Differential Responses of Food Web Properties to Opposite Assembly Rules and Species Richness
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Source
2.2. Data Acquisition
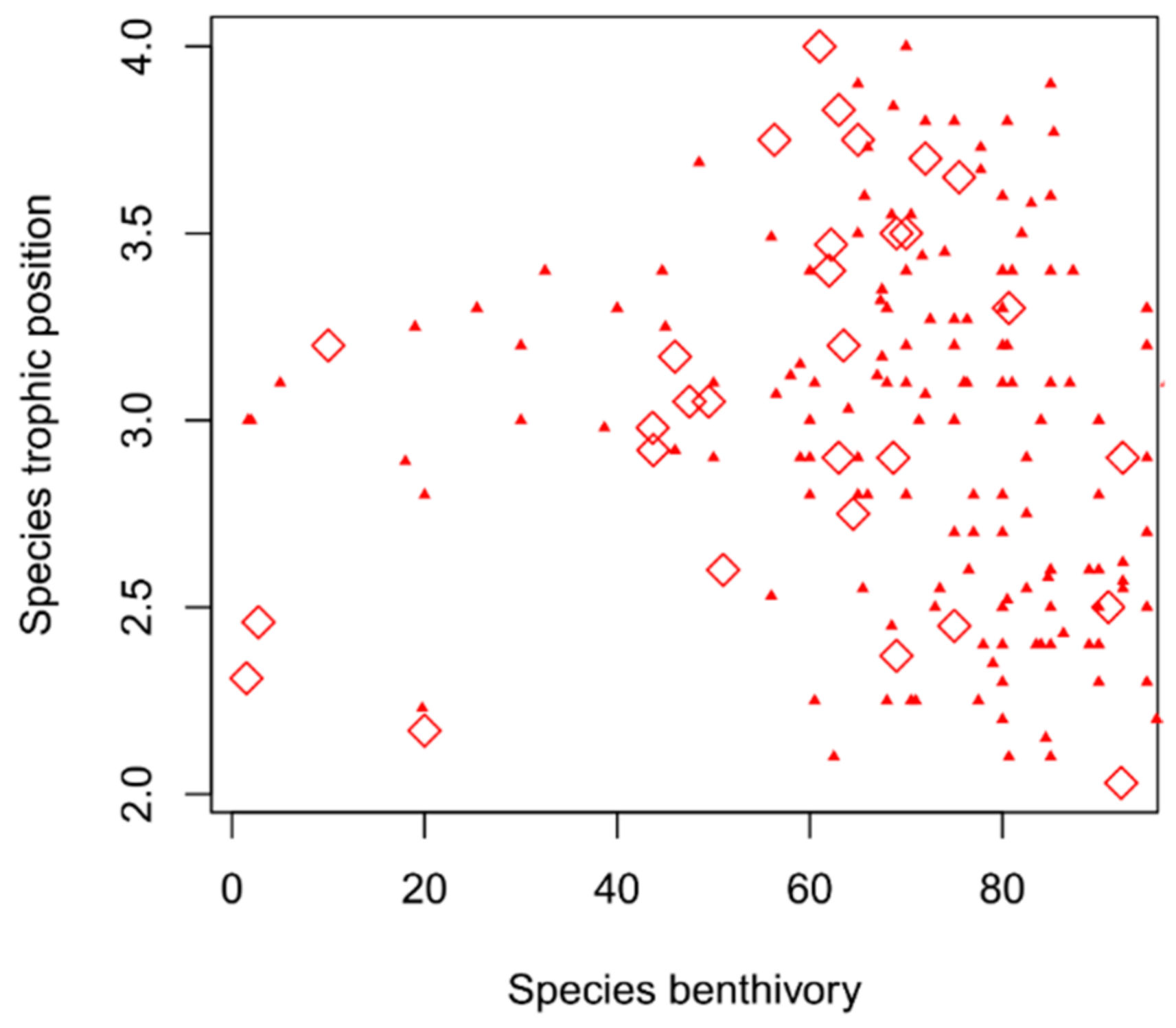
2.3. Niche Determination
2.4. Food Web Properties
- (1)
- Richness of trophic niche space. This metric is defined by the convex hull volume of an assemblage in trophic niche space where the most extreme species (i.e., vertices) are linked to build the convex hull and calculate the corresponding volume inside. Therefore, by including all species in trophic niche space, it represents a measure of the total amount of niche space occupied, and a proxy for the trophic richness of a food web [34,35,36].
- (2)
- Evenness of trophic niche space. We measured trophic evenness following the regularity index proposed for the estimation of functional evenness [34,37]. This index measures the regularity of spacing between species in trophic niche space. A value of 1 is achieved when the distances of all nearest neighbor species pairs are identical. Conversely, when a large proportion of species are characterized by similar trophic ecologies (i.e., similar occupancy of the trophic niche space), trophic evenness will exhibit a smaller value, indicative of increased trophic redundancy.
- (3)
- Divergence of trophic niche space. This represents a measure of the species distance to the center of gravity of the assemblage’s trophic niche space defined by the convex hull, and thus it is a proxy for trophic niche specialization at community level. Trophic divergence is the highest if all the species are on the convex hull and at equal distance to its center of gravity. It has the advantage of being independent from trophic evenness [34]. Specialization has also been outlined as a key concept to predict the adaptive response of populations in heterogeneous or fluctuating environments [38].
2.5. Simulation
2.6. Response Thresholds
2.7. Testing for Non-Randomization
3. Results
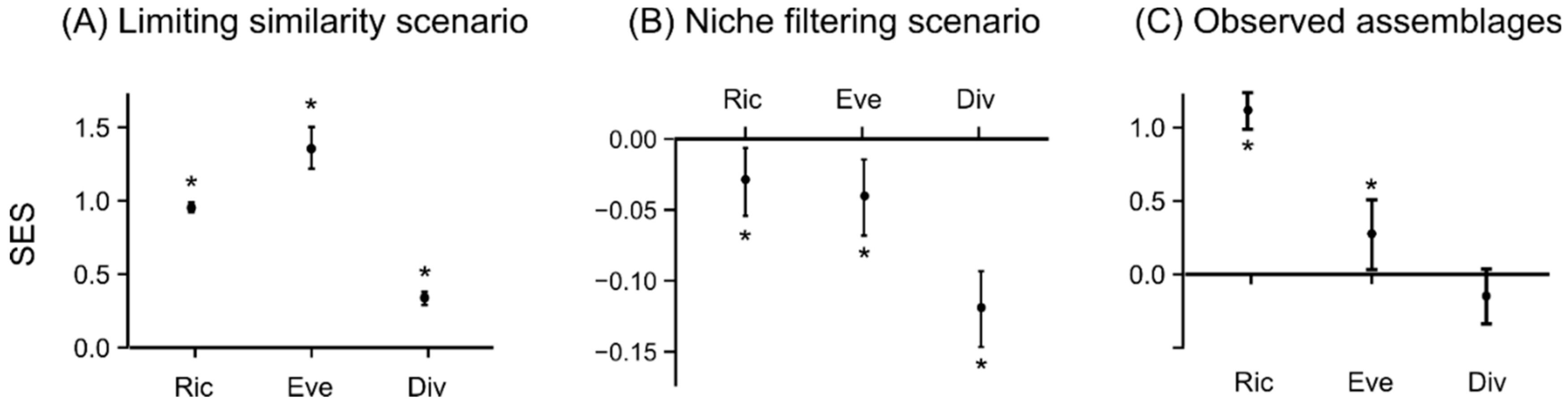
3.1. Assembly Rules in Modelled Scenarios
3.2. Food Web Properties of Lake Fish Assemblages
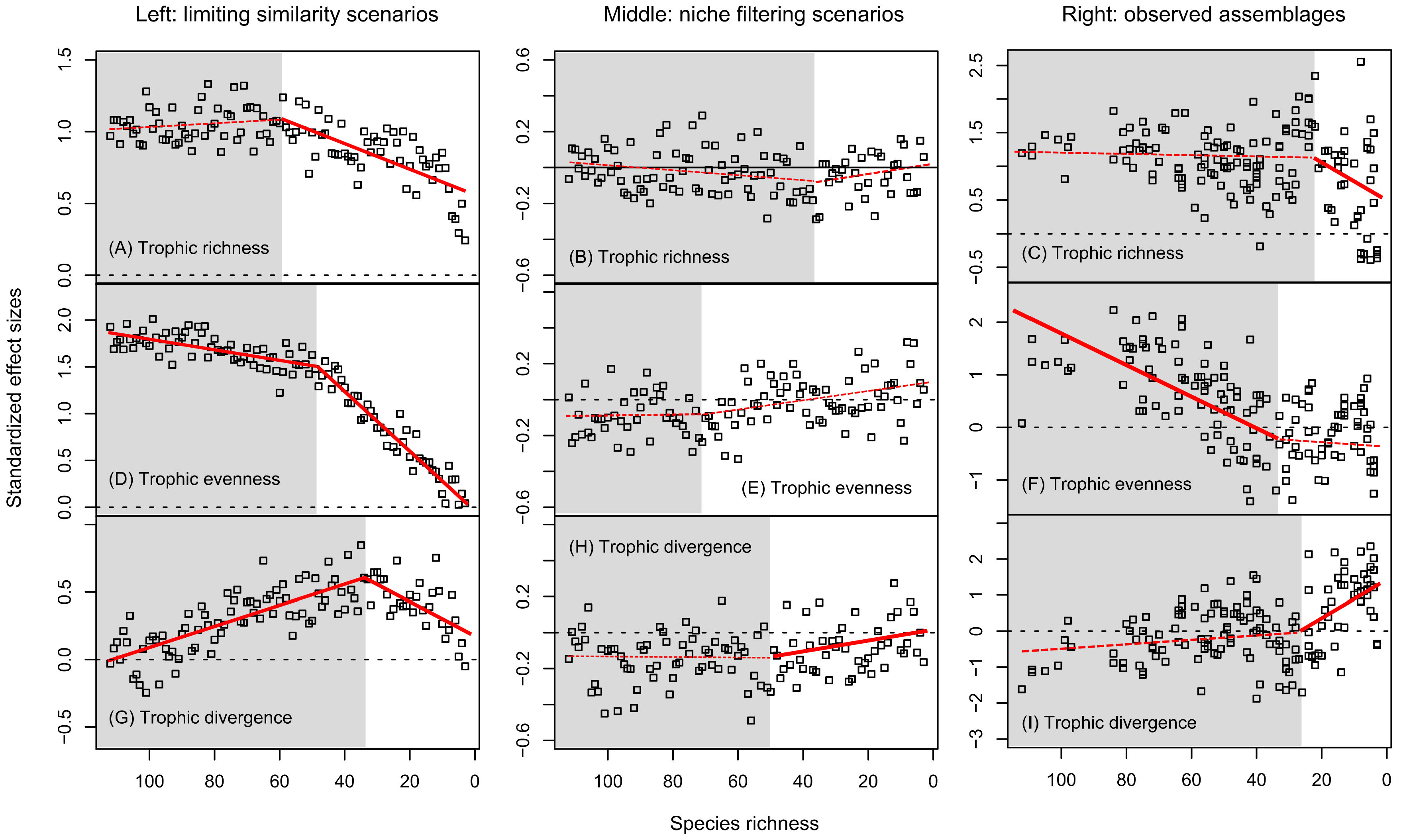
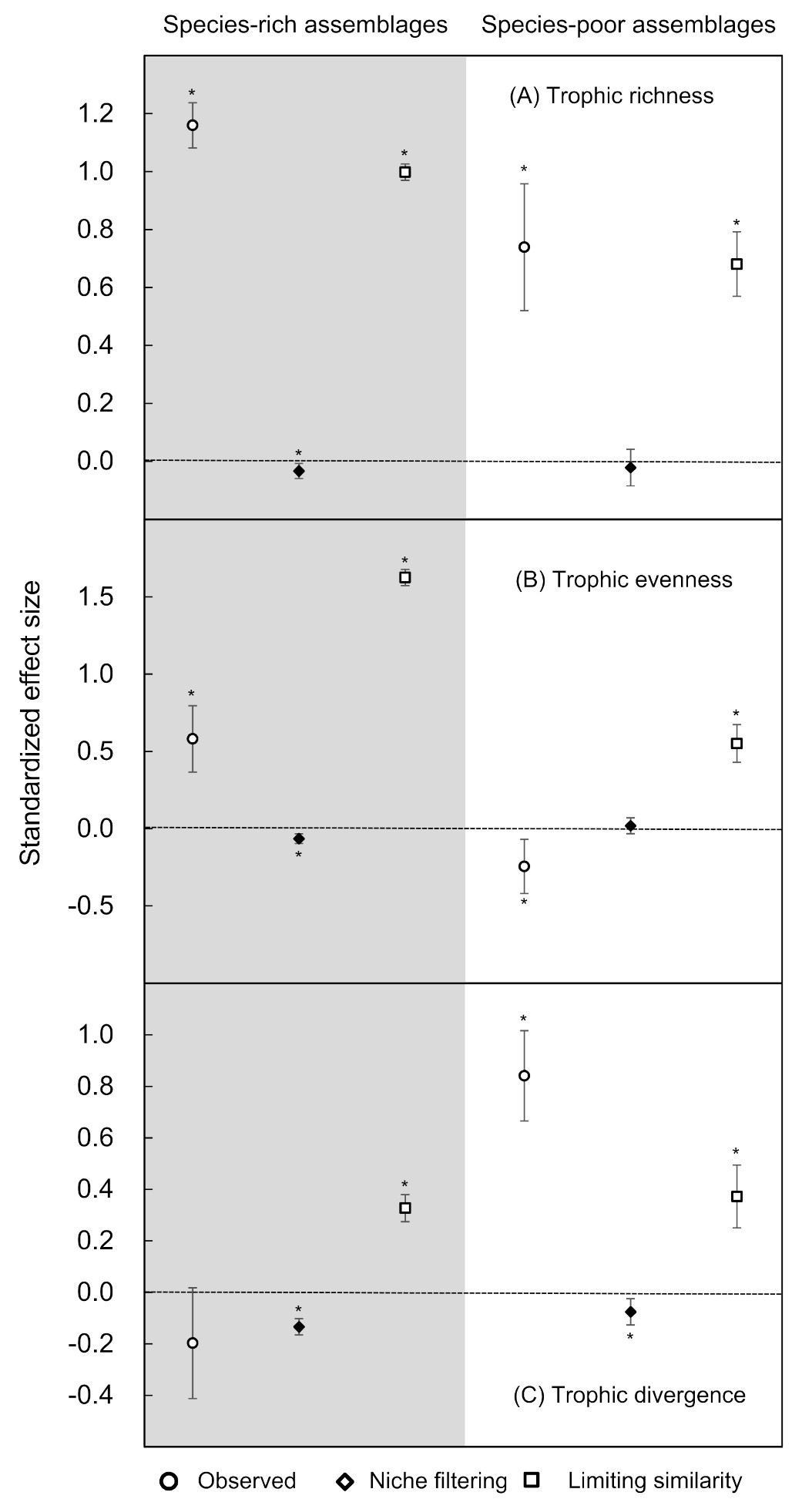
3.3. Differential Response to Variation in Species Richness
4. Discussion
4.1. Assembly Rules and Modelled Scenarios
4.2. Influence of Assembly Rules on Food Web Properties of Fish Assemblages
4.3. Response to Variation in Species Richness
4.4. Trophy-Based Conservation Implications
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Physicochemical Parameters | Mean | Max | Min | Stddev | Median |
|---|---|---|---|---|---|
| surface area (km2) | 190.372 | 2933.000 | 10.200 | 470.023 | 45.000 |
| pH | 8.341 | 10.010 | 6.891 | 0.476 | 8.323 |
| DO (mg/L) | 9.473 | 40.080 | 0.540 | 2.284 | 9.300 |
| Chla (µg/L) | 10.462 | 224.189 | 0.911 | 13.703 | 6.592 |
| TP (mg/L) | 0.106 | 4.996 | 0.010 | 0.269 | 0.070 |
| TN (mg/L) | 1.672 | 6.866 | 0.211 | 1.232 | 1.264 |
| NOx-N (mg/L) | 0.622 | 4.123 | 0.000 | 0.896 | 0.284 |
| NO2-N (mg/L) | 0.017 | 0.342 | 0.000 | 0.036 | 0.002 |
| NH4-N (mg/L) | 0.249 | 2.685 | 0.003 | 0.353 | 0.143 |
| PO4-P (µg/L) | 16.917 | 628.480 | 0.307 | 46.748 | 3.973 |
| CODMn (mg/L) | 5.436 | 20.912 | 1.684 | 1.934 | 5.333 |
| Scenario | Food Web Properties | Species Richness Changepoint (CI 95%) | Species-Poor Slope (CI 95%) | Species-Rich Slope (CI 95%) |
|---|---|---|---|---|
| Limiting similarity | Trophic richness | 59.0 (10.2–74.0) | 0.0088 (0.0062–0.0872) | −0.0013 (−0.0046–0.0030) |
| Trophic evenness | 48.6 (44.0–52.8) | 0.0312 (0.0287–0.0347) | 0.0056 (0.0039–0.0074) | |
| Trophic divergence | 33.1 (17.3–52.0) | 0.0135 (0.0036–0.0446) | −0.0214 (−0.0106–−0.0061) | |
| Niche filtering | Trophic richness | 36.0 (14.2–90.0) | −0.0025 (−0.0161–0.0018) | 0.0014 (−0.0013–0.0038) |
| Trophic evenness | 71.0 (14.2–90.1) | −0.0024 (−0.0047–0.0010) | −0.0003 (−0.0035–0.0022) | |
| Trophic divergence | 50.0 (19.0–103.5) | −0.0030 (−0.0174–−0.0005) | 0.0003 (−0.0025–0.0274) | |
| Observed assemblages | Trophic richness | 22.0 (8.0–96.4) | 0.0308 (0.0008–0.1801) | 0.0005 (−0.0187–0.0087) |
| Trophic evenness | 33.0 (31.0–108.2) | 0.0044 (−0.0099–0.0283) | 0.0305 (0.0003–0.0412) | |
| Trophic divergence | 26.0 (20.7–99.0) | −0.0553 (−0.0864–−0.0144) | −0.0059 (−0.0722–0.0003) |
References
- Dirzo, R.; Young, H.S.; Galetti, M.; Ceballos, G.; Isaac, N.J.B.; Collen, B. Defaunation in the Anthropocene. Science 2014, 345, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Martins, G.M.; Arenas, F.; Neto, A.I.; Jenkins, S.R. Effects of Fishing and Regional Species Pool on the Functional Diversity of Fish Communities. PLoS ONE 2012, 7, e44297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myers, R.A.; Worm, B. Rapid worldwide depletion of predatory fish communities. Nature 2003, 423, 280–283. [Google Scholar] [CrossRef] [PubMed]
- May, R.M. Food-web assembly and collapse: Mathematical models and implications for conservation. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1643–1646. [Google Scholar] [CrossRef]
- Duffy, E.J.; Cardinale, B.J.; France, K.E.; McIntyre, P.B.; Thébault, E.; Loreau, M. The functional role of biodiversity in ecosystems: Incorporating trophic complexity. Ecol. Lett. 2007, 10, 522–538. [Google Scholar] [CrossRef] [Green Version]
- Holt, R.D.; Lawton, J.H.; Polis, G.A.; Martinez, N.D. Trophic Rank and the Species-Area Relationship. Ecology 1999, 80, 1495. [Google Scholar] [CrossRef] [Green Version]
- Post, D.M.; Takimoto, G. Proximate structural mechanisms for variation in food-chain length. Oikos 2007, 116, 775–782. [Google Scholar] [CrossRef]
- Shmida, A.; Wilson, M.V. Biological Determinants of Species Diversity. J. Biogeogr. 1985, 12, 1. [Google Scholar] [CrossRef]
- Paine, R.T. Food Web Complexity and Species Diversity. Am. Nat. 1966, 100, 65–75. [Google Scholar] [CrossRef]
- Riede, J.O.; Rall, B.C.; Banasek-Richter, C.; Navarrete, S.A.; Wieters, E.A.; Emmerson, M.C.; Jacob, U.; Brose, U. Scaling of Food-Web Properties with Diversity and Complexity Across Ecosystems. In Resilience in Complex Socio-Ecological Systems; Elsevier B.V.: Amsterdam, The Netherlands, 2010; Volume 42. [Google Scholar]
- Estes, J.A.; Terborgh, J.; Brashares, J.S.; Power, M.E.; Berger, J.; Bond, W.J.; Carpenter, S.R.; Essington, T.E.; Holt, R.D.; Jackson, J.B.C.; et al. Trophic Downgrading of Planet Earth. Science 2011, 333, 301–306. [Google Scholar] [CrossRef] [Green Version]
- Logue, J.B.; Mouquet, N.; Peter, H.; Hillebrand, H. Empirical approaches to metacommunities: A review and comparison with theory. Trends Ecol. Evol. 2011, 26, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Calcagno, V.; Massol, F.; Mouquet, N.; Jarne, P.; David, P. Constraints on Food Chain Length Arising from Regional Metacommunity Dynamics. Available online: https://royalsocietypublishing.org/doi/full/10.1098/rspb.2011.0112 (accessed on 18 September 2020).
- Calcagno, V.; Mouquet, N.; Jarne, P.; David, P. Coexistence in a metacommunity: The competition–colonization trade-off is not dead. Ecol. Lett. 2006, 9, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Holt, R.D.; Hoopes, M.E. Food Web Dynamics in a Metacommunity Context-Modules and Beyond. In Metacommunities: Spatial Dynamics and Ecological Communities; University of Chicago Press: Chicago, IL, USA, 2005. [Google Scholar]
- Hubbell, S.P. Neutral theory and the evolution of ecological equivalence. Ecology 2006, 87, 1387–1398. [Google Scholar] [CrossRef] [Green Version]
- Kembel, S.W. Disentangling niche and neutral influences on community assembly: Assessing the performance of community phylogenetic structure tests. Ecol. Lett. 2009, 12, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Weiher, E.; Keddy, P.A. Assembly rules, null models, and trait dispersion—New questions front old patterns. Oikos 1995, 74, 159–164. [Google Scholar] [CrossRef] [Green Version]
- MacArthur, R.; Levins, R. The Limiting Similarity, Convergence, and Divergence of Coexisting Species. Am. Nat. 1967, 101, 377–385. [Google Scholar] [CrossRef]
- Zobel, M. The relative role of species pools in determining plant species richness. An alternative explanation of species coexistence? Trends Ecol. Evol. 1997, 12, 266–269. [Google Scholar] [CrossRef]
- Xu, J.; Wen, Z.; Gong, Z.; Zhang, M.; Xie, P.; Hansson, L.-A. Seasonal Trophic Niche Shift and Cascading Effect of a Generalist Predator Fish. PLoS ONE 2012, 7, e49691. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Wen, Z.; Ke, Z.; Zhang, M.; Zhang, M.; Guo, N.; Hansson, L.-A.; Xie, P. Contrasting energy pathways at the community level as a consequence of regime shifts. Oecologia 2014, 175, 231–241. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wu, G.; Zhang, P.; Xu, J. Trophic fingerprint of fish communities in subtropical floodplain lakes. Ecol. Freshw. Fish 2013, 22, 246–256. [Google Scholar] [CrossRef]
- Chen, Y. Fauna Sinica, Osteichthyes, Cypriniformes II; Science Press: Beijing, China, 1998. [Google Scholar]
- Chu, X.; Zheng, B.; Dai, D. Fauna Sinica, Osteichthyes, Siluriformes; Science Press: Beijing, China, 1999. [Google Scholar]
- Yue, P. Fauna Sinica. Osteichthyes Cypriniformes III; Science Press: Beijing, China, 2000. [Google Scholar]
- Elton, C. The Journal of Animal Ecology. J. Ecol. 1947, 35, 266. [Google Scholar] [CrossRef]
- Leibold, M.A. The Niche Concept Revisited: Mechanistic Models and Community Context. Ecology 1995, 76, 1371–1382. [Google Scholar] [CrossRef]
- Leibold, M.A.; Holyoak, M.; Mouquet, N.; Amarasekare, P.; Chase, J.M.; Hoopes, M.F.; Holt, R.D.; Shurin, J.B.; Law, R.; Tilman, D.; et al. The metacommunity concept: A framework for multi-scale community ecology. Ecol. Lett. 2004, 7, 601–613. [Google Scholar] [CrossRef]
- Bonhommeau, S.; Dubroca, L.; Le Pape, O.; Barde, J.; Kaplan, D.M.; Chassot, E.; Nieblas, A.E. Eating up the world’s food web and the human trophic level. Proc. Natl. Acad. Sci. USA 2013, 110, 20617–20620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vander Zanden, M.J.; Vadeboncoeur, Y. Fishes as integrators of benthic and pelagic food webs in lakes. Ecology 2002, 83, 2152–2161. [Google Scholar] [CrossRef]
- Vander Zanden, M.J.; Cabana, G.; Rasmussen, J.B. Comparing trophic position of freshwater fish calculated using stable nitrogen isotope ratios (delta N-15) and literature dietary data. Can. J. Fish. Aquat.Sci. 1997, 54, 1142–1158. [Google Scholar] [CrossRef]
- Winemiller, K.O. Spatial and Temporal Variation in Tropical Fish Trophic Networks. Ecol. Monogr. 1990, 60, 331–367. [Google Scholar] [CrossRef]
- Villéger, S.; Mason, N.W.H.; Mouillot, D. New Multidimensional Functional Diversity Indices for a Multifaceted Framework In Functional Ecology. Ecology 2008, 89, 2290–2301. [Google Scholar] [CrossRef] [Green Version]
- Cornwell, W.K.; Schwilk, D.W.; Ackerly, D. A trait-based test for habitat filtering: Convex hull volume. Ecology 2006, 87, 1465–1471. [Google Scholar] [CrossRef]
- Layman, C.A.; Arrington, D.A.; Montana, C.G.; Post, D.M. Can stable isotope ratios provide for community-wide measures of trophic structure? Ecology 2007, 88, 42–48. [Google Scholar] [CrossRef]
- Mouillot, D.; Mason, W.H.N.; Dumay, O.; Wilson, J.B. Functional regularity: A neglected aspect of functional diversity. Oecologia 2004, 142, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Levins, R. Evolution in Changing Environments: Some Theorethical Explorations; Princeton University Press: Princeton, NJ, USA, 1968. [Google Scholar]
- Toms, J.D.; Lesperance, M.L. Piecewise regression: A tool for identifying ecological thresholds. Ecology 2003, 84, 2034–2041. [Google Scholar] [CrossRef]
- Gotelli, N.J.; McCabe, D.J. Species co-occurrence: A meta-analysis of J. M. Diamond’s assembly rules model. Ecology 2002, 83, 2091–2096. [Google Scholar] [CrossRef]
- SiZer: Significant Zero Crossings. R Package Version 0.1-4. Available online: http://CRANR-projectorg/package=SiZer (accessed on 18 September 2020).
- Sonderegger, D.L.; Wang, H.; Clements, W.H.; Noon, B.R. Using SiZer to detect thresholds in ecological data. Front. Ecol. Environ. 2009, 7, 190–195. [Google Scholar] [CrossRef]
- Kraft, N.J.B.; Ackerly, D.D. Functional trait and phylogenetic tests of community assembly across spatial scales in an Amazonian forest. Ecol. Monogr. 2010, 80, 401–422. [Google Scholar] [CrossRef]
- Post, W.; Pimm, S. Community assembly and food web stability. Math. Biosci. 1983, 64, 169–192. [Google Scholar] [CrossRef]
- Pimm, S.L.; Lawton, J.H. On feeding on more than one trophic level. Nature 1978, 275, 542–544. [Google Scholar] [CrossRef]
- Yodzis, P. How Rare is Omnivory? Ecology 1984, 65, 321–323. [Google Scholar] [CrossRef]
- Mouillot, D.; Dumay, O.; Tomasini, J.A. Limiting similarity, niche filtering and functional diversity in coastal lagoon fish communities. Estuarine Coast. Shelf Sci. 2007, 71, 443–456. [Google Scholar] [CrossRef]
- Zhong, Y.G.; Power, G. Fisheries in China: Progress, problems, and prospects. Can. J. Fish. Aquat. Sci. 1997, 54, 224–238. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Wang, T.; Jorge, G.M.; Zhang, H.; Zhang, P.; Zhang, M.; Xu, J. Differential Responses of Food Web Properties to Opposite Assembly Rules and Species Richness. Water 2020, 12, 2828. https://doi.org/10.3390/w12102828
Guo Y, Wang T, Jorge GM, Zhang H, Zhang P, Zhang M, Xu J. Differential Responses of Food Web Properties to Opposite Assembly Rules and Species Richness. Water. 2020; 12(10):2828. https://doi.org/10.3390/w12102828
Chicago/Turabian StyleGuo, Yulun, Tao Wang, García Molinos Jorge, Huan Zhang, Peiyu Zhang, Min Zhang, and Jun Xu. 2020. "Differential Responses of Food Web Properties to Opposite Assembly Rules and Species Richness" Water 12, no. 10: 2828. https://doi.org/10.3390/w12102828





