Correlating Microbial Community Characteristics with Environmental Factors along a Two-Stage Biological Aerated Filter
Abstract
:1. Introduction
2. Materials and Methods
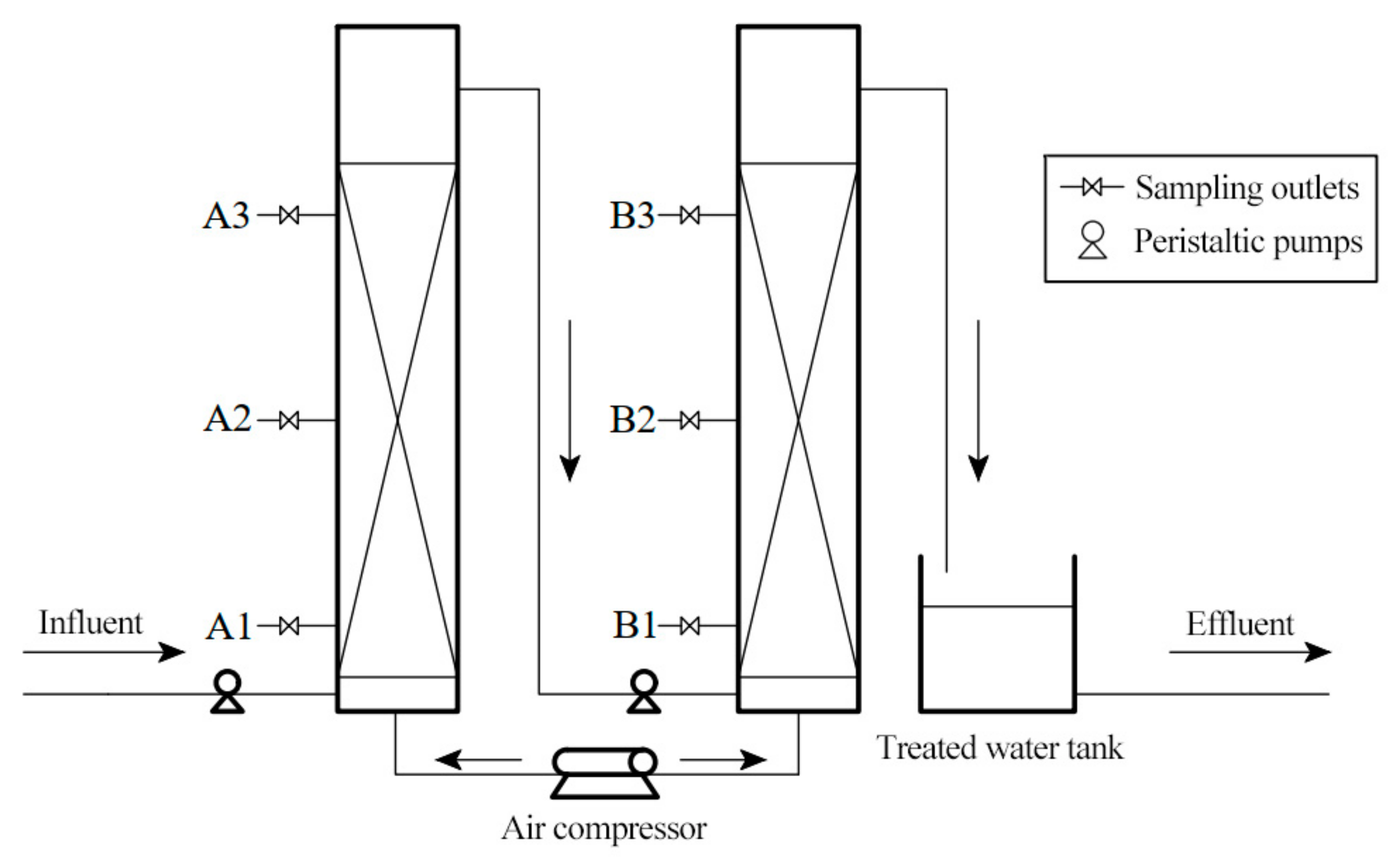
2.1. Description of the Two-Stage BAF System
2.2. Operation of the Two-Stage BAF
2.3. Synthetic Wastewater
2.4. Sample Collection and Analysis
2.5. Data Analysis
3. Results and Discussion
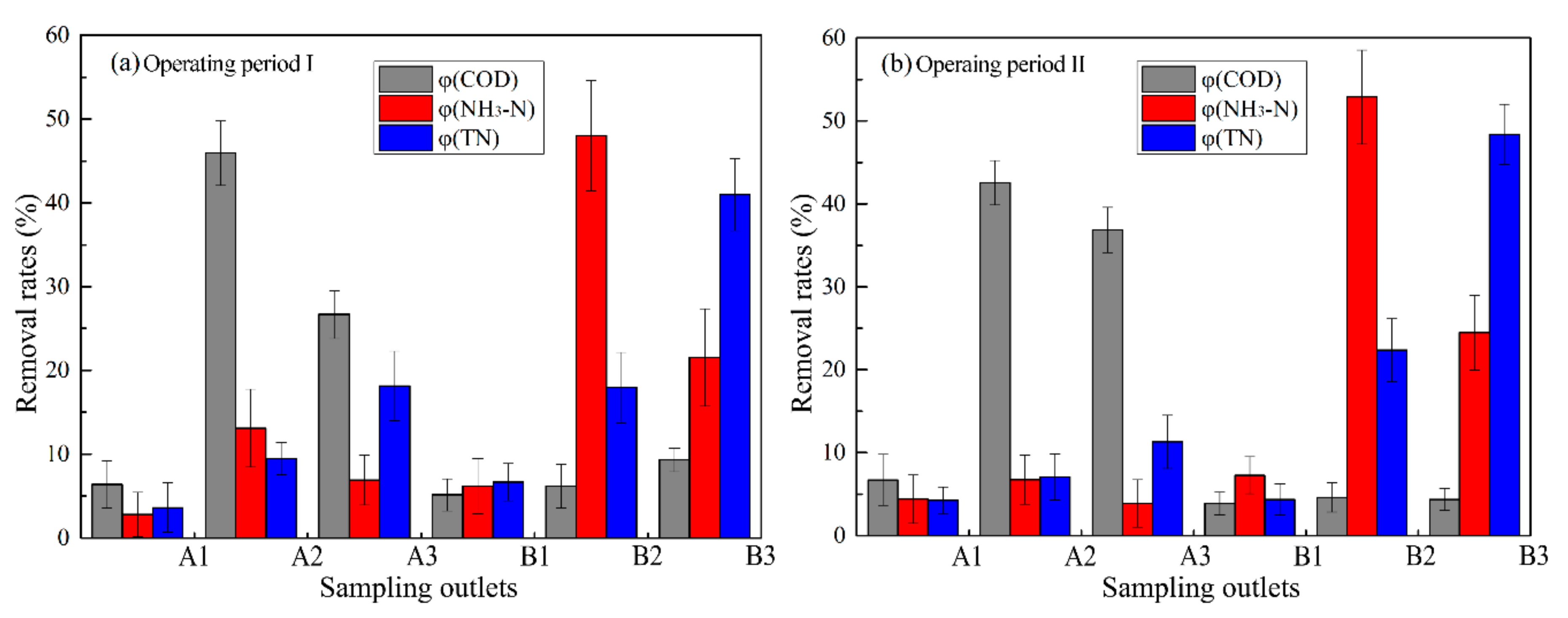
3.1. Characteristics of Wastewater Parameters and Pollutant Removal Rates along the Filter
3.2. Abundance and Diversity of Microbial Community along the Filter
3.3. Correlation between Microbial Communities and Environmental Factors
4. Conclusions
- (1)
- In the A-stage filter, the crucial organic-degrading bacteria were of the genus Novosphingobium, which had a significant increase in terms of relative abundance at sampling outlet A3 after the enhancement of organic load. The DO and COD concentrations were the main driving forces leading to differences in the microbial community structure of the A-stage filter.
- (2)
- In the B-stage filter, the microbial communities at different positions were similarly affected by environmental factors. The main bacteria associated with nitrogen removal in the B-stage filter were Zoogloea and Rhodocyclus.
- (3)
- To improve the pollutant removal performance of this two-stage biological aerated filter, a strategy of adding an internal circulation in the B-stage filter can be adopted.
Author Contributions
Funding
Conflicts of Interest
References
- Zhou, Y.; Zhou, Y.; Sun, X.; Fu, L. The recent development of advanced wastewater treatment by ozone and biological aerated filter. Environ. Sci. Pollut. Res. 2018, 25, 8315–8329. [Google Scholar] [CrossRef]
- Abou-Elela, S.I.; Abo-El-Enein, S.A.; Hellal, M.S. Utilization of autoclaved aerated concrete solid waste as a bio-carrier in immobilized bioreactor for municipal wastewater treatment. Desalin. Water Treat. 2019, 168, 108–116. [Google Scholar] [CrossRef]
- Vieira, A.; Marques, R.; Galinha, C.; Povoa, P.; Carvalho, G.; Oehmen, A. Nitrous oxide emissions from a full-scale biological aerated filter (BAF) subject to seawater infiltration. Environ. Sci. Pollut. Res. 2019, 26, 20939–20948. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghouti, M.A.; Al-Kaabi, M.A.; Ashfaq, M.Y.; Da’Na, D.A. Produced water characteristics, treatment and reuse: A review. J. Water Process. Eng. 2019, 28, 222–239. [Google Scholar] [CrossRef]
- Fdz-Polanco, F.; Mendez, E.; Urueña, M.; Villaverde, S.; García, P. Spatial distribution of heterotrophs and nitrifiers in a submerged biofilter for nitrification. Water Res. 2000, 34, 4081–4089. [Google Scholar] [CrossRef]
- Madoni, P.; Davoli, D.; Fontani, N.; Cucchi, A.; Rossi, F. Spatial Distribution of Microorganisms and Measurements of Oxygen Uptake Rate and Ammonia Uptake Rate Activity in a Drinking Water Biofilter. Environ. Technol. 2001, 22, 455–462. [Google Scholar] [CrossRef]
- Benáková, A.; Johanidesová, I.; Kelbich, P.; Pospíšil, V.; Wanner, J. The increase of process stability in removing ammonia nitrogen from wastewater. Water Sci. Technol. 2018, 77, 2213–2219. [Google Scholar] [CrossRef]
- Sun, J.; Li, Y.; Zhang, Y. Study on Treatment Process Improvement of Herbal Drug Wastewater. Asian J. Chem. 2013, 25, 951–953. [Google Scholar] [CrossRef]
- Pan, L.T.; Han, Y. A novel anoxic–aerobic biofilter process using new composite packing material for the treatment of rural domestic wastewater. Water Sci. Technol. 2016, 73, 2486–2492. [Google Scholar] [CrossRef]
- Yang, B.; Wang, J.; Wang, J.-F.; Xu, H.; Song, X.; Wang, Y.; Li, F.; Liu, Y.; Bai, J. Correlating microbial community structure with operational conditions in biological aerated filter reactor for efficient nitrogen removal of municipal wastewater. Bioresour. Technol. 2018, 250, 374–381. [Google Scholar] [CrossRef]
- Wang, C.; Wang, B.; Wang, L. The simultaneous nitrification and denitrification characteristics of two-stage biological aerated filter. China Environ. Sci. 2005, 25, 70–74, (In Chinese Journal). [Google Scholar]
- White, C.P.; DeBry, R.W.; Lytle, D.A. Microbial Survey of a Full-Scale, Biologically Active Filter for Treatment of Drinking Water. Appl. Environ. Microbiol. 2012, 78, 6390–6394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, X.; Wang, X.; Chen, Z.; Chen, J. Nitrogen removal from iron oxide red wastewater via partial nitritation-Anammox based on two-stage zeolite biological aerated filter. Bioresour. Technol. 2019, 279, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Dean, S.L.; Farrer, E.C.; Porras-Alfaro, A.; Suding, K.N.; Sinsabaugh, R.L. Assembly of root-associated bacteria communities: Interactions between abiotic and biotic factors. Environ. Microbiol. Rep. 2014, 7, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Tisthammer, K.H.; Cobian, G.M.; Amend, A.S. Global biogeography of marine fungi is shaped by the environment. Fungal Ecol. 2016, 19, 39–46. [Google Scholar] [CrossRef]
- Ju, F.; Guo, F.; Ye, L.; Xia, Y.; Zhang, T. Metagenomic analysis on seasonal microbial variations of activated sludge from a full-scale wastewater treatment plant over 4 years. Environ. Microbiol. Rep. 2014, 6, 80–89. [Google Scholar] [CrossRef]
- Yan, G.; Xu, X.; Yao, L.; Lu, L.; Zhao, T.; Zhang, W. Process of inorganic nitrogen transformation and design of kinetics model in the biological aerated filter reactor. Bioresour. Technol. 2011, 102, 4628–4632. [Google Scholar] [CrossRef]
- Ma, W.; Han, Y.; Ma, W.; Han, H.; Zhu, H.; Xu, C.; Li, K.; Wang, D. Enhanced nitrogen removal from coal gasification wastewater by simultaneous nitrification and denitrification (SND) in an oxygen-limited aeration sequencing batch biofilm reactor. Bioresour. Technol. 2017, 244, 84–91. [Google Scholar] [CrossRef]
- Li, L.; Sun, Y.; Yuan, Z.; Kong, X.; Wao, Y.; Yang, L.; Zhang, Y.; Li, D. Effect of microalgae supplementation on the silage quality and anaerobic digestion performance of Manyflower silvergrass. Bioresour. Technol. 2015, 189, 334–340. [Google Scholar] [CrossRef]
- Hocaoglu, S.M.; Insel, G.; Cokgor, E.U.; Orhon, D. Effect of low dissolved oxygen on simultaneous nitrification and denitrification in a membrane bioreactor treating black water. Bioresour. Technol. 2011, 102, 4333–4340. [Google Scholar] [CrossRef]
- Lima, P.S.; Dezotti, M.; Bassin, J.P. Interpreting the effect of increasing COD loading rates on the performance of a pre-anoxic MBBR system: Implications on the attached and suspended biomass dynamics and nitrification–denitrification activity. Bioprocess. Biosyst. Eng. 2016, 39, 945–957. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Cheng, W.; Wan, T.; Wang, M.; Zhang, C. Effect of Aeration Rates and Filter Media Heights on the Performance of Pollutant Removal in an Up-Flow Biological Aerated Filter. Water 2018, 10, 1244. [Google Scholar] [CrossRef] [Green Version]
- Contreras, C.R.; López, D.; Leiva, A.M.; Domínguez, C.; Bayona, J.M.; Vidal, G. Removal of Organic Micropollutants in Wastewater Treated by Activated Sludge and Constructed Wetlands: A Comparative Study. Water 2019, 11, 2515. [Google Scholar] [CrossRef] [Green Version]
- Celmer, D.; Oleszkiewicz, J.; Cicek, N. Impact of shear force on the biofilm structure and performance of a membrane biofilm reactor for tertiary hydrogen-driven denitrification of municipal wastewater. Water Res. 2008, 42, 3057–3065. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Xu, W.; Sontag, P.; Li, X.; Xue, G.; Liu, T.; Sun, W. Correlating microbial community compositions with environmental factors in activated sludge from four full-scale municipal wastewater treatment plants in Shanghai, China. Appl. Microbiol. Biotechnol. 2016, 100, 4663–4673. [Google Scholar] [CrossRef] [PubMed]
- Freixa, A.; Ejarque, E.; Crognale, S.; Amalfitano, S.; Fazi, S.; Butturini, A.; Romaní, A.M. Sediment microbial communities rely on different dissolved organic matter sources along a Mediterranean river continuum. Limnol. Oceanogr. 2016, 61, 1389–1405. [Google Scholar] [CrossRef] [Green Version]
- Callejas, C.; Fernández, A.; Passeggi, M.; Wenzel, J.; Bovio, P.; Borzacconi, L.; Etchebehere, C. Microbiota adaptation after an alkaline pH perturbation in a full-scale UASB anaerobic reactor treating dairy wastewater. Bioprocess. Biosyst. Eng. 2019, 42, 2035–2046. [Google Scholar] [CrossRef]
- Pelaez, A.I.; Fernández-Nava, Y.; Soons, J.; Martinez-Toledo, M.V.; Maranon, E.; Sanchez, J. Population analysis in high-nitrate wastewater treatment in sequencing batch reactors. Environ. Eng. Manag. J. 2016, 15, 93–103. [Google Scholar] [CrossRef]
- Sul, W.-J.; Kim, I.-S.; Ekpeghere, K.I.; Song, B.; Kim, B.-S.; Kim, H.-G.; Kim, J.-T.; Koh, S.-C. Metagenomic insight of nitrogen metabolism in a tannery wastewater treatment plant bioaugmented with the microbial consortium BM-S-1. J. Environ. Sci. Heal. Part. A 2016, 51, 1164–1172. [Google Scholar] [CrossRef]
- Das, S.; Jeong, S.T.; Das, S.; Kim, P.J. Composted Cattle Manure Increases Microbial Activity and Soil Fertility More Than Composted Swine Manure in a Submerged Rice Paddy. Front. Microbiol. 2017, 8, 1702. [Google Scholar] [CrossRef]
- Chettri, B.; Singh, A.K. Kinetics of hydrocarbon degradation by a newly isolated heavy metal tolerant bacterium Novosphingobium panipatense P5:ABC. Bioresour. Technol. 2019, 294, 122190. [Google Scholar] [CrossRef] [PubMed]
- Ibero, J.; Galán, B.; Diaz, E.; García, J.L. Testosterone Degradative Pathway of Novosphingobium tardaugens. Genes 2019, 10, 871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulhane, M.; Pandit, P.; Khardenavis, A.; Singh, D.; Purohit, H. Study of microbial community plasticity for anaerobic digestion of vegetable waste in Anaerobic Baffled Reactor. Renew. Energy 2017, 101, 59–66. [Google Scholar] [CrossRef]
- Szabó, E.; Liébana, R.; Hermansson, M.; Modin, O.; Persson, F.; Wilén, B.-M. Microbial Population Dynamics and Ecosystem Functions of Anoxic/Aerobic Granular Sludge in Sequencing Batch Reactors Operated at Different Organic Loading Rates. Front. Microbiol. 2017, 8, 770. [Google Scholar] [CrossRef]
- Kim, E.; Shin, S.G.; Jannat, A.H.; Tongco, J.V.; Hwang, S. Use of food waste-recycling wastewater as an alternative carbon source for denitrification process: A full-scale study. Bioresour. Technol. 2017, 245, 1016–1021. [Google Scholar] [CrossRef]
- Poddar, K.; Sarkar, D.; Sarkar, A. Construction of potential bacterial consortia for efficient hydrocarbon degradation. Int. Biodeterior. Biodegrad. 2019, 144. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, C.; Jiang, L.; Qi, J.; Wang, J.; He, X. Impact of dissolved oxygen on the microbial community structure of an intermittent biological aerated filter (IBAF) and the removal efficiency of gasification wastewater. Bioresour. Technol. 2018, 255, 198–204. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Han, J.; Qian, Y. Removal regularity of textile wastewater pollutants in biological aerated filter. Chin. J. Environ. Eng. 2012, 6, 1167–1170, (In Chinese Journal). [Google Scholar]
- Zhang, X.; Li, Q.; Wang, J.; Wang, X.; Lin, Y. Research progress process improvement of biological aerated filter: A review. Chem. Ind. Eng. Prog. 2015, 34, 2023–2030, (In Chinese Journal). [Google Scholar]
- Wang, C.; Peng, Z.; Feng, K.; Chen, Z.; Liu, H. A study on the treatment efficiency of internal circulation biological aerated filters for refinery wastewater and the transformation of main organic pollutants. Environ. Sci. Pollut. Res. 2020, 27, 22902–22912. [Google Scholar] [CrossRef]



| Operating Period | Average Organic Load (kg/m3·d) | COD (mg/L) | NH3-N (mg/L) | TN (mg/L) | NO2−-N (mg/L) | NO3−-N (mg/L) | NOx−-N (mg/L) |
|---|---|---|---|---|---|---|---|
| I | 1.02 | 260 (23) | 27.59 (3.12) | 32.13 (3.76) | 0.17 (0.08) | 3.98 (1.23) | 4.15 (1.30) |
| II | 1.55 | 392 (25) | 28.77 (2.94) | 33.42 (3.17) | 0.13 (0.06) | 4.06 (0.99) | 4.20 (1.02) |
| Operating Period | Sampling Outlet | DO (mg/L) | pH (mg/L) | COD (mg/L) | NH3−N (mg/L) | TN (mg/L) | NOx−-N (mg/L) |
|---|---|---|---|---|---|---|---|
| I | A1 | 4.33 (0.55) | 7.86 (0.26) | 246 (22) | 26.90 (2.72) | 31.32 (3.76) | 4.05 (1.47) |
| A2 | 1.18 (0.21) | 7.84 (0.26) | 141 (16) | 23.90 (2.14) | 29.14 (3.47) | 5.00 (1.81) | |
| A3 | 0.87 (0.10) | 7.91 (0.17) | 81 (13) | 22.32 (2.03) | 24.86 (2.38) | 2.53 (1.51) | |
| B1 | 1.13 (0.18) | 7.64 (0.24) | 69 (12) | 20.89 (1.79) | 23.29 (2.02) | 2.27 (1.67) | |
| B2 | 0.59 (0.10) | 7.12 (0.26) | 55 (7) | 10.12 (1.60) | 19.20 (1.59) | 8.59 (1.98) | |
| B3 | 0.40 (0.08) | 6.96 (0.24) | 34 (6) | 5.31 (0.76) | 9.79 (0.92) | 4.20 (0.99) | |
| II | A1 | 4.34 (0.51) | 7.83 (0.19) | 369 (28) *** | 27.74 (2.96) | 32.40 (3.04) | 4.33 (1.13) |
| A2 | 1.07 (0.23) | 7.87 (0.24) | 225 (19) *** | 26.12 (2.54) * | 30.72 (3.11) | 4.33 (1.60) | |
| A3 | 0.60 (0.10) *** | 7.95 (0.15) | 99 (11) *** | 25.15 (2.14) *** | 28.04 (3.11) ** | 1.78 (0.85) | |
| B1 | 1.04 (0.16) | 7.67 (0.16) | 86 (12) *** | 23.41 (1.87) *** | 26.97 (2.86) *** | 2.89 (1.28) | |
| B2 | 0.56 (0.11) | 7.06 (0.20) | 70 (11) *** | 10.98 (1.41) | 21.55 (1.83) ** | 9.80 (1.60) | |
| B3 | 0.37 (0.08) | 6.92 (0.18) | 55 (10) *** | 5.22 (0.60) | 9.98 (0.80) | 4.53 (0.75) |
| Sample | OTU | ACE | Chao | Simpson | Coverage |
|---|---|---|---|---|---|
| LA1 | 91 | 168 | 162 | 0.18 | 0.999 |
| LA2 | 221 | 259 | 295 | 0.41 | 0.998 |
| LA3 | 229 | 270 | 271 | 0.08 | 0.999 |
| LB1 | 236 | 266 | 260 | 0.09 | 0.999 |
| LB2 | 160 | 192 | 184 | 0.19 | 0.999 |
| LB3 | 191 | 204 | 209 | 0.12 | 0.999 |
| HA1 | 116 | 234 | 162 | 0.16 | 0.999 |
| HA2 | 229 | 266 | 263 | 0.40 | 0.998 |
| HA3 | 210 | 258 | 251 | 0.34 | 0.998 |
| HB1 | 218 | 252 | 259 | 0.07 | 0.999 |
| HB2 | 169 | 258 | 214 | 0.14 | 0.999 |
| HB3 | 191 | 219 | 216 | 0.13 | 0.999 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, Y.; Li, S.; Wang, X.; Liu, Y.; Wang, R. Correlating Microbial Community Characteristics with Environmental Factors along a Two-Stage Biological Aerated Filter. Water 2020, 12, 3317. https://doi.org/10.3390/w12123317
An Y, Li S, Wang X, Liu Y, Wang R. Correlating Microbial Community Characteristics with Environmental Factors along a Two-Stage Biological Aerated Filter. Water. 2020; 12(12):3317. https://doi.org/10.3390/w12123317
Chicago/Turabian StyleAn, Yuchen, Songmin Li, Xiaoling Wang, Yuyang Liu, and Ruonan Wang. 2020. "Correlating Microbial Community Characteristics with Environmental Factors along a Two-Stage Biological Aerated Filter" Water 12, no. 12: 3317. https://doi.org/10.3390/w12123317
APA StyleAn, Y., Li, S., Wang, X., Liu, Y., & Wang, R. (2020). Correlating Microbial Community Characteristics with Environmental Factors along a Two-Stage Biological Aerated Filter. Water, 12(12), 3317. https://doi.org/10.3390/w12123317




