Literature Review: Global Neonicotinoid Insecticide Occurrence in Aquatic Environments
Abstract
1. Introduction
1.1. Background of Neonicotinoids
1.2. Global Economic Impacts and Commercialization
2. Physical and Chemical Structure of Neonicotinoids
3. Application Methods
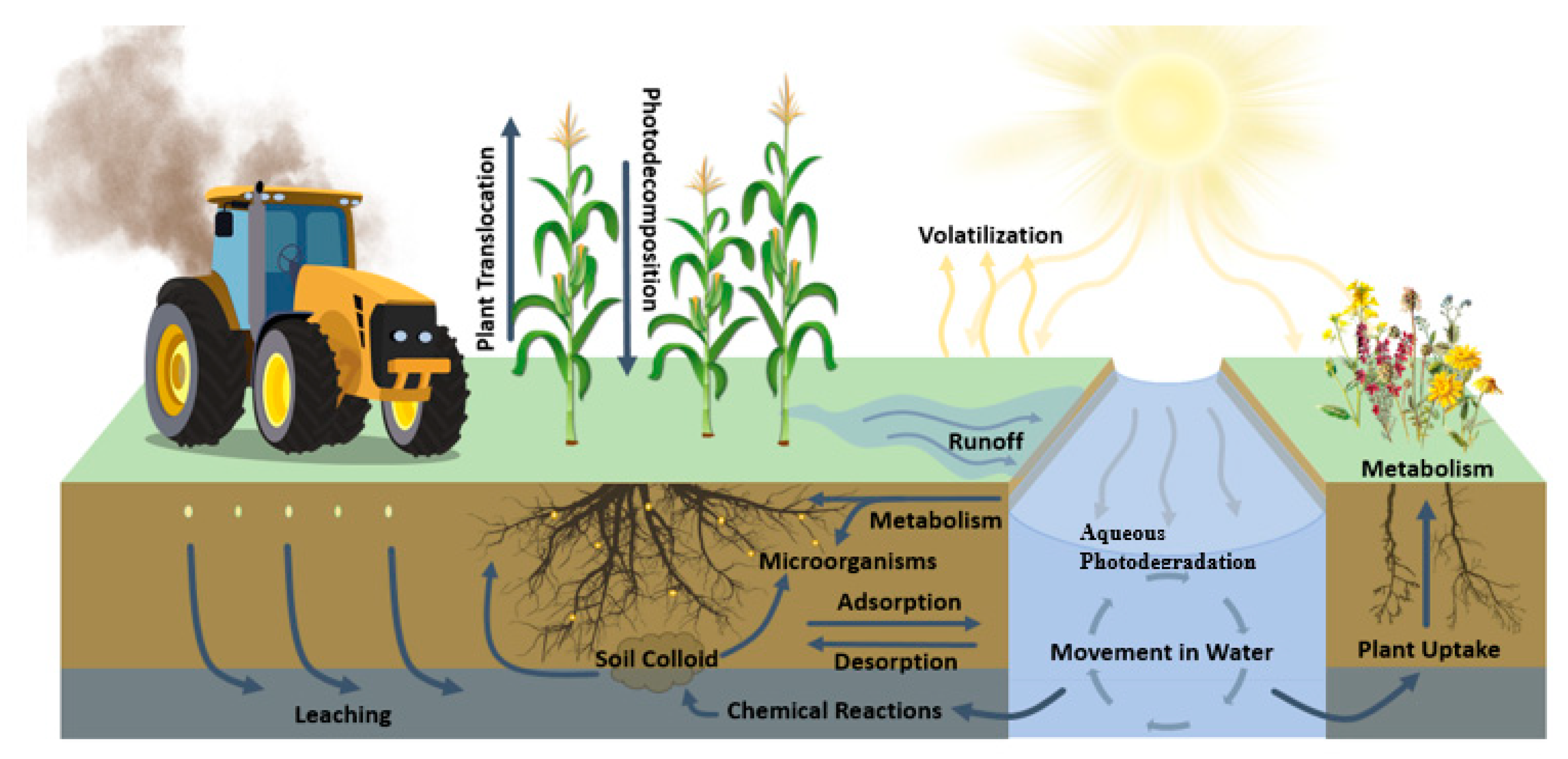
3.1. Fate and Transport in Soil Environments
3.2. Fate and Transport in Water Environments
3.3. Neonicotinoid Degradation Pathways
4. Occurrence and Persistence of Neonicotinoids in Global Surface Waters
5. Toxicity of Neonicotinoids towards Organisms
6. Exposure Risks to Humans
7. Future Work/Knowledge Gap
- 1)
- Quantify the potential roles of realistic field conditions on degradation along various neonicotinoid insecticide fate and transport scenarios.
- 2)
- Improve our understanding of the role of river geomorphology on photochemical transformation and degradation of neonicotinoids along path-specific environmental conditions.
- 3)
- Investigate the long-term exposure implications to non-target organisms (including humans).
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blacquière, T.; Smagghe, G.; Van Gestel, C.A.M.; Mommaerts, V. Neonicotinoids in bees: A review on concentrations, side-effects and risk assessment. Ecotoxicology 2012, 21, 973–992. [Google Scholar] [CrossRef]
- Hladik, M.L.; Kolpin, D.W.; Kuivila, K.M. Widespread occurrence of neonicotinoid insecticides in streams in a high corn and soybean producing region, USA. Environ. Pollut. 2014, 193, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Goulson, D. An overview of the environmental risks posed by neonicotinoid insecticides. J. Appl. Ecol. 2013, 50, 977–987. [Google Scholar] [CrossRef]
- Elbert, A.; Nauen, R.; Leicht, W. Imidacloprid, a novel chloronicotinyl insecticide: Biological activity and agricultural importance. In Insecticides with Novel Modes of Action; Springer: Berlin/Heidelberg, Germany, 1998; pp. 50–73. [Google Scholar]
- Mahai, G.; Wan, Y.; Xia, W.; Yang, S.; He, Z.; Xu, S. Neonicotinoid insecticides in surface water from the central Yangtze River, China. Chemosphere 2019, 229, 452–460. [Google Scholar] [CrossRef]
- Jeschke, P.; Nauen, R.; Schindler, M.; Elbert, A. Overview of the status and global strategy for neonicotinoids. J. Agric. Food Chem. 2011, 59, 2897–2908. [Google Scholar] [CrossRef]
- Jeschke, P.; Nauen, R. Thiamethoxam: A neonicotinoid precursor converted to clothianidin in insects and plants. Acs Symp. Ser. 2007, 948, 51–65. [Google Scholar]
- Anderson, J.C.; Dubetz, C.; Palace, V.P. Neonicotinoids in the Canadian aquatic environment: A literature review on current use products with a focus on fate, exposure, and biological effects. Sci. Total Environ. 2015, 505, 409–422. [Google Scholar] [CrossRef]
- Žabar, R.; Komel, T.; Fabjan, J.; Kralj, M.B.; Trebše, P. Photocatalytic degradation with immobilised TiO2 of three selected neonicotinoid insecticides: Imidacloprid, thiamethoxam and clothianidin. Chemosphere 2012, 89, 293–301. [Google Scholar] [CrossRef]
- Craddock, H.A.; Huang, D.; Turner, P.C.; Quirós-Alcalá, L.; Payne-Sturges, D.C. Trends in neonicotinoid pesticide residues in food and water in the United States, 1999–2015. Environ. Heal. Glob. Access Sci. Source 2019, 18, 1–16. [Google Scholar] [CrossRef]
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef]
- Pisa, L.W.; Amaral-Rogers, V.L.; Belzunces, P.; Bonmatin, J.M.; Downs, C.A.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; McField, M.; et al. Effects of neonicotinoids and fipronil on non-target invertebrates. Environ. Sci. Pollut. Res. 2014, 22, 68–102. [Google Scholar] [CrossRef]
- Radolinski, J.; Wu, J.; Xia, K.; Hession, W.C.; Stewart, R.D. Plants mediate precipitation-driven transport of a neonicotinoid pesticide. Chemosphere 2019, 222, 445–452. [Google Scholar] [CrossRef]
- Simon-Delso, N.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Chagnon, M.; Downs, C.; Furlan, L.; Gibbons, D.W.; Giorio, C.; Girolami, V.; et al. Systemic insecticides (Neonicotinoids and fipronil): Trends, uses, mode of action and metabolites. Environ. Sci. Pollut. Res. 2015, 22, 5–34. [Google Scholar] [CrossRef]
- Sultana, T.; Murray, C.; Kleywegt, S.; Metcalfe, C.D. Neonicotinoid pesticides in drinking water in agricultural regions of southern Ontario, Canada. Chemosphere 2018, 202, 506–513. [Google Scholar] [CrossRef]
- Ford, K.A.; Casida, J.E. Unique and common metabolites of thiamethoxam, clothianidin, and dinotefuran in mice. Chem. Res. Toxicol. 2006, 19, 1549–1556. [Google Scholar] [CrossRef]
- Tomizawa, M.; Casida, J.E. S elective T oxicity of N eonicotinoids a ttributable to S pecificity of I Nsect and M ammalian N icotinic, R. eceptors. Annu. Rev. Entomol. 2003, 48, 339–364. [Google Scholar] [CrossRef]
- Duke, S.O.; Powles, S.B. Glyphosate: A once-in-a-century herbicide. Pest. Manag. Sci. 2008, 63, 1100–1106. [Google Scholar] [CrossRef]
- Kanne, D.B.; Dick, R.A.; Tomizawa, M.; Casida, J.E. Neonicotinoid nitroguanidine insecticide metabolites: Synthesis and nicotinic receptor potency of guanidines, aminoguanidines, and their derivatives. Chem. Res. Toxicol. 2005, 18, 1479–1484. [Google Scholar] [CrossRef]
- Karlin, A. Ion channel structure: Emerging structure of the nicotinic acetylcholine receptors. Nat. Rev. Neurosci. 2002, 3, 102–114. [Google Scholar] [CrossRef]
- Atsuda, K.M.; Himomura, M.S.; Hara, M.I.; Kamatsu, M.A.; Attelle, D.B.S. Neonicotinoids show selective and diverse actions on their nicotinic receptor targets: Electrophysiology, molecular biology, and receptor modeling studies I. insect nicotinic acetylcholine receptors II. physicochemical and structural proper-ties of. Receptor 2005, 69, 1442–1452. [Google Scholar]
- Morrissey, C.A.; Mineau, P.; Devries, J.H.; Sanchez-Bayo, F.; Liess, M.; Cavallaro, M.C.; Liber, K. Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: A review. Environ. Int. 2015, 74, 291–303. [Google Scholar] [CrossRef]
- Miranda, G.R.B.; Raetano, C.G.; Silva, E.; Daam, M.A.; Cerejeira, M.J. Environmental fate article: Environmental fate of neonicotinoids and classification of their potential risks to hypogean, epygean, and surface water ecosystems in Brazil. Hum. Ecol. Risk Assess. 2011, 17, 981–995. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Hyne, R.V. Detection and analysis of neonicotinoids in river waters—Development of a passive sampler for three commonly used insecticides. Chemosphere 2014, 99, 143–151. [Google Scholar] [CrossRef]
- Robin, S.U.R.; Stork, A. Uptake, translocation and metabolism of imidacloprid in plants. Bull. Insectology 2003, 56, 35–40. [Google Scholar]
- Tišler, T.; Jemec, A.; Mozetič, B.; Trebše, P. Hazard identification of imidacloprid to aquatic environment. Chemosphere 2009, 76, 907–914. [Google Scholar] [CrossRef]
- Lamers, M.; Anyusheva, M.; La, N.; Nguyen, V.V.; Streck, T. Pesticide Pollution in Surface-and Groundwater by Paddy Rice Cultivation: A Case Study from Northern Vietnam. Clean Soil Air Water 2011, 39, 356–361. [Google Scholar] [CrossRef]
- Gupta, S.; Gajbhiye, V.T.; Agnihotri, N.P. Leaching behavior of imidacloprid formulations in soil. Bull. Environ. Contam. Toxicol. 2002, 68, 502–508. [Google Scholar] [CrossRef]
- Huseth, A.S.; Groves, R.L. Environmental fate of soil applied neonicotinoid insecticides in an irrigated potato agroecosystem. PLoS ONE 2014, 9, e97081. [Google Scholar] [CrossRef]
- Bonmatin, J.M.; Giorio, C.; Girolami, V.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; Long, E.; Marzaro, M.; Mitchell, E.A.D.; et al. Environmental fate and exposure; neonicotinoids and fipronil. Environ. Sci. Pollut. Res. 2015, 22, 35–67. [Google Scholar] [CrossRef]
- Wamhoff, H.; Schneider, V. Photodegradation of imidacloprid. J. Agric. Food Chem. 1999, 47, 1730–1734. [Google Scholar] [CrossRef]
- Comfort, S.D.; Shea, P.J.; Roeth, F.W. Understanding pesticides and water quality in Nebraska. EC (Nebraska Cooperative Extension Service). 1994. Available online: https://digitalcommons.unl.edu/extensionhist/1635/ (accessed on 27 November 2020).
- Carbo, L.; Martins, E.L.; Dores, E.F.G.C.; Spadotto, C.A.; Weber, O.L.S.; De-Lamonica-Freire, E.M. Acetamiprid, carbendazim, diuron and thiamethoxam sorption in two Brazilian tropical soils. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2007, 42, 499–507. [Google Scholar] [CrossRef]
- Banerjee, K.; Patil, S.H.; Dasgupta, S.; Oulkar, D.P.; Adsule, P.G. Sorption of thiamethoxam in three Indian soils. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2008, 43, 151–156. [Google Scholar] [CrossRef]
- Wettstein, F.E.; Kasteel, R.; Garcia Delgado, M.F.; Hanke, I.; Huntscha, S.; Balmer, M.E.; Bucheli, T.D. Leaching of the neonicotinoids thiamethoxam and imidacloprid from sugar beet seed dressings to subsurface tile drains. J. Agric. Food Chem. 2016, 64, 6407–6415. [Google Scholar] [CrossRef]
- Peña, A.; Rodríguez-Liébana, J.A.; Mingorance, M.D. Persistence of two neonicotinoid insecticides in wastewater, and in aqueous solutions of surfactants and dissolved organic matter. Chemosphere 2011, 84, 464–470. [Google Scholar] [CrossRef]
- Dabrowski, J.M.; Peall, S.K.C.; Van Niekerk, A.; Reinecke, A.J.; Day, J.A.; Schulz, R. Predicting runoff-induced pesticide input in agricultural sub-catchment surface waters: Linking catchment variables and contamination. Water Res. 2002, 36, 4975–4984. [Google Scholar] [CrossRef]
- Zhang, P.; Ren, C.; Sun, H.; Min, L. Sorption, desorption and degradation of neonicotinoids in four agricultural soils and their effects on soil microorganisms. Sci. Total Environ. 2018, 615, 59–69. [Google Scholar] [CrossRef]
- Balsari, P.; Marucco, P. Internal and external contamination of sprayers: Causes and strategies to minimise negative effects on the environment. Chem. Eng. Trans. 2017, 58, 793–798. [Google Scholar]
- Occurrence, P. Pesticides in the Nation’s Streams and Ground Water, 1992–2001—A Summary; USGS: Reston, VA, USA, 2006. [Google Scholar]
- Hallberg, G.R. Pesticides pollution of groundwater in the humid United States. Agric. Ecosyst. Environ. 1989, 26, 299–367. [Google Scholar] [CrossRef]
- Reichenberger, S.; Bach, M.; Skitschak, A.; Frede, H.G. Mitigation strategies to reduce pesticide inputs into ground- and surface water and their effectiveness: A review. Sci. Total Environ. 2007, 384, 1–35. [Google Scholar] [CrossRef]
- Kurwadkar, S.; Evans, A.; DeWinne, D.; White, P.; Mitchell, F. Modeling photodegradation kinetics of three systemic neonicotinoids—Dinotefuran, imidacloprid, and thiamethoxam—In aqueous and soil environment. Environ. Toxicol. Chem. 2016, 35, 1718–1726. [Google Scholar] [CrossRef]
- Guzsvány, V.; Csanádi, J.; Gaál, F. NMR study of the influence of pH on the persistence of some neonicotinoids in water. Acta Chim. Slov. 2006, 53, 52–57. [Google Scholar]
- Kumar, S. Estimating spatial distribution of soil organic carbon for the Midwestern United States using historical database. Chemosphere 2015, 127, 49–57. [Google Scholar] [CrossRef]
- Satkowski, L.E.; Goyne, K.W.; Anderson, S.H.; Lerch, R.N.; Webb, E.B.; Snow, D.D. Imidacloprid Sorption and Transport in Cropland, Grass Buffer, and Riparian Buffer Soils. Vadose Zone J. 2018, 17, 170139. [Google Scholar] [CrossRef]
- Moza, P.N.; Hustert, K.; Feicht, E.; Kettrup, A. Photolysis of imidacloprid in aqueous solution. Chemosphere 1998, 36, 497–502. [Google Scholar] [CrossRef]
- Sakata, S.; Mikami, N.; Matsuda, T.; Miyamoto, J. Degradation and Leaching Behavior of the Pyrethroid Insecticide Cypermethrin in Soils. J. Pestic. Sci. 1986, 11, 71–79. [Google Scholar] [CrossRef]
- Schwartz, B.J.; Sparrow, F.K.; Heard, N.E.; Thede, B.M. Simultaneous derivatization and trappning of volatile products from aqueous photolysis of thiamethoxam insecticide. J. Agric. Food Chem. 2000, 48, 4671–4675. [Google Scholar] [CrossRef]
- Zeng, T.; Arnold, W.A. Pesticide photolysis in prairie potholes: Probing photosensitized processes. Environ. Sci. Technol. 2013, 47, 6735–6745. [Google Scholar] [CrossRef]
- Zhang, C.; Tian, D.; Yi, X.; Zhang, T.; Ruan, J.; Wu, R.; Chen, C.; Huang, M.; Ying, G. Occurrence, distribution and seasonal variation of five neonicotinoid insecticides in surface water and sediment of the Pearl Rivers, South China. Chemosphere 2019, 217, 437–446. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Goka, K.; Hayasaka, D. Contamination of the aquatic environment with neonicotinoids and its implication for ecosystems. Front. Environ. Sci. 2016, 4, 71. [Google Scholar] [CrossRef]
- Starner, K.; Goh, K.S. Detections of the neonicotinoid insecticide imidacloprid in surface waters of three agricultural regions of California, USA, 2010–2011. Bull. Environ. Contam. Toxicol. 2012, 88, 316–321. [Google Scholar] [CrossRef]
- Struger, J.; Grabuski, J.; Cagampan, S.; Sverko, E.; McGoldrick, D.; Marvin, C.H. Factors influencing the occurrence and distribution of neonicotinoid insecticides in surface waters of southern Ontario, Canada. Chemosphere 2017, 169, 516–523. [Google Scholar] [CrossRef]
- Ali, M.H.; Sumon, K.A.; Sultana, M.; Rashid, H. Toxicity of cypermethrin on the embryo and larvae of Gangetic mystus, Mystus cavasius. Environ. Sci. Pollut. Res. 2018, 25, 3193–3199. [Google Scholar] [CrossRef]
- Van Dijk, T.C.; Van Staalduinen, M.A.; Van der Sluijs, J.P. Macro-Invertebrate Decline in Surface Water Polluted with Imidacloprid. PLoS ONE 2013, 8, e62374. [Google Scholar] [CrossRef]
- Sumon, K.A.; Ritika, A.K.; Peeters, E.T.; Rashid, H.; Bosma, R.H.; Rahman, M.S.; Fatema, K.M.; Van den Brink, P.J. Effects of imidacloprid on the ecology of sub-tropical freshwater microcosms. Environ. Pollut. 2018, 236, 432–441. [Google Scholar] [CrossRef]
- Rahman, S. Pesticide consumption and productivity and the potential of IPM in Bangladesh. Sci. Total Environ. 2013, 445, 48–56. [Google Scholar] [CrossRef]
- Hladik, M.L.; Kolpin, D.W. First national-scale reconnaissance of neonicotinoid insecticides in streams across the USA. Environ. Chem. 2016, 13, 12–20. [Google Scholar] [CrossRef]
- Metcalfe, C.D.; Helm, P.; Paterson, G.; Kaltenecker, G.; Murray, C.; Nowierski, M.; Sultana, T. Pesticides related to land use in watersheds of the Great Lakes basin. Sci. Total Environ. 2019, 648, 681–692. [Google Scholar] [CrossRef]
- Yamamoto, A.; Terao, T.; Hisatomi, H.; Kawasaki, H.; Arakawa, R. Evaluation of river pollution of neonicotinoids in Osaka City (Japan) by LC/MS with dopant-assisted photoionisation. J. Environ. Monit. 2012, 14, 2189–2194. [Google Scholar] [CrossRef]
- Hauer, M.; Hansen, A.L.; Manderyck, B.; Olsson, Å.; Raaijmakers, E.; Hanse, B.; Stockfischa, N.; Märländer, B. Neonicotinoids in sugar beet cultivation in Central and Northern Europe: Efficacy and environmental impact of neonicotinoid seed treatments and alternative measures. Crop. Prot. 2017, 93, 132–142. [Google Scholar] [CrossRef]
- Gibbons, D.; Morrissey, C.; Mineau, P. A review of the direct and indirect effects of neonicotinoids and fipronil on vertebrate wildlife. Environ. Sci. Pollut. Res. 2015, 22, 103–118. [Google Scholar] [CrossRef]
- Han, W.; Tian, Y.; Shen, X. Human exposure to neonicotinoid insecticides and the evaluation of their potential toxicity: An overview. Chemosphere 2018, 192, 59–65. [Google Scholar] [CrossRef]
- Raby, M.; Nowierski, M.; Perlov, D.; Zhao, X.; Hao, C.; Poirier, D.G.; Sibley, P.K. Acute toxicity of 6 neonicotinoid insecticides to freshwater invertebrates. Environ. Toxicol. Chem. 2018, 37, 1430–1445. [Google Scholar] [CrossRef]
- USEPA. Aquatic Life Benchmarks and Ecological Risk Assessments for Registered Pesticides|Pesticide Science and Assessing Pesticide Risks|US EPA. 2017. Available online: https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/aquatic-life-benchmarks-and-ecological-risk (accessed on 27 November 2020).
- CCME. Canadian Environmental Quality Guidelines. 2017. Available online: https://www.ccme.ca/en/resources/canadian_environmental_quality_guidelines/ (accessed on 27 November 2020).
- Spivak, M.; Mader, E.; Vaughan, M.; Euliss, N.H., Jr. The plight of the bees. Environ. Sci. Technol. 2011, 45, 34–38. [Google Scholar] [CrossRef]
- Cox-foster, D.L.; Conlan, S.; Holmes, E.C.; Palacios, G.; Evans, J.D.; Moran, N.A.; Phenix-Lan, Q.; Briese, T.; Hornig, M.; Martinson, V. Collapse disorder. Science 2007, 318, 283–288. [Google Scholar] [CrossRef]
- Naug, D. Nutritional stress due to habitat loss may explain recent honeybee colony collapses. Biol. Conserv. 2009, 142, 2369–2372. [Google Scholar] [CrossRef]
- Oldroyd, B.P. What’s killing American honey bees? PLoS Biol. 2007, 5, 1195–1199. [Google Scholar] [CrossRef]
- Henry, M.; Beguin, M.; Requier, F.; Rollin, O.; Odoux, J.F.; Aupinel, P.; Aptel, J.; Tchamitchian, S.; Decourtye, A. A common pesticide decreases foraging success and survival in honey bees. Science 2012, 336, 348–350. [Google Scholar] [CrossRef]
- Whitehorn, P.R.; O’Connor, S.; Wackers, F.L.; Goulson, D. Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science 2012, 336, 351–352. [Google Scholar] [CrossRef]
- Charpentier, G.; Louat, F.; Bonmatin, J.M.; Marchand, P.A.; Vanier, F.; Locker, D.; Decoville, M. Lethal and sublethal effects of imidacloprid, after chronic exposure, on the insect model drosophila melanogaster. Environ. Sci. Technol. 2014, 48, 4096–4102. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Z.; Chang, C.H.; Lou, J.L.; Zhao, M.R.; Lu, C. Potential human exposures to neonicotinoid insecticides: A review. Environ. Pollut. 2018, 236, 71–81. [Google Scholar] [CrossRef]
- Chen, M.; Tao, L.; McLean, J.; Lu, C. Quantitative analysis of neonicotinoid insecticide residues in foods: Implication for dietary exposures. J. Agric. Food Chem. 2014, 62, 6082–6090. [Google Scholar] [CrossRef]
- WHO. Guidelines for Predicting Dietary Intake of Pesticide Residues; WHO: Geneva, Switzerland, 1997; p. 41. [Google Scholar]
- Hou, R.Y.; Hu, J.F.; Qian, X.S.; Su, T.; Wang, X.H.; Zhao, X.X.; Wan, X.C. Comparison of the dissipation behaviour of three neonicotinoid insecticides in tea. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2013, 30, 1761–1769. [Google Scholar] [CrossRef]
- Storkstad, E. European Union Expands Ban of Three Neonicotinoid Pesticides|Science|AAAS. 2018. Available online: https://www.sciencemag.org/news/2018/04/european-union-expands-ban-three-neonicotinoid-pesticides (accessed on 27 November 2020).





| Compound | Structures | Molecular Mass (g/mol) | Water Solubility (mg/L) | Half-Life in Water (Days) | Half-Life in Soil (Days) | |||
|---|---|---|---|---|---|---|---|---|
| Acetamiprid |  | 222.7 | 2950 | 0.80 | 169.05 | NA | 31–450 [22] | High [23] |
| Clothianidin |  | 249.7 | 340 | 0.91 | 215 | 385–408 [22] | 9–1250 [22] | Moderate [23] |
| Dinotefuran |  | 202.2 | 39830 | −0.55 | NA | NA | 75–82 [22] | NA |
| Imidacloprid |  | 255.7 | 610 | 0.57 | 260 | 0–365 [22] | 17–6931 [22] | Moderate [23] |
| Nitenpyram |  | 270.7 | 590000 | −0.66 | NA | NA | 0–8 [22] | NA |
| Thiacloprid |  | 252.7 | 184 | 1.26 | 615 | NA | 3.4–1000 [22] | Low [23] |
| Thiamethoxam |  | 291.7 | 4100 | −0.13 | 70 | 385–408 [22] | 6–3001 [22] | High [23] |
| Study Location | Year | THM | IMI | ACE | CLO | THA | DNT | NTP | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Elkhorn River, Midwestern, USA | 2018–2019 (Range) | nd | 7–81 | nd | 9–49 | nd | nd | - | Ongoing study |
| Neuse River, Southeastern USA | 2019 Median (Range) | nd | 14–42 | nd | nd | nd | nd | nd | Ongoing study |
| Nationwide River, USA | 2012–2014 Range for 38 streams | nd–190 | nd–142 | nd–45.6 | nd–66.3 | nd | nd–13.8 | nd | [59] |
| Seven Stream Basins, Iowa, USA | 2013 Median (Range) | <(nd–185) | <2 (nd–42.7) | nd | 8.2 (nd–257) | nd | nd | - | [2] |
| Seven watersheds, Ontario, Canada | 2016 Range | nd–1607 | nd–1333 | nd–109 | nd–778 | nd–7 | nd–18 | nd | [60] |
| Stream, Southern Ontario, Canada | 2012–2014 Range | <1.4–12.9 | <1.3–364 | <0.2–8.5 | <1.8–31.4 | <0.5–7.97 | - | - | [54] |
| Pearl River, Guangzhou, China | 2017 Median (Range) | 30.6 (4.97–102) | 31.0 (nd−180) | 17.1 (3.13–67.6) | 16.6 (0.55–67.2) | 1.33 (nd–12.4) | [51] | ||
| Yangtze River, China | 2015 Median (Range) | 1.10 (nd–236) | 4.37 (0.02–44.4) | 2.50 (0.26–2.0) | 0.10 (nd–10.5) | 0.02 (nd–0.26) | nd | 0.34 (nd–3.50) | [5] |
| River, Osaka, Japan | 2009–2010 Median | 2.65 (nd–11) | 5.55 (nd–25) | 1.4 (nd–1.4) | 3.2 (nd–12) | - | 20.0 (3.7–220) | - | [61] |
| River, Sydney, Australia | 2013 Median (Range) | 0.1 (nd–0.2) | 0.2 (nd–4.56) | 0.08 (nd–0.32) | 0.06 (nd–0.42) | 0.1 (nd–0.2) | nd | - | [24] |
| Compounds | Fish | Invertebrates | ||
|---|---|---|---|---|
| Acute (µg/L) | Chronic (µg/L) | Acute (µg/L) | Chronic (µg/L) | |
| Acetamiprid | >50,000 | 19,200 | 10.5 | 2.1 |
| Clothianidin | >50,750 | 9700 | 11 | 0.05 |
| Dinotefuran | >49,550 | 6360 | >484,150 | >95,300 |
| Imidacloprid | 114,500 | 9000 | 0.385 | 0.1 |
| Nitenpyram | - | - | - | - |
| Thiacloprid | 12,600 | 918 | 81.9 | 0.97 |
| Thiamethoxam | >57,000 | 20,000 | 17.5 | 0.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borsuah, J.F.; Messer, T.L.; Snow, D.D.; Comfort, S.D.; Mittelstet, A.R. Literature Review: Global Neonicotinoid Insecticide Occurrence in Aquatic Environments. Water 2020, 12, 3388. https://doi.org/10.3390/w12123388
Borsuah JF, Messer TL, Snow DD, Comfort SD, Mittelstet AR. Literature Review: Global Neonicotinoid Insecticide Occurrence in Aquatic Environments. Water. 2020; 12(12):3388. https://doi.org/10.3390/w12123388
Chicago/Turabian StyleBorsuah, Josephus F., Tiffany L. Messer, Daniel D. Snow, Steve D. Comfort, and Aaron R. Mittelstet. 2020. "Literature Review: Global Neonicotinoid Insecticide Occurrence in Aquatic Environments" Water 12, no. 12: 3388. https://doi.org/10.3390/w12123388
APA StyleBorsuah, J. F., Messer, T. L., Snow, D. D., Comfort, S. D., & Mittelstet, A. R. (2020). Literature Review: Global Neonicotinoid Insecticide Occurrence in Aquatic Environments. Water, 12(12), 3388. https://doi.org/10.3390/w12123388






