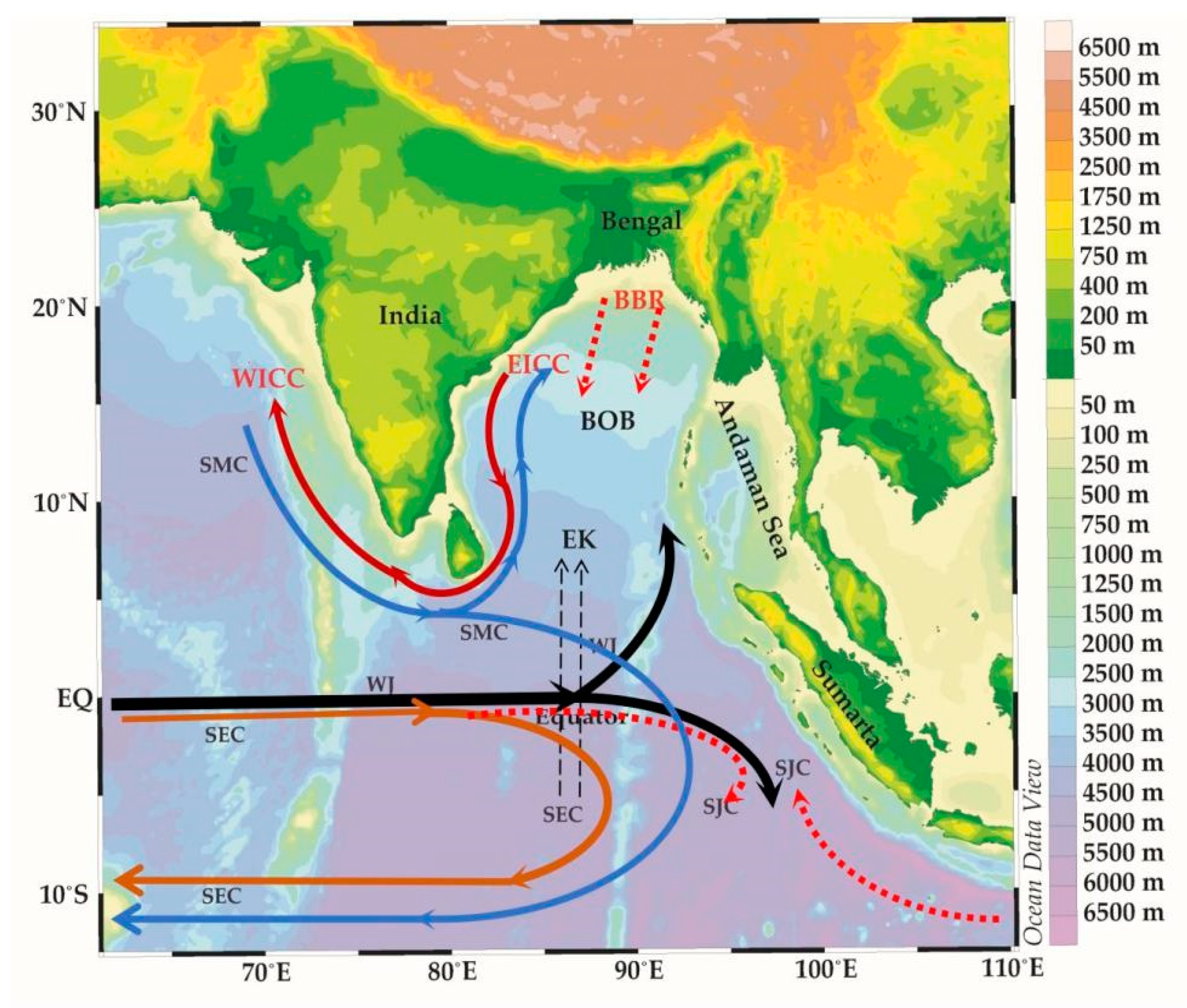
Figure 1.
The equatorial currents of the Eastern Indian Ocean. BBR (Bay of Bengal runoffs), EICC (east India coastal current), WICC (west India coastal currents), EK (Ekman transport), SEC (south equatorial counter current), SJS (south Java current), SMC (northeast and southwest monsoon currents) and WJ (Wyrtki jets).
Figure 1.
The equatorial currents of the Eastern Indian Ocean. BBR (Bay of Bengal runoffs), EICC (east India coastal current), WICC (west India coastal currents), EK (Ekman transport), SEC (south equatorial counter current), SJS (south Java current), SMC (northeast and southwest monsoon currents) and WJ (Wyrtki jets).
Figure 2.
Map of the Eastern Indian Ocean, showing the sampling stations in the study area across the three transects during cruise in spring 2014.
Figure 2.
Map of the Eastern Indian Ocean, showing the sampling stations in the study area across the three transects during cruise in spring 2014.
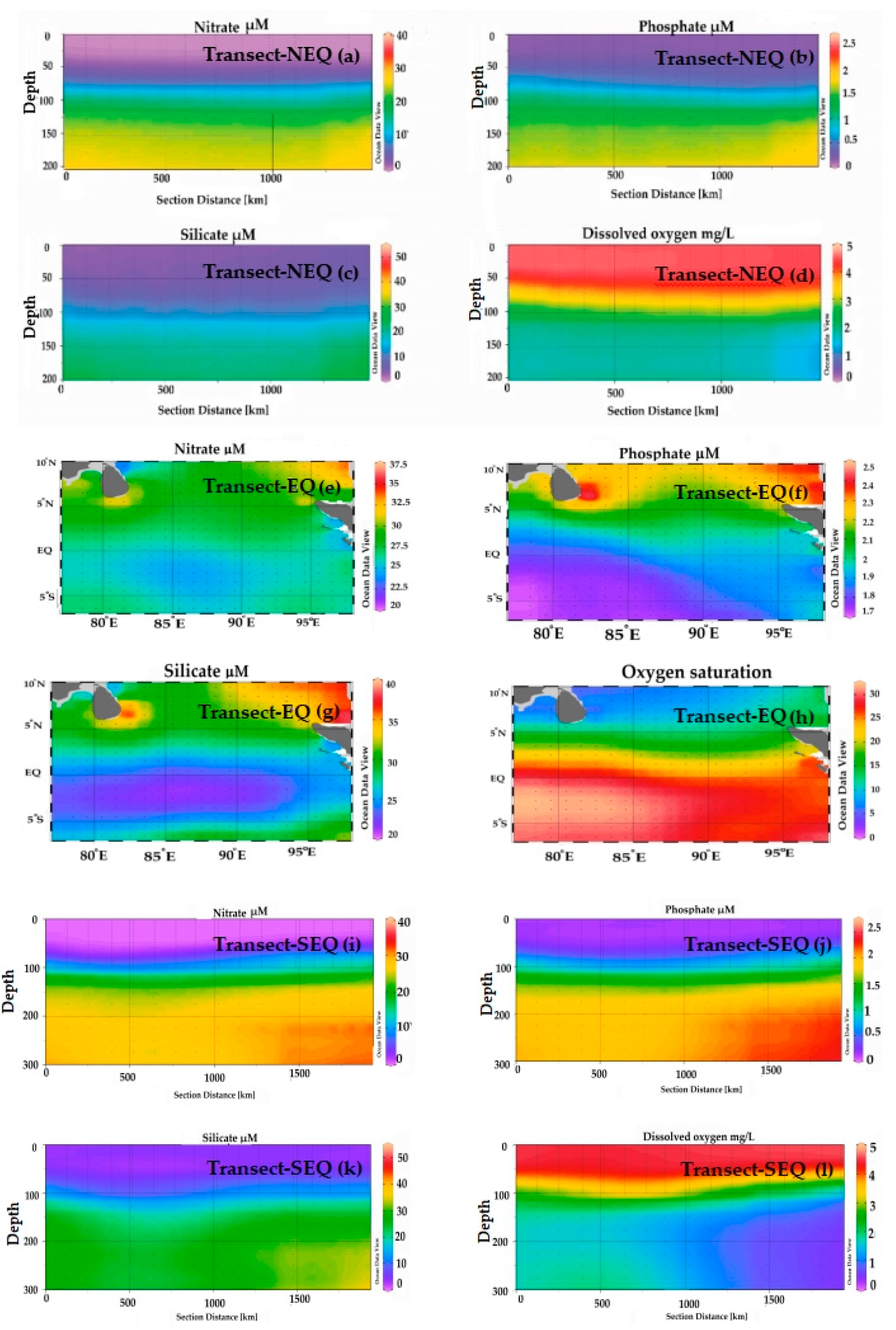
Figure 3.
The vertical profile of nitrate (a,e,i), phosphate (b,f,j), silicate (c,g,k) and dissolved oxygen (d,h,l) in three transects of the Eastern Indian Ocean. (a) Nitrate concentrations (µM) in the North Equatorial transect (NEQ-transect); (b) Phosphate concentrations (µM) in the NEQ-transect; (c) Silicate concentrations (µM) in the NEQ-transect; (d) Dissolved oxygen (mg/L) in the NEQ-transect; (e) Nitrate concentrations (µM) in the Equatorial transect (EQ-transect); (f) Phosphate concentrations (µM) in the EQ-transect; (g) Silicate concentrations (µM) in the EQ-transect; (h) Dissolved oxygen unit (mg/L) in the EQ-transect; (i) Nitrate concentrations (µM) in the South-Equatorial transect (SEQ-transect); (j) Phosphate concentrations (µM) in the SEQ-transect; (k) Silicate concentrations (µM) in the SEQ-transect; (l) Dissolved oxygen (mg/L) in the SEQ-transect.
Figure 3.
The vertical profile of nitrate (a,e,i), phosphate (b,f,j), silicate (c,g,k) and dissolved oxygen (d,h,l) in three transects of the Eastern Indian Ocean. (a) Nitrate concentrations (µM) in the North Equatorial transect (NEQ-transect); (b) Phosphate concentrations (µM) in the NEQ-transect; (c) Silicate concentrations (µM) in the NEQ-transect; (d) Dissolved oxygen (mg/L) in the NEQ-transect; (e) Nitrate concentrations (µM) in the Equatorial transect (EQ-transect); (f) Phosphate concentrations (µM) in the EQ-transect; (g) Silicate concentrations (µM) in the EQ-transect; (h) Dissolved oxygen unit (mg/L) in the EQ-transect; (i) Nitrate concentrations (µM) in the South-Equatorial transect (SEQ-transect); (j) Phosphate concentrations (µM) in the SEQ-transect; (k) Silicate concentrations (µM) in the SEQ-transect; (l) Dissolved oxygen (mg/L) in the SEQ-transect.
Figure 4.
The vertical distribution of temperature (
a,
d,
g), salinity (
b,
e,
h) and chlorophyll
a (
c,
f,
i) in three transects described from
Table 1. (
a) Temperature (°C) in the North Equatorial transect (NEQ-transect); (
b) Salinity (psu) in the NEQ-transect; (
c) Chlorophyll
a concentration (µg/L) in the NEQ-transect; (
d) Temperature (°C) in the Equatorial transect (EQ-transect); (
e) Salinity (psu) in the EQ-transect; (
f) Chlorophyll
a concentration (µg/L) in the EQ-transect; (
g) Temperature (°C) in the South-Equatorial transect (SEQ-transect); (
h) Salinity (psu) in the SEQ-transect; (
i) Chlorophyll
a concentration (µg/L) in the SEQ-transect.
Figure 4.
The vertical distribution of temperature (
a,
d,
g), salinity (
b,
e,
h) and chlorophyll
a (
c,
f,
i) in three transects described from
Table 1. (
a) Temperature (°C) in the North Equatorial transect (NEQ-transect); (
b) Salinity (psu) in the NEQ-transect; (
c) Chlorophyll
a concentration (µg/L) in the NEQ-transect; (
d) Temperature (°C) in the Equatorial transect (EQ-transect); (
e) Salinity (psu) in the EQ-transect; (
f) Chlorophyll
a concentration (µg/L) in the EQ-transect; (
g) Temperature (°C) in the South-Equatorial transect (SEQ-transect); (
h) Salinity (psu) in the SEQ-transect; (
i) Chlorophyll
a concentration (µg/L) in the SEQ-transect.
Figure 5.
The horizontal distribution of sea-surface temperature (a), salinity (b) and chlorophyll a (c) in the Eastern Indian Ocean. Panels (a,b) represents the high temperature (°C) and salinity (psu) values in BOB stations and SEQ stations at the transect 90° E and 80° E, and (c) represent the maximum Chlorophyll a (µg/L) concentration in the Equator station.
Figure 5.
The horizontal distribution of sea-surface temperature (a), salinity (b) and chlorophyll a (c) in the Eastern Indian Ocean. Panels (a,b) represents the high temperature (°C) and salinity (psu) values in BOB stations and SEQ stations at the transect 90° E and 80° E, and (c) represent the maximum Chlorophyll a (µg/L) concentration in the Equator station.
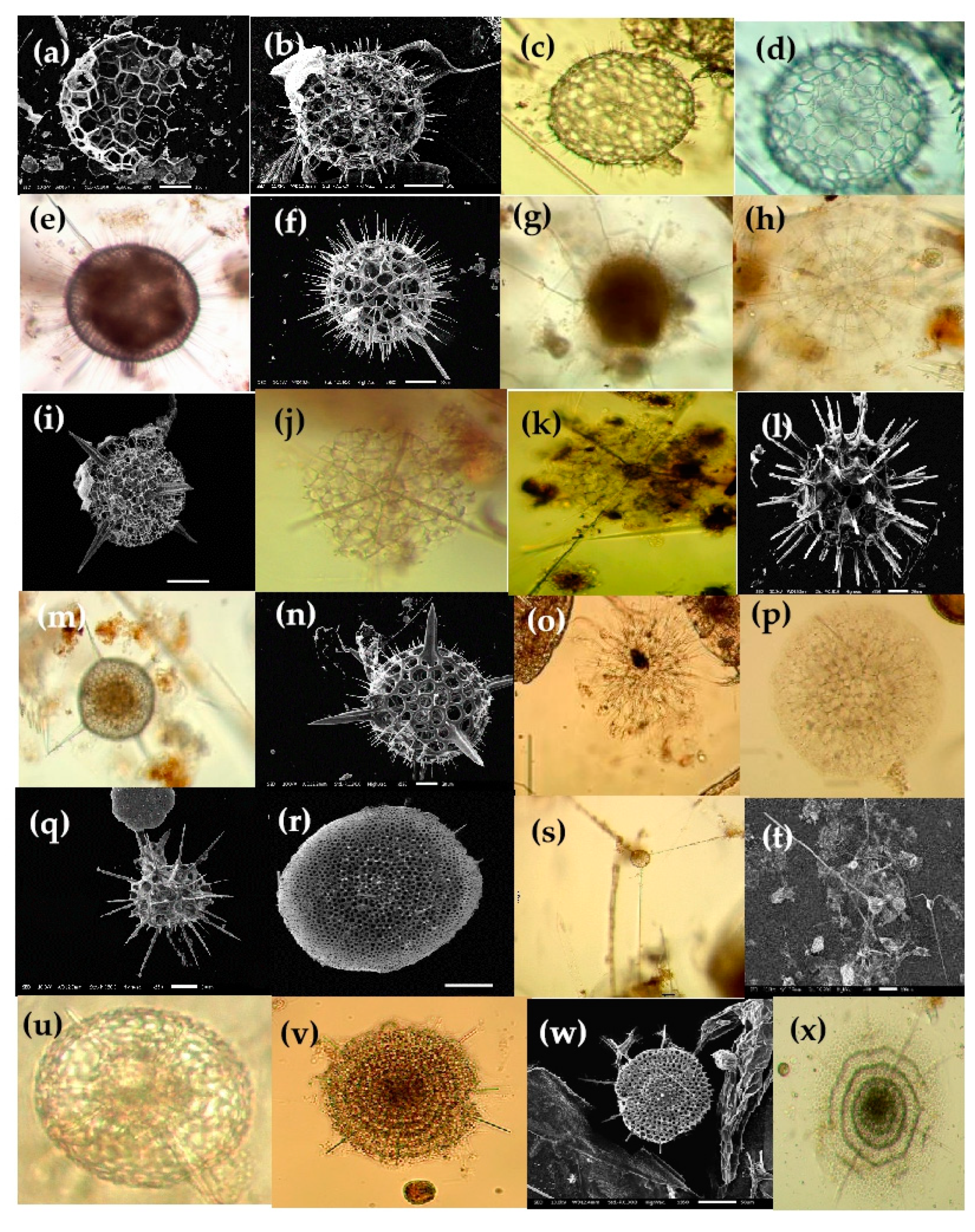
Figure 6.
Photomicrographs of Collozeoum (a) and Sphaerozoum species (b–g) and Thallosoxanthium species (h,i) in the Eastern Indian Ocean. (a) Collozoum inerme; (b,c) Sphaerozoum punctatum; (c) SEM of the spicules (Sk); (e,f) Sphaerozoum fuscum; (g) Sphaerozoum cf. ovodimare; (h) Thalassoxanthium cervicorne; (i) Thalassoxanthium octoceras. Scale bars: (c) 20 µm.
Figure 6.
Photomicrographs of Collozeoum (a) and Sphaerozoum species (b–g) and Thallosoxanthium species (h,i) in the Eastern Indian Ocean. (a) Collozoum inerme; (b,c) Sphaerozoum punctatum; (c) SEM of the spicules (Sk); (e,f) Sphaerozoum fuscum; (g) Sphaerozoum cf. ovodimare; (h) Thalassoxanthium cervicorne; (i) Thalassoxanthium octoceras. Scale bars: (c) 20 µm.
Figure 7.
Photographs of Collosphera and Siphonosphera species of Collodaria (a–o) in the Eastern Indian Ocean. (a,b) Siphonosphaera magnisphaera; (c,f) Siphonosphaera polysiphonia; (g,h) Siphonosphaera socialis; (j) and (k) Solenosphaera zanguebarica; (l) Collosphaera macropora; (m) Collosphaera tuberosa; (n,o) Collosphaera huxleyi. Scale bars: (b,h) 50 µm, (e) 10 µm, (f,i–o) 20 µm.
Figure 7.
Photographs of Collosphera and Siphonosphera species of Collodaria (a–o) in the Eastern Indian Ocean. (a,b) Siphonosphaera magnisphaera; (c,f) Siphonosphaera polysiphonia; (g,h) Siphonosphaera socialis; (j) and (k) Solenosphaera zanguebarica; (l) Collosphaera macropora; (m) Collosphaera tuberosa; (n,o) Collosphaera huxleyi. Scale bars: (b,h) 50 µm, (e) 10 µm, (f,i–o) 20 µm.
Figure 8.
Photomicrographs of Spumellarian species in the Eastern Indian Ocean, e.g., (a) Acanthosphaera actinota; (b) Acanthosphaera pinchuda; (c,d) Actinosphaera capillacea; (e,f) Actinosphaera tenella; (g) Astrosphaera hexagonalis; (h) Arachnosphaera myriacantha; (i) Centrocubus cladostylus; (j,k) Cromyechinus circumtextum; (l) Elatomma penicillus; (m,n) Hexalonche amphisiphon; (o) Styptosphaera spongiacea; (p) Spongodictyon spongiosum; (q) Streblacantha circumtexta; (r) Spongurus pylomaticus; (s,t) Xiphosphaera tessaractis; (u) Xiphatractus sp; (v) Stylodictya multispina; (w,x) Stylochlamydium venustum. Scale bars: (a,b,f,i,n,q) 20 µm, (r,w) 50 µm, (t) 100 µm.
Figure 8.
Photomicrographs of Spumellarian species in the Eastern Indian Ocean, e.g., (a) Acanthosphaera actinota; (b) Acanthosphaera pinchuda; (c,d) Actinosphaera capillacea; (e,f) Actinosphaera tenella; (g) Astrosphaera hexagonalis; (h) Arachnosphaera myriacantha; (i) Centrocubus cladostylus; (j,k) Cromyechinus circumtextum; (l) Elatomma penicillus; (m,n) Hexalonche amphisiphon; (o) Styptosphaera spongiacea; (p) Spongodictyon spongiosum; (q) Streblacantha circumtexta; (r) Spongurus pylomaticus; (s,t) Xiphosphaera tessaractis; (u) Xiphatractus sp; (v) Stylodictya multispina; (w,x) Stylochlamydium venustum. Scale bars: (a,b,f,i,n,q) 20 µm, (r,w) 50 µm, (t) 100 µm.
Figure 9.
Photomicrographs of Nassellarian species, e.g., (a,b) Callimitra solocicribrata; (c,d) Clathrocorys murrayi; (e,f) Lophophaena capito; (g,h) Lampromitra schultzei; (i) Phormacantha hystrix, (j) Pseudodictyophimus gracilipes, (k) Pteroscenium pinnatum, (l) Tetraphormis dodecaster; (m) Tetraplecta pinigera; (n) Archibursa tripodiscus; (o) Pterocanium korotnevi; (p) Stichopilium bicorne; (q) Conarachnium parabolicum; (r) Litharachnium tentorium; (s) Eucecryphalus clinatus; (t) Eucyrtidium dictyopodium; (u) Dictyocodon palladius; (v) Sethoconus venosum; (w) Theopilium tricostatum; (x) Cephalospyris cancellata. Scale bars: (a,m,p,x) 50 µm, (f,h–k,o,r–w) 20 µm and (n) 10 µm.
Figure 9.
Photomicrographs of Nassellarian species, e.g., (a,b) Callimitra solocicribrata; (c,d) Clathrocorys murrayi; (e,f) Lophophaena capito; (g,h) Lampromitra schultzei; (i) Phormacantha hystrix, (j) Pseudodictyophimus gracilipes, (k) Pteroscenium pinnatum, (l) Tetraphormis dodecaster; (m) Tetraplecta pinigera; (n) Archibursa tripodiscus; (o) Pterocanium korotnevi; (p) Stichopilium bicorne; (q) Conarachnium parabolicum; (r) Litharachnium tentorium; (s) Eucecryphalus clinatus; (t) Eucyrtidium dictyopodium; (u) Dictyocodon palladius; (v) Sethoconus venosum; (w) Theopilium tricostatum; (x) Cephalospyris cancellata. Scale bars: (a,m,p,x) 50 µm, (f,h–k,o,r–w) 20 µm and (n) 10 µm.
Figure 10.
Photomicrographs of Acanthrian species (a–f) and Taxopodida species (e,f) in the Eastern Indian Ocean. (a) Acanthochiasma fusiforme; (b) Trizona brandti; (c) Amphilonche concreta/elongate; (d) Diploconus cylindrus; (e,f) Sticholonche zanclea. Scale bar: (f) 50 µm.
Figure 10.
Photomicrographs of Acanthrian species (a–f) and Taxopodida species (e,f) in the Eastern Indian Ocean. (a) Acanthochiasma fusiforme; (b) Trizona brandti; (c) Amphilonche concreta/elongate; (d) Diploconus cylindrus; (e,f) Sticholonche zanclea. Scale bar: (f) 50 µm.
Figure 11.
Photomicrographs of Phaeodarian species (a–l) in the Eastern Indian Ocean. (a) Aulacantha scolymantha; (b) Auloceras arborescens subelegens; (c,d) Aulatractus ternaria; (e) Coelodendrum ramosissimum; (f,g) Castanidium longispiniim; (h) Conchidium compressa; (i) Conchidium capsula; (j) Conchidium caudatum; (k) Challengeron radians; (l) Pharyngella gastrula. Scale bars: (a,d,g) : 100 µm, (k) 10 µm.
Figure 11.
Photomicrographs of Phaeodarian species (a–l) in the Eastern Indian Ocean. (a) Aulacantha scolymantha; (b) Auloceras arborescens subelegens; (c,d) Aulatractus ternaria; (e) Coelodendrum ramosissimum; (f,g) Castanidium longispiniim; (h) Conchidium compressa; (i) Conchidium capsula; (j) Conchidium caudatum; (k) Challengeron radians; (l) Pharyngella gastrula. Scale bars: (a,d,g) : 100 µm, (k) 10 µm.
Figure 12.
Species richness (a) and Shannon-diversity H’ (b), Total abundance ind/m−3 of radiolarian (c), % of Collodaria (d), % of Taxopodida (e), % of Acantharia (f), % of Spumellaria (g), % of Nassellaria (h) and % of Phaeodaria (i) in the Eastern Indian Ocean. Panels (d–i) represent the percentage contribution of representative groups to total radiolarian among stations of the Eastern Indian Ocean.
Figure 12.
Species richness (a) and Shannon-diversity H’ (b), Total abundance ind/m−3 of radiolarian (c), % of Collodaria (d), % of Taxopodida (e), % of Acantharia (f), % of Spumellaria (g), % of Nassellaria (h) and % of Phaeodaria (i) in the Eastern Indian Ocean. Panels (d–i) represent the percentage contribution of representative groups to total radiolarian among stations of the Eastern Indian Ocean.
Figure 13.
Horizontal distribution and abundance (ind/m−3) of dominant species of radiolarian (a–i) in the Eastern Indian Ocean. (a) Sphaerozoum punctatum, (b) Sticholonche zanclea, (c) Siphonosphaera polysiphonia, (d) L. pentagona pentagona, (e) Callimitra sociliatorchia, (f) Hexacontium armatum-hostile, (g) Didymocyrtis tetrathalmus, (h) Astrosphaera hexagonalis, (i) Eucytidium hexastichum.
Figure 13.
Horizontal distribution and abundance (ind/m−3) of dominant species of radiolarian (a–i) in the Eastern Indian Ocean. (a) Sphaerozoum punctatum, (b) Sticholonche zanclea, (c) Siphonosphaera polysiphonia, (d) L. pentagona pentagona, (e) Callimitra sociliatorchia, (f) Hexacontium armatum-hostile, (g) Didymocyrtis tetrathalmus, (h) Astrosphaera hexagonalis, (i) Eucytidium hexastichum.
Figure 14.
Plots for (a) the full species dataset, (b) the Long-90 transect, (c) the Long-80 transect and (d) the Lat-0 transect. The left-hand column contains plots of the eigenvalues with the average eigenvalue shown as a red line (Guttman–Kaiser Criterion): The number of values exceeding the average is an indication of the number of explanatory variables. The right-hand column has the percentage variance (yellow) against the broken stick estimates (red): The number of values exceeding the broken stick estimates indicate the probable number of explanatory variables.
Figure 14.
Plots for (a) the full species dataset, (b) the Long-90 transect, (c) the Long-80 transect and (d) the Lat-0 transect. The left-hand column contains plots of the eigenvalues with the average eigenvalue shown as a red line (Guttman–Kaiser Criterion): The number of values exceeding the average is an indication of the number of explanatory variables. The right-hand column has the percentage variance (yellow) against the broken stick estimates (red): The number of values exceeding the broken stick estimates indicate the probable number of explanatory variables.
Figure 15.
Redundancy analysis (RDA) plot between the dominant radiolarian species and environmental variables.
Figure 15.
Redundancy analysis (RDA) plot between the dominant radiolarian species and environmental variables.
Table 1.
The station designators and coordinates of the three transects.
Table 1.
The station designators and coordinates of the three transects.
| NEQ-Long-90 | SEQ-Long-80 | EQ-Lat-0 |
|---|
| Station | Longitude | Latitude | Station | Longitude | Latitude | Station | Longitude | Latitude |
|---|
| HFA01 | 6.502 | 88.002 | I302 | 2.415 | 80.003 | I101 | 0.485 | 92.685 |
| HFA03 | 7.505 | 88.003 | I304 | 1.502 | 80 | I401 | 0.002 | 80.004 |
| HFA03 | 8.5 | 88 | I308 | −0.997 | 80.006 | I403 | 0 | 82.007 |
| I101 | 0.485 | 92.685 | I310 | −1.996 | 80.004 | I404 | 0 | 83.016 |
| I103 | 1.5 | 92.233 | I312 | −2.999 | 80 | I406 | 0 | 85.002 |
| I105 | 2.499 | 91.632 | I314 | −4 | 80.003 | I407 | 0 | 86.002 |
| I107 | 3.5 | 91.03 | I316 | −5 | 80 | I410 | 0 | 89.002 |
| I109 | 4.5 | 90.434 | I318 | −6 | 80.002 | I412 | 0 | 91.003 |
| I501 | −0.5 | 93.103 | I401 | 0.002 | 80.004 | I413 | 0 | 92.014 |
| I503 | −1.5 | 93.81 | I807 | −0.5 | 81.003 | I501 | −0.5 | 93.103 |
| I505 | −2.5 | 94.337 | I809 | −1.499 | 81.002 | I807 | −0.5 | 81.003 |
| I507 | −3.5 | 94.935 | I811 | −2.499 | 80.998 | | | |
| I509 | −4.5 | 95.532 | I813 | −3.5 | 80.999 | | | |
| I511 | −5.499 | 96.098 | I815 | −4.499 | 81 | | | |
| I601 | 6 | 89.5 | I817 | −5.5 | 81 | | | |
| I603 | 5.999 | 88.502 | | | | | | |
| IQ2 | 10.078 | 88.133 | | | | | | |
Table 2.
The relative abundance (%), frequency distribution and dominant index (Y) of the selected radiolarian species in EIO.
Table 2.
The relative abundance (%), frequency distribution and dominant index (Y) of the selected radiolarian species in EIO.
| Species | Frequency (fi)/% | Relative Abundance (P)/% | Dominance Index Y |
|---|
| Sphaerozoum punctatum | 52 | 22.2 | 5.1 |
| Sphaerozoum fuscum | 50 | 15.8 | 3.49 |
| Didymocyrtis tetrathalamus | 52 | 3.5 | 0.8 |
| Sticholonche zanclea | 39 | 7.7 | 1.32 |
| Siphonsphaera polysiphonia | 32 | 3.3 | 0.46 |
| Lophospyris pentagona pentagone | 36 | 2.3 | 0.37 |
| Eucytidium hexastichum | 36 | 1.9 | 0.31 |
| Anthocyrtidium cineraria | 20 | 0.8 | 0.07 |
| Pterocanium pretextum | 25 | 0.8 | 0.09 |
| Eucecryphalus gegenbauri | 20 | 1.2 | 0.11 |
| Lophophaena dodecantha | 20 | 0.7 | 0.07 |
| Arachnocorys circumtexta | 23 | 1.2 | 0.12 |
| Callimitra sociliatorchia | 27 | 2 | 0.24 |
| Amphirrhopalum ypsilon | 20 | 0.7 | 0.06 |
| Hexacontium armatum-hostile | 23 | 1.4 | 0.14 |
| Acanthsphaera actinote | 23 | 0.7 | 0.07 |
| Euchitonia elegans | 18 | 0.6 | 0.05 |
| Spongodiscus resurgens | 18 | 0.6 | 0.05 |
Table 3.
The inertia and first ten eigenvalues were generated by RDA from the full species data and the three transect (see text). Two sets of data are given for each source: That for the full, Hellinger-transformed, species set and that for the reduced to include the dominant species only.
Table 3.
The inertia and first ten eigenvalues were generated by RDA from the full species data and the three transect (see text). Two sets of data are given for each source: That for the full, Hellinger-transformed, species set and that for the reduced to include the dominant species only.
| Source | Dataset | Inertia | PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | PC7 | PC8 | PC9 | PC10 |
|---|
| All | Hellinger | 0.786 | 0.098 | 0.074 | 0.054 | 0.048 | 0.042 | 0.038 | 0.033 | 0.029 | 0.027 | 0.026 |
| All | Dominant | 0.786 | 0.124 | 0.099 | 0.081 | 0.069 | 0.050 | 0.048 | 0.045 | 0.033 | 0.030 | 0.026 |
| Long-90 | Hellinger | 0.755 | 0.127 | 0.099 | 0.073 | 0.064 | 0.056 | 0.052 | 0.044 | 0.038 | 0.036 | 0.030 |
| Long-90 | Dominant | 0.755 | 0.147 | 0.112 | 0.085 | 0.076 | 0.055 | 0.044 | 0.040 | 0.035 | 0.021 | 0.017 |
| Long-80 | Hellinger | 0.774 | 0.164 | 0.108 | 0.095 | 0.078 | 0.065 | 0.055 | 0.052 | 0.040 | 0.033 | 0.028 |
| Long-80 | Dominant | 0.774 | 0.185 | 0.124 | 0.102 | 0.077 | 0.064 | 0.047 | 0.023 | 0.020 | 0.016 | 0.008 |
| Lat-0 | Hellinger | 0.820 | 0.154 | 0.123 | 0.111 | 0.093 | 0.090 | 0.078 | 0.067 | 0.046 | 0.033 | 0.023 |
| Lat-0 | Dominant | 0.820 | 0.176 | 0.124 | 0.106 | 0.091 | 0.089 | 0.063 | 0.048 | 0.029 | 0.016 | 0.011 |
Table 4.
The potential explanatory environmental variables for the Lat-0 and Long-80 transects with. Details of the variance explained and the associated significance statistics.
Table 4.
The potential explanatory environmental variables for the Lat-0 and Long-80 transects with. Details of the variance explained and the associated significance statistics.
| Transect | Explanatory Variable | Depth (m bsl) | Total Variance | Variance Explained | % Variance Explained | F Statistic | Pr(>F) | Λ1/Λ2 |
|---|
| Lat-0 | Chlorophyll a | 0 | 0.8085 | 0.1399 | 17.3 | 1.884 | 0.0001 | 1.13 |
| Lat-0 | Chlorophyll a | 25 | 0.8085 | 0.1312 | 16.2 | 1.743 | 0.0015 | 1.05 |
| Long-80 | Nitrate | 75 | 0.7939 | 0.1020 | 12.9 | 1.917 | 0.0013 | 1.03 |
| Long-80 | Diss. Oxygen | 25 | 0.7939 | 0.1077 | 13.6 | 2.041 | 0.0006 | 1.06 |
| Long-80 | Diss. Oxygen | 50 | 0.7939 | 0.1077 | 13.6 | 2.042 | 0.0008 | 1.09 |
| Long-80 | Silicate | 0 | 0.7939 | 0.1065 | 13.4 | 2.014 | 0.0003 | 1.06 |
Table 5.
The Pearson coefficients for the correlations between the six ocean environmental variables used in this study.
Table 5.
The Pearson coefficients for the correlations between the six ocean environmental variables used in this study.
| Correlation Coefficients (43 Degrees of Freedom) | Nitrate µM | Phosphate µM | Salinity psu | Silicate µM | Temperature °C | Dissolved Oxygen mg/L |
|---|
| Chlorophyll a µg/L | 0.131 | −0.326 | −0.164 | −0.126 | 0.149 | −0.124 |
| Nitrate µM | | −0.540 | −0.339 | −0.556 | 0.280 | 0.129 |
| Phosphate µM | | | 0.430 | 0.662 | −0.465 | −0.159 |
| Salinity psu | | | | 0.672 | −0.551 | −0.406 |
| Silicate µM | | | | | −0.676 | −0.622 |
| Temperature °C | | | | | | 0.590 |
| Dissolved Oxygen mg/L | | | | | | |
Table 6.
The Pearson coefficients for the correlations between the dominant species and integrated six ocean environmental variables used in this study.
Table 6.
The Pearson coefficients for the correlations between the dominant species and integrated six ocean environmental variables used in this study.
| Dominant Species | Temperature °C | Salinity psu | Chlorophyll a µg/L | Phosphate µM | Silicate µM | Nitrate µM |
|---|
| Sticholonche zanclea | 0.118 | 0.130 | 0.208 | 0.031 | 0.121 | −0.020 |
| Siphonosphaera polysiphonia | 0.177 | −0.471** | −0.213 | 0.004* | 0.130 | 0.068 |
| Sphaerozoum punctatum | −0.312* | −0.043 | −0.187 | 0.085 | 0.092 | −0.048 |
| Sphaerozoum fuscum | 0.007 | 0.117 | 0.027 | −0.014 | −0.237 | −0.097 |
| Acanthosphaera actinota | −0.062 | 0.089 | −0.094 | 0.059 | −0.100 | 0.055 |
| Astrosphaera hexagonalis | −0.028 | 0.065 | 0.136 | −0.058 | −0.291 | −0.191 |
| Hexacontium armatum-hostile | 0.051 | 0.164 | −0.015 | 0.066 | −0.318* | −0.272 |
| Didymocyrtis tetrathalamus | 0.102 | 0.290 | 0.226 | −0.290 | −0.158 | −0.070 |
| Amphirhopalum ypsilon | −0.058 | 0.036 | −0.087 | 0.054 | 0.019 | −0.066 |
| Euchitonia elegans | 0.104 | 0.071 | 0.117 | 0.032 | −0.035 | −0.111 |
| Spongodiscus resurgens | 0.261 | 0.207 | −0.094 | −0.100 | −0.022 | −0.119 |
| Arachnocorys circumtexta | −0.132 | −0.200 | −0.067 | −0.028 | 0.117 | 0.195 |
| Callimitra sociliatorchia | 0.030 | −0.123 | −0.131 | −0.018 | 0.178 | 0.061 |
| Lophospyris pentagona pentagone | −0.026 | 0.061 | 0.100* | −0.045 | 0.045* | 0.062 |
| Lophophaena dodecantha | −0.066 | 0.099 | 0.360 | −0.063 | −0.309 | −0.163 |
| Anthocyrtidium cineraria | −0.308* | 0.154 | −0.098 | −0.196 | −0.050 | −0.049 |
| Pterocanium pretextum | −0.029 | 0.054 | −0.109 | −0.060 | 0.081 | −0.103 |
| Eucytidium hexastichum | −0.013 | 0.285 | 0.171 | −0.123 | −0.056 | −0.275 |
| Eucecryphalus gegenbauri | −0.080 | 0.043 | −0.158 | −0.077 | −0.043 | 0.207 |
Table 7.
Geographical records of newly identified taxa from other ocean basins.
Table 7.
Geographical records of newly identified taxa from other ocean basins.
| Class | Taxa | Worldwide Distribution Records | References |
|---|
| Acantharia | | | |
| | Acanthochiasma fusiforme | Mediterranean Sea, Atlantic and Pacific Ocean | [54] |
| | Acanthostaurus conacanthus | South Atlantic Ocean | [54] |
| | Trizona brandti | Mediterranean Sea, the Red Sea, the English Channel and the West Pacific Ocean | [38] |
| | Dictyacantha tabulate | Pacific Ocean | [38] |
| | Amphibelone anomala | East China Sea, Australia | [38,55] |
| | Amphilonche concreta | Mediterranean Sea, Atlantic and Pacific Ocean | [38] |
| | Diploconus cylindrus | North Atlantic Ocean, Pacific Ocean | [14,38] |
| | Diploconus faces | North Pacific Ocean, southward Pacific, Atlantic and Indian Ocean | [1] |
| Taxopodida | | | |
| | Sticholonche zanclea | East China Sea; Mediterranean Sea | [56,57] |
| Collodaria | Solenosphaera zanguebarica | Atlantic, Pacific Ocean, eastern Indian Ocean, China Sea | [25,58] |
| | Collosphaera huxleyi | Atlantic, Pacific Ocean | [25,58] |
| | Collosphaera macropora | China Sea | [25,58] |
| | Collosphaera tuberosa | China Sea | [25,58] |
| | Siphonosphaera polysiphonia | Pacific, Atlantic Ocean | [25,58] |
| | Siphonosphaera socialis | Gulf of California, Atlantic Ocean | [39] |
| | Siphonosphera magnisphera | Atlantic Ocean | [39] |
| | Sphaerozoum punctatum | Gulf of California; Southern Indian Ocean | [27] |
| | Sphaerozoum cf. ovodimare | Mediterranean Sea, Naples, Messina, Atlantic, Canary Islands, Cape Verde Islands, West Coast of Africa | [59] |
| | Sphaerozoum fuscum | East China Sea | [60] |
| | Collozoum inerme | Northern Norwegian Sea, the South Atlantic Ocean | [59] |
| | Thalassoxanthium cervicorne | Atlantic Ocean | [14] |
| | Thalassoxanthium octoceras | Madagascar, Rabbe Island, Indian Ocean | [38] |
| Phaeodaria | Aularia ternaria | North Pacific Ocean, south Atlantic and Pacific Ocean | [39] |
| | Aulacantha scolymantha | Pacific, Atlantic, Indian and Mediterranean Sea | [14,61] |
| | Auloceros arborescens subelegens | Atlantic and Arctic Ocean | [14] |
| | Coelodendrum ramosissimum | China Sea, Eastern Mediterranean Sea | [58] |
| | Castanella longispinium | South Atlantic Ocean, Gulf of Oman | [62] |
| | Conchopsis compressa | North Pacific (Japan and San Francisco), South Pacific Ocean | [14] |
| | Conchellium capsula | Atlantic Ocean and Pacific Ocean | [14] |
| | Conchidium caudatum | Atlantic Ocean | [14] |
| | Challengeron radians | Pacific, Atlantic Ocean and Japan Sea | [39] |
| | Pharyngella gastrula | Pacific and Atlantic Ocean | [39] |
| Spumellaria | Acanthosphaera dodecastyla | Atlantic | [39] |
| | Acanthosphaera pinchuda | Equatorial Pacific Ocean | [13] |
| | Actinosphaera capillacea | Pacific and Atlantic Ocean; East China Sea | [21,39] |
| | Astrosphaera hexagonalis | Pacific and Atlantic Ocean | [39] |
| | Centrocubus cladostylus | Gulf of California, Pacific Ocean | [1] |
| | Cladococcus scoparius | Central Pacific Ocean | [38] |
| | Cyrtidosphaera reticulata | Japan | [63] |
| | Spongurus pylomaticus | Gulf of California | [64] |
| | Streblacantha circumtexta | Arctic Sea, Nordic Sea; North Atlantic Ocean | [16,65] |
| Nassellaria | Archibursa tripodiscus | Central Pacific Ocean | [38] |
| | Callimitra solocicribrata | Central Pacific Ocean | [38] |
| | Cephalospyris cancellata | Central Pacific Ocean | [38] |
| | Cladoscenium limbatum | Nordic Seas | [39] |
| | Conarachnium parabolicum | Pacific and Atlantic Ocean | [39] |
| | Dictyocodon palladius | Central Pacific Ocean | [59] |
| | Eucecryphalus clinatus | Gulf of California | [39] |
| | Lampromitra schultzei | Pacific and Atlantic Ocean | [14,39] |
| | Litharachnium tentorium | Mediterranean Sea | [38] |
| | Pterocanium korotnevi | Central Equatorial Pacific Ocean | [1] |
| | Pteroscenium pinnatum | Pacific and Atlantic Ocean | [13,38] |
| | Pseudodictyophimus gracilipes | Arctic Ocean; Gulf of California; Nordic Sea; North Atlantic; North Pacific Ocean | [63,64] |
| | Phormacantha hystrix | Arctic Ocean, Gulf of California, Nordic Sea | [64,65] |
| | Stichopilium bicorne | Gulf of California, | [64] |
| | Tetraplecta pinigera | Central Pacific Ocean | [38] |
| | Theocorys veneris | Pacific and Atlantic Ocean | [39] |