland, 2016; p. 95. [Google Scholar]
- Madigan, M.T.; Marinko, J.M.; Parker, J. Brock Biology of Microorganisms, 9th ed.; Prentice Hall: New York, NY, USA, 2000. [Google Scholar]
- APHA 2540-G: Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association; American Water Works Association and Water Environment Federation: Washington, DC, USA, 1998.
- Buswell, A.M.; Müller, H.F. Mechanism of methane fermentation. Ind. Eng. Chem. 1952, 44, 550–552. [Google Scholar] [CrossRef]
- Boyle, W.C. Energy recovery from sanitary landfills a review. In Microbial Energy Conversion; Schlegel, H.G., Barnea, S., Eds.; Pergamon Press: Oxford, UK, 1976. [Google Scholar]
- Kouas, M.; Torrijos, M.; Sousbie, P.; Steyer, J.P.; Sayadi, S.; Harmand, J. Robust assessment of both biochemical methane potential and degradation kinetics of solid residues in successive batches. Waste Manag. 2017, 70, 59–70. [Google Scholar] [CrossRef]
- Martinez-Sosa, D.; Torrijos, M.; Buitron, G.; Sousbie, P.; Devillers, P.H.; Delgenès, J.P. Treatment of fatty solid waste from the meat industry in an anaerobic sequencing batch reactor: Start-up period and establishment of the design criteria. Water Sci Technol. 2009, 60, 2245–2251. [Google Scholar] [CrossRef] [Green Version]
- Pearse, L.F.; Hettiaratchi, J.P.; Kumar, S.; Moreno-Andrade, I.; Buitrón, G. Towards developing a representative biochemical methane potential (BMP) assay for landfilled municipal solid waste–A review. Water Sci. Technol. 2018, 49, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Raposo, F.; De La Rubia, M.A.; Fernández-Cegrí, V.; Borja, R. Anaerobic digestion of solid organic substrates in batch mode: An overview relating to methane yields and experimental procedures. Renew. Sustain. Energy Rev. 2012, 16, 861–877. [Google Scholar] [CrossRef]
- Nilsson Påledal, S.; Hellman, E.; Moestedt, J. The effect of temperature, storage time and collection method on biomethane potential of source separated household food waste. Waste Manag. 2018, 71, 636–643. [Google Scholar] [CrossRef]
- Elbeshbishy, E.; Nakhla, G.; Hafez, H. Biochemical methane potential (BMP) of food waste and primary sludge: Influence of inoculum pre-incubation and inoculum source. Bioresour. Technol. 2012, 110, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Dechrugsa, S.; Kantachote, D.; Chaiprapat, S. Effects of inoculum to substrate ratio, substrate mix ratio and inoculum source on batch co-digestion of grass and pig manure. Bioresour. Technol. 2013, 146, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, A.; Serranti, S.; Bonifazi, G. Biochemical methane potential (BMP) of artichoke waste: The inoculum effect. Waste Manag. Res. J. Sustain. Circ. Econ. 2014, 32, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Hülsemann, B.; Zhou, L.; Merkle, W.; Hassa, J.; Müller, J.; Oechsner, H. Biomethane potential test: Influence of inoculum and the digestion system. Appl. Sci. 2020, 10, 2589. [Google Scholar] [CrossRef] [Green Version]
- De Vrieze, J.; Raport, L.; Willems, B.; Verbrugge, S.; Volcke, E.; Meers, E.; Angenent, L.T.; Boon, N. Inoculum selection influences the biochemical methane potential of agro-industrial substrates. Microb. Biotechnol. 2015, 8, 776–786. [Google Scholar] [CrossRef]
- Hagen, L.H.; Vivekanand, V.; Pope, P.B.; Eijsink, V.G.H.; Horn, S.J. The effect of storage conditions on microbial community composition and biomethane potential in a biogas starter culture. Appl. Microbiol. Biotechnol. 2015, 99, 5749–5761. [Google Scholar] [CrossRef]
- Steinmetz, R.L.R.; Mezzari, M.P.; Busi da Silva, M.L.; Airton Kunz, A.; Cestonaro do Amaral, A.; Tápparo, D.C.; Moreira Soares, H. Enrichment and acclimation of an anaerobic mesophilic microorganism’s inoculum for standardization of BMP assays. Bioresour. Technol. 2016, 219, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Strömberg, S.; Nges, I.A.; Nistor, M.; Liu, J. Impacts of inoculum pre-treatments on enzyme activity and biochemical methane potential. J. Biosci. Bioeng. 2016, 121, 557–560. [Google Scholar] [CrossRef]
- Koch, K.; Lippert, T.; Drewes, J.E. The role of inoculum’s origin on the methane yield of different substrates in biochemical methane potential (BMP) tests. Bioresour. Technol. 2017, 243, 457–463. [Google Scholar] [CrossRef]
- Astals, S.; Koch, K.; Weinrich, S.; Hafner, S.D.; Tait, S.; Peces, M. Impact of storage conditions on the methanogenic activity of anaerobic digestion inocula. Water 2020, 12, 1321. [Google Scholar] [CrossRef]
- Hafner, S.D.; Richards, B.K.; Astals, S.; Holliger, C.; Koch, K.; Weinrich, S. Calculation of Methane Production from Gravimetric Measurements. Standard BMP Methods Document 203, Version 1.0. Available online: https://www.dbfz.de/en/BMP (accessed on 19 April 2020).
- Wang, B.; Björn, A.; Strömberg, S.; Achu Nges, I.; Nistor, M.; Liu, J. Evaluating the influences of mixing strategies on the Biochemical Methane Potential test. J. Environ. Manag. 2017, 185, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Valero, D.; Montes, J.A.; Rico, J.L.; Rico, C. Influence of headspace pressure on methane production in Biochemical Methane Potential (BMP) tests. Waste Manag. 2016, 48, 193–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Himanshu, H.; Voelklein, M.A.; Murphy, J.D.; Grant, J.; O’Kiely, P. Factors controlling headspace pressure in a manual manometric BMP method can be used to produce a methane output comparable to AMPTS. Bioresour. Technol. 2017, 238, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Koch, K.; Bajón Fernandez, Y.; Drewes, J.E. Influence of headspace flushing on methane production in Biochemical Methane Potential (BMP) tests. Bioresour. Technol. 2015, 186, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Koch, K.; Hafner, S.D.; Astals, S.; Weinrich, S. Evaluation of common supermarket products as positive controls in Biochemical Methane Potential (BMP) Tests. Water 2020, 12, 1223. [Google Scholar] [CrossRef]
- Kleinheinz, G.; Hernandez, G. Comparison of two laboratory methods for the determination of biomethane potential of organic feedstocks. J. Microbiol. Methods 2016, 130, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Koch, K.; Hafner, S.D.; Weinrich, S.; Astals, S. Identification of critical problems in biochemical methane potential (BMP) tests from methane production curves. Front. Environ. Sci. 2019, 7, 178. [Google Scholar] [CrossRef]
- Cresson, R.; Pommier, S.; Béline, F.; Bouchez, T.; Bougrier, C.; Buffière, P.; Cacho, J.; Camacho, P.; Mazéas, L.; Pauss, A.; et al. Etude Interlaboratoires pour L’harmonisation des Protocoles de Mesure du Potentiel Bio-Methanogène des Matrices Solides Hétérogènes—Rapport Final; ADEME: Paris, France, 2014; pp. 1–121. [Google Scholar]
- Cresson, R.; Pommier, S.; Beline, F.; Bouchez, T.; Bougrier, C.; Buffière, P.; Pauss, A.; Pouech, P.; Preys, S.; Ribeiro, T. Results from a French Inter-Laboratory Campaign on the Biological Methane Potential of Solid Substrates. 2015. Available online: https://hal.archives-ouvertes.fr/hal-01947463/ (accessed on 27 August 2020).









| Values for the 1st Phase | SA | SA’ | SB |
|---|---|---|---|
| Number of expected values | 78 | 78 | 78 |
| Number of removed values | 17 | 10 | 19 |
| Number of considered values | 61 | 68 | 59 |
| Values for the 2nd Phase | SA’ | SB | SC |
|---|---|---|---|
| Number of expected values | 75 | 69 | 69 |
| Number of removed values | 6 | 16 | 13 |
| Number of considered values | 69 | 53 | 56 |
| Substrate | Composition (% w) | Main Characteristics | |
|---|---|---|---|
| SA | Potato Grain maize Beef Wheat straw Total | 40% 20% 30% 10% 100% | Complex composition (mix of compounds easily and slowly biodegradable) High heterogeneity High content of moisture |
| SA’ | Idem SA but dried and shredded (1 mm) | 100% | Complex composition (same as SA) Homogeneous Dry |
| SB | Wheat straw | 100% | Unique substrate slowly biodegradable Homogeneous Dry |
| SC | Mayonnaise | 100% | Lipidic substrate Homogeneous Immiscible with water |
| Chemicals | Concentration (g L−1) |
|---|---|
| NH4Cl | 26.6 |
| KH2PO4 | 10 |
| MgCl2·6H2O | 6 |
| CaCl2·2H2O | 3 |
| FeCl2·4H2O | 2 |
| CoCl2·6H2O | 0.5 |
| MnCl2·4H2O | 0.1 |
| NiCl2·6H2O | 0.1 |
| ZnCl2 | 0.05 |
| H3BO3 | 0.05 |
| Na2SeO3 | 0.05 |
| CuCl2·2H2O | 0.04 |
| Na2MoO4·2H2O | 0.01 |
| Substrate | n * | TS (%) | VS (% TS) | Theoretical BMP (NmL CH4/gVS) ** |
|---|---|---|---|---|
| SA | 11 | 30.20 ± 2.28 | 95.22 ± 1.25 | 476 |
| SA’ | 21 | 97.09 ± 0.92 | 94.43 ± 1.06 | 476 |
| SB | 22 | 91.86 ± 1.79 | 92.13 ± 2.87 | 499 |
| SC | 10 | 74.49 ± 1.79 | 98.66 ± 0.26 | 1013 |
| BMP (NmLCH4 gVS−1) | SA | SA’ | SB |
|---|---|---|---|
| Mean | 425 | 403 | 267 |
| Median | 422 | 407 | 267 |
| Min | 289 | 250 | 175 |
| Max | 629 | 481 | 370 |
| Intra-laboratory repeatability RSD | 7% | 4% | 6% |
| Intra-laboratory reproducibility RSD | 9% | 6% | 8% |
| Inter-laboratory reproducibility RSD | 20% | 17% | 20% |
| BMP (NmLCH4 gVS−1) | SA’ | SB | SC |
|---|---|---|---|
| Mean | 405 | 277 | 848 |
| Median | 408 | 282 | 828 |
| Min | 260 | 195 | 660 |
| Max | 525 | 370 | 1026 |
| Intra-laboratory repeatability RSD | 4% | 4% | 4% |
| Intra-laboratory reproducibility RSD | 5% | 7% | 5% |
| Inter-laboratory reproducibility RSD | 19% | 21% | 13% |
| Intra- and Inter-Laboratory RSDs | Harmonized Protocol |
|---|---|
| Intra-laboratory repeatability RSD | 4% |
| Intra-laboratory reproducibility RSD | 6% |
| Inter-laboratory reproducibility RSD | 18% |
| Variable | Factor | Data Number | ||
|---|---|---|---|---|
| SA’ | SB | SC | ||
| Total | 69 | 53 | 56 | |
| Method | Automatic | 22 | 14 | 11 |
| Manual | 47 | 39 | 45 | |
| Gas measurement | Manometric | 29 | 24 | 27 |
| Volumetric | 40 | 29 | 29 | |
| Factor | Substrate | FD * | Sum of Squares | F | p-Value |
|---|---|---|---|---|---|
| Method | SA’ | 1 | 37,297.734 | 18.207 | <0.0001 |
| SB | 1 | 31,758.846 | 24.33 | <0.0001 | |
| SC | 1 | 70,302.028 | 13.349 | 0.001 | |
| Gas measurement | SA’ | 1 | 11,076.371 | 4.506 | 0.038 |
| SB | 1 | 12,932.688 | 7.619 | 0.008 | |
| SC | 1 | 100,817.886 | 21.958 | <0.0001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, T.; Cresson, R.; Pommier, S.; Preys, S.; André, L.; Béline, F.; Bouchez, T.; Bougrier, C.; Buffière, P.; Cacho, J.; et al. Measurement of Biochemical Methane Potential of Heterogeneous Solid Substrates: Results of a Two-Phase French Inter-Laboratory Study. Water 2020, 12, 2814. https://doi.org/10.3390/w12102814
Ribeiro T, Cresson R, Pommier S, Preys S, André L, Béline F, Bouchez T, Bougrier C, Buffière P, Cacho J, et al. Measurement of Biochemical Methane Potential of Heterogeneous Solid Substrates: Results of a Two-Phase French Inter-Laboratory Study. Water. 2020; 12(10):2814. https://doi.org/10.3390/w12102814
Chicago/Turabian StyleRibeiro, Thierry, Romain Cresson, Sébastien Pommier, Sébastien Preys, Laura André, Fabrice Béline, Théodore Bouchez, Claire Bougrier, Pierre Buffière, Jesús Cacho, and et al. 2020. "Measurement of Biochemical Methane Potential of Heterogeneous Solid Substrates: Results of a Two-Phase French Inter-Laboratory Study" Water 12, no. 10: 2814. https://doi.org/10.3390/w12102814
APA StyleRibeiro, T., Cresson, R., Pommier, S., Preys, S., André, L., Béline, F., Bouchez, T., Bougrier, C., Buffière, P., Cacho, J., Camacho, P., Mazéas, L., Pauss, A., Pouech, P., Rouez, M., & Torrijos, M. (2020). Measurement of Biochemical Methane Potential of Heterogeneous Solid Substrates: Results of a Two-Phase French Inter-Laboratory Study. Water, 12(10), 2814. https://doi.org/10.3390/w12102814









