Potential Sources of Particulate Iron in Surface and Deep Waters of the Terra Nova Bay (Ross Sea, Antarctica)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling
2.3. Particulate Metals Analysis
2.4. Additional Parameters
2.5. Data Processing
3. Results
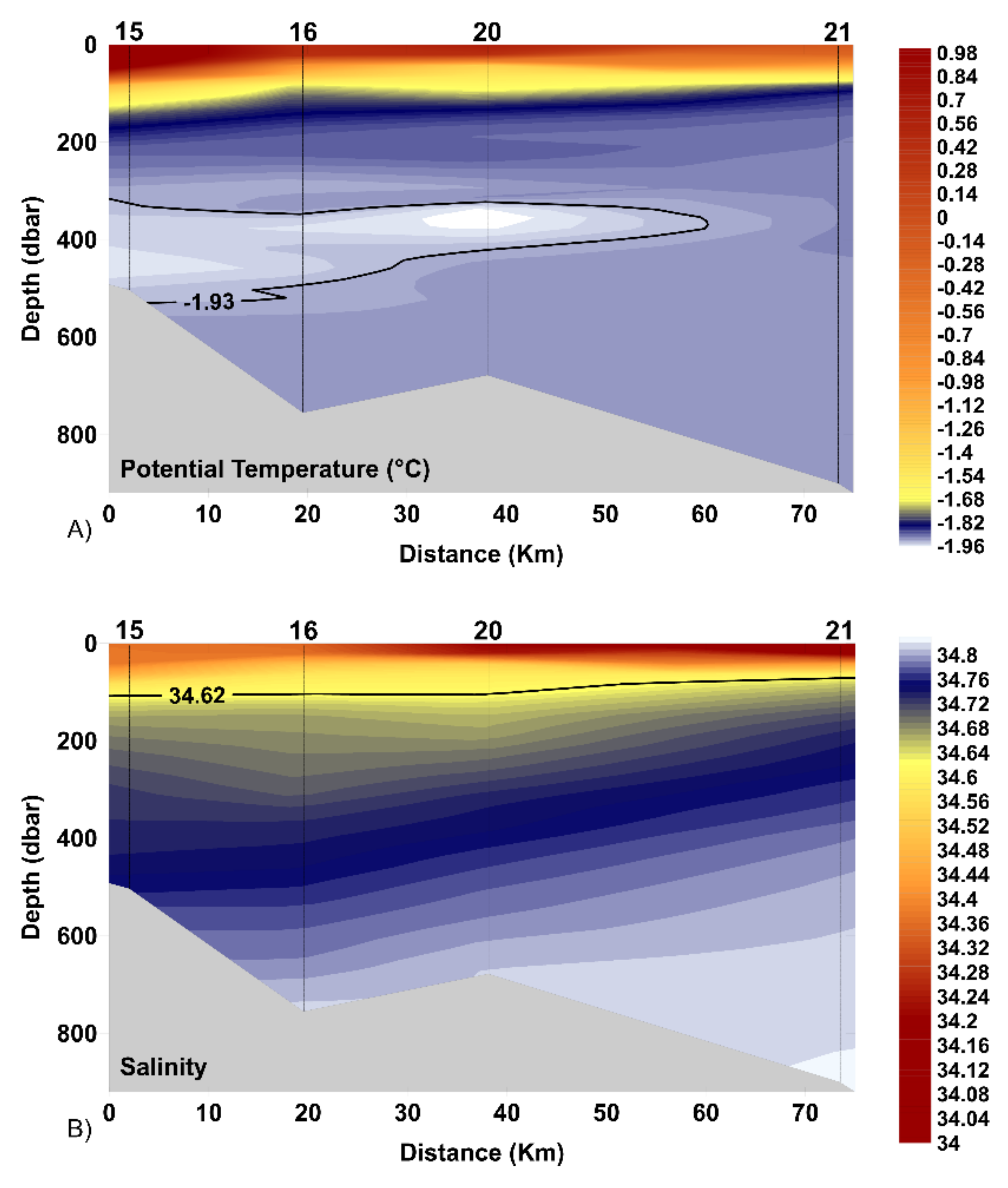
3.1. Water Masses
3.2. Particulate Trace Metals
4. Discussion
4.1. Particulate Iron in the ASSW
4.2. Particulate Iron in the Shelf Waters
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Parekh, P.; Follows, M.J.; Boyle, E.A. Decoupling of iron and phosphate in the global ocean. Glob. Biogeochem. Cycles 2005, 19. [Google Scholar] [CrossRef]
- Gerringa, L.; Rijkenberg, M.; Schoemann, V.; Laan, P.; De Baar, H. Organic complexation of iron in the West Atlantic Ocean. Mar. Chem. 2015, 177, 434–446. [Google Scholar] [CrossRef]
- McGillicuddy, D.J.J.; Sedwick, P.N.; Dinniman, M.S.; Arrigo, K.R.; Bibby, T.S.; Greenan, B.J.W.; E Hofmann, E.; Klinck, J.M.; O Smith, W.; Mack, S.L.; et al. Iron supply and demand in an Antarctic shelf ecosystem. Geophys. Res. Lett. 2015, 42, 8088–8097. [Google Scholar] [CrossRef]
- De Jong, J.; Schoemann, V.; Maricq, N.; Mattielli, N.; Langhorne, P.; Haskell, T.; Tison, J.-L. Iron in land-fast sea ice of McMurdo Sound derived from sediment resuspension and wind-blown dust attributes to primary productivity in the Ross Sea, Antarctica. Mar. Chem. 2013, 157, 24–40. [Google Scholar] [CrossRef]
- Noble, A.E.; Moran, D.M.; Allen, A.E.; Saito, M.A. Dissolved and particulate trace metal micronutrients under the McMurdo Sound seasonal sea ice: Basal sea ice communities as a capacitor for iron. Front. Chem. 2012, 1, 25. [Google Scholar] [CrossRef] [Green Version]
- Marsay, C.M.; Barrett, P.M.; McGillicuddy, D.J.J.; Sedwick, P.N. Distributions, sources, and transformations of dissolved and particulate iron on the Ross Sea continental shelf during summer. J. Geophys. Res. Oceans 2017, 122, 6371–6393. [Google Scholar] [CrossRef]
- Fitzwater, S.; Johnson, K.; Gordon, R.; Coale, K.; Smith, W. Trace metal concentrations in the Ross Sea and their relationship with nutrients and phytoplankton growth. Deep. Sea Res. Part II: Top. Stud. Oceanogr. 2000, 47, 3159–3179. [Google Scholar] [CrossRef]
- Atkins, C.B.; Dunbar, G.B. Aeolian sediment fl ux from sea ice into Southern McMurdo Sound, Antarctica. Glob. Planet. Chang. 2009, 69, 133–141. [Google Scholar] [CrossRef]
- Smith, W.; Sedwick, P.; Arrigo, K.; Ainley, D.; Orsi, A. The Ross Sea in a Sea of Change. Oceanography 2012, 25, 90–103. [Google Scholar] [CrossRef] [Green Version]
- Rignot, E.; Jacobs, S.; Mouginot, J.; Scheuchl, B. Ice-Shelf Melting Around Antarctica. Science 2013, 341, 266–270. [Google Scholar] [CrossRef] [Green Version]
- Grotti, M.; Soggia, F.; Ianni, C.; Frache, R. Trace metals distributions in coastal sea ice of Terra Nova Bay, Ross Sea, Antarctica. Antarct. Sci. 2005, 17, 289–300. [Google Scholar] [CrossRef]
- Rivaro, P.; Ianni, C.; Massolo, S.; Abelmoschi, M.L.; De Vittor, C.; Frache, R. Distribution of dissolved labile and particulate iron and copper in Terra Nova Bay polynya (Ross Sea, Antarctica) surface waters in relation to nutrients and phytoplankton growth. Cont. Shelf Res. 2011, 31, 879–889. [Google Scholar] [CrossRef]
- Rivaro, P.; Abelmoschi, M.L.; Grotti, M.; Ianni, C.; Magi, E.; Margiotta, F.; Massolo, S.; Saggiomo, V. Combined effects of hydrographic structure and iron and copper availability on the phytoplankton growth in Terra Nova Bay Polynya (Ross Sea, Antarctica). Deep. Sea Res. Part I: Oceanogr. Res. Pap. 2012, 62, 97–110. [Google Scholar] [CrossRef]
- Rivaro, P.; Ardini, F.; Grotti, M.; Aulicino, G.; Cotroneo, Y.; Fusco, G.; Mangoni, O.; Bolinesi, F.; Saggiomo, M.; Celussi, M. Mesoscale variability related to iron speciation in a coastal Ross Sea area (Antarctica) during summer 2014. Chem. Ecol. 2019, 35, 1–19. [Google Scholar] [CrossRef]
- Budillon, G.; Gremes Cordero, S.; Salusti, E. On The Dense Water Spreading Off The Ross Sea Shelf (Antarctica). J. Mar. Syst. 2002, 35, 207–227. [Google Scholar] [CrossRef]
- Petrelli, P.; Bindoff, N.L.; Bergamasco, A. The sea ice dynamics of Terra Nova Bay and Ross Ice Shelf Polynyas during a spring and winter simulation. J. Geophys. Res. Space Phys. 2008, 113, 1–16. [Google Scholar] [CrossRef]
- Frezzotti, M.; Mabin, M.C.G. 20th century behaviour of Drygalski Ice Tongue, Ross Sea, Antarctica. Ann. Glaciol. 1994, 20, 397–400. [Google Scholar] [CrossRef]
- Sansiviero, M.; Maqueda, M.M.; Fusco, G.; Aulicino, G.; Flocco, D.; Budillon, G. Modelling sea ice formation in the Terra Nova Bay polynya. J. Mar. Syst. 2017, 166, 4–25. [Google Scholar] [CrossRef]
- Misic, C.; Harriague, A.C.; Mangoni, O.; Aulicino, G.; Castagno, P.; Cotroneo, Y. Effects of physical constraints on the lability of POM during summer in the Ross Sea. J. Mar. Syst. 2017, 166, 132–143. [Google Scholar] [CrossRef]
- Budillon, G.; Castagno, P.; Aliani, S.; Spezie, G.; Padman, L. Thermohaline variability and Antarctic bottom water formation at the Ross Sea shelf break. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2011, 58, 1002–1018. [Google Scholar] [CrossRef]
- Castagno, P.; Capozzi, V.; DiTullio, G.R.; Falco, P.; Fusco, G.; Rintoul, S.R.; Spezie, G.; Budillon, G. Rebound of shelf water salinity in the Ross Sea. Nat. Commun. 2019, 10, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Silvano, A.; Foppert, A.; Rintoul, S.R.; Holland, P.R.; Tamura, T.; Kimura, N.; Castagno, P.; Falco, P.; Budillon, G.; Haumann, F.A.; et al. Recent recovery of Antarctic Bottom Water formation in the Ross Sea driven by climate anomalies. Nat. Geosci. 2020, 13, 780–786. [Google Scholar] [CrossRef]
- Orsi, A.H.; Wiederwohl, C.L. A recount of Ross Sea waters. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2009, 56, 778–795. [Google Scholar] [CrossRef]
- Budillon, G.; Spezie, G. Thermohaline structure and variability in the Terra Nova Bay polynya, Ross Sea. Antarct. Sci. 2000, 12, 493–508. [Google Scholar] [CrossRef]
- Tremblay, J.-E.; Smith, W. Primary Production and Nutrient Dynamics in Polynyas; Elsevier Oceanography Series; Elsevier: Amsterdam, The Netherlands, 2007; Chapter 8; Volume 74, pp. 239–269. [Google Scholar]
- Saggiomo, M.; Poulin, M.; Mangoni, O.; Lazzara, L.; De Stefano, M.; Sarno, D.; Zingone, A. Spring-time dynamics of diatom communities in landfast and underlying platelet ice in Terra Nova Bay, Ross Sea, Antarctica. J. Mar. Syst. 2017, 166, 26–36. [Google Scholar] [CrossRef]
- SCOR Working Group. The Acquisition, Calibration and Analysis of CTD Data; UNESCO: Paris, France, 1988; p. 102. [Google Scholar]
- Fofonoff, N.P.; Millard, R.C., Jr. Algorithms for Computation of Fundamental Properties of Seawater; UNESCO: Paris, France, 1983; Volume 44. [Google Scholar]
- Planquette, H.; Sherrell, R.M. Sampling for particulate trace element determination using water sampling bottles: Methodology and comparison to in situ pumps. Limnol. Oceanogr. Methods 2012, 10, 367–388. [Google Scholar]
- Grasshoff, K.; Kremling, K.; Ehrhardt, M. Methods of Seawate Analysis; Wiley Online Library: Hoboken, NJ, USA, 1983. [Google Scholar]
- Bazzano, A.; Rivaro, P.; Soggia, F.; Ardini, F.; Grotti, M. Anthropogenic and natural sources of particulate trace elements in the coastal marine environment of Kongsfjorden, Svalbard. Mar. Chem. 2014, 163, 28–35. [Google Scholar] [CrossRef]
- Jackett, D.R.; McDougall, T.J. A Neutral Density Variable for the World’s Oceans. J. Phys. Oceanogr. 1997, 27, 237–263. [Google Scholar] [CrossRef]
- Leardi, R.; Melzi, C.; Polotti, G. Chemometric Agile Tool (CAT). 2017. Available online: http://gruppochemiometria.it/index.php/software (accessed on 14 November 2020).
- Bolinesi, F.; Saggiomo, M.; Ardini, F.; Castagno, P.; Cordone, A.; Fusco, G.; Mangoni, O. Spatial-Related Community Structure and Dynamics in Phytoplankton of The Ross Sea, Antarctica. Front. Mar. Sci. 2020, 7, 1092. [Google Scholar]
- Ianni, C.; Rivaro, P.; Frache, R. Distribution of Dissolved and Particulate Iron, Copper and Manganese in the Shelf Waters of the Ross Sea (Antarctica). Mar. Ecol. 2002, 23, 210–219. [Google Scholar] [CrossRef]
- Corami, F.; Capodaglio, G.; Turetta, C.; Soggia, F.; Magi, E.; Grotti, M. Summer distribution of trace metals in the western sector of the Ross Sea, Antarctica. J. Environ. Monit. 2005, 7, 1256. [Google Scholar] [CrossRef] [PubMed]
- Rivaro, P.; Ianni, C.; Langone, L.; Ori, C.; Aulicino, G.; Cotroneo, Y.; Saggiomo, M.; Mangoni, O. Physical and biological forcing of mesoscale variability in the carbonate system of the Ross Sea (Antarctica) during summer 2014. J. Mar. Syst. 2017, 166, 144–158. [Google Scholar] [CrossRef]
- Dini, M.; Stenni, B. Oxygen Isotope Characterization of Terra Nova Bay Seawater. In Ross Sea Ecology; Springer: Berlin/Heidelberg, Germany, 2000; pp. 27–37. [Google Scholar]
- Meredith, M.P.; Brandon, M.A.; Wallace, M.I.; Clarke, A.; Leng, M.J.; Renfrew, I.A.; King, J.C. Variability in the freshwater balance of northern Marguerite Bay, Antarctic Peninsula: Results from delta-18O. Deep Sea Res. Part II Top. Stud. Oceanogr. 2008, 55, 309–322. [Google Scholar] [CrossRef] [Green Version]
- Lannuzel, D.; Schoemann, V.; De Jong, J.; Tison, J.-L.; Chou, L. Distribution and biogeochemical behaviour of iron in the East Antarctic sea ice. Mar. Chem. 2007, 106, 18–32. [Google Scholar] [CrossRef]
- Wedepohl, K.H. The composition of the continental crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Planquette, H.; Sherrell, R.M.; E Stammerjohn, S.; Field, M.P. Particulate iron delivery to the water column of the Amundsen Sea, Antarctica. Mar. Chem. 2013, 153, 15–30. [Google Scholar] [CrossRef]
- Planquette, H.; Fones, G.R.; Statham, P.J.; Morris, P.J. Origin of iron and aluminium in large particles (>53 µm) in the Crozet region, Southern Ocean. Mar. Chem. 2009, 115, 31–42. [Google Scholar] [CrossRef]
- Sherrell, R.M.; Lagerstrom, M.; Forsch, K.; E Stammerjohn, S.; Yager, P.L. Dynamics of dissolved iron and other bioactive trace metals (Mn, Ni, Cu, Zn) in the Amundsen Sea Polynya, Antarctica. Elem. Sci. Anth. 2015, 3, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Umani, S.F.; Monti, M.; Nuccio, C. Microzooplankton biomass distribution in Terra Nova Bay, Ross Sea (Antarctica). J. Mar. Syst. 1998, 17, 289–303. [Google Scholar] [CrossRef]
- Celussi, M.; Cataletto, B.; Umani, S.F.; Del Negro, P. Deep-Sea Research I Depth profiles of bacterioplankton assemblages and their activities in the Ross Sea. Deep Sea Res. Part I Oceanogr. Res. Pap. 2009, 56, 2193–2205. [Google Scholar] [CrossRef]
- Boyd, P.W.; Ellwood, M.J. The biogeochemical cycle of iron in the ocean. Nat. Geosci. 2010, 3, 675–682. [Google Scholar] [CrossRef]
- Sedwick, P.N.; DiTullio, G.R.; Mackey, D.J. Iron and manganese in the Ross Sea, Antarctica: Seasonal iron limitation in Antarctic shelf waters. J. Geophys. Res. Space Phys. 2000, 105, 11321–11336. [Google Scholar] [CrossRef]
- Langone, L.; Frignani, M.; Ravaioli, M.; Bianchi, C. Particle fluxes and biogeochemical processes in an area influenced by seasonal retreat of the ice margin (northwestern Ross Sea, Antarctica). J. Mar. Syst. 2000, 27, 221–234. [Google Scholar] [CrossRef]
- Biagioni, C.; Pasero, M. The systematics of the spinel-type minerals: An overview. Am. Mineral. 2014, 99, 1254–1264. [Google Scholar] [CrossRef]
- Monien, D.; Monien, P.; Brünjes, R.; Widmer, T.; Kappenberg, A.; Busso, A.A.S.; Schnetger, B.; Brumsack, H.-J. Meltwater as a source of potentially bioavailable iron to Antarctica waters. Antarct. Sci. 2017, 29, 277–291. [Google Scholar] [CrossRef] [Green Version]
- Malandrino, M.; Abollino, O.; Buoso, S.; Casalino, C.E.; Gasparon, M.; Giacomino, A.; La Gioia, C.; Mentasti, E. Geochemical characterisation of Antarctic soils and lacustrine sediments from Terra Nova Bay. Microchem. J. 2009, 92, 21–31. [Google Scholar] [CrossRef]
- Hawkings, J.; Benning, L.G.; Raiswell, R.; Kaulich, B.; Araki, T.; Kazemian, M.; Stockdale, A.; Koch-Müller, M.; Wadham, J.L.; Tranter, M. Biolabile ferrous iron bearing nanoparticles in glacial sediments. Earth Planet. Sci. Lett. 2018, 493, 92–101. [Google Scholar] [CrossRef]







| Station | Sampling Date | Latitude S (°) | Longitude E (°) | Bottom Depth (m) | Sampling Depth (m) |
|---|---|---|---|---|---|
| 2 | 9 January 2017 | −75.5858 | 165.4712 | 842 | 30–100–225–300 |
| 3 | 9 January 2017 | −75.5273 | 165.7213 | 789 | 20–100–200–350 |
| 6 | 12 January 2017 | −75.2055 | 163.5515 | 1108 | 20–100–240–320–440 |
| 7 | 12 January 2017 | −75.2982 | 164.0772 | 1201 | 20–100–442 |
| 8 | 12 January 2017 | −75.3495 | 164.6843 | 683 | 212–482 |
| 9 | 12 January 2017 | −75.3630 | 165.1157 | 653 | 10–220–513 |
| 10 | 12 January 2017 | −75.3947 | 165.9260 | 778 | 169–242–500 |
| 11 | 12 January 2017 | −75.1160 | 164.1530 | 971 | 200–240–389 |
| 12 | 13 January 2017 | −75.0720 | 163.7043 | 867 | 35–280–394 |
| 14 | 13January 2017 | −74.9277 | 163.9963 | 342 | 20–205–320 |
| 15 | 13 January 2017 | −74.7115 | 164.2308 | 498 | 40–200–390 |
| 16 | 13 January 2017 | −74.7837 | 164.7640 | 771 | 20–300–379 |
| 17 | 14 January 2017 | −74.9670 | 164.7920 | 919 | 20–240–300 |
| 19 | 14 January 2017 | −75.0050 | 165.1255 | 925 | 20–191–400 |
| 20 | 14 January 2017 | −74.7960 | 165.3995 | 662 | 20–288–330 |
| 21 | 14 January 2017 | −74.8748 | 166.5780 | 886 | 30–300 |
| 22 | 15 January 2017 | −75.0757 | 166.4043 | 854 | 15–300 |
| 23 | 15 January 2017 | −75.2368 | 166.1813 | 852 | 15–252 |
| Station | Depth (m) | SPM (mg L−1) | Al (nM) | Ba (nM) | Cd (nM) | Cu (nM) | Fe (nM) | Mn (nM) | Ni (nM) | Ti (nM) | Zn (nM) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 30 | 0.090 | 7.07 | 0.61 | 0.02 | 0.36 | 2.11 | 0.05 | 0.07 | 1.25 | 0.76 |
| 2 | 100 | 0.14 | 11.4 | 0.33 | 0.01 | 0.27 | 4.36 | 0.15 | 0.18 | 2.55 | 1.04 |
| 2 | 225 | 4.53 | 9.71 | 0.20 | 0.07 | 0.45 | 11.1 | 0.34 | 0.23 | 5.74 | 2.09 |
| 2 | 300 | 1.18 | 10.6 | 0.52 | 0.01 | 0.15 | 3.96 | 0.25 | 0.24 | 0.38 | 1.14 |
| 3 | 20 | 0.15 | 5.48 | 0.42 | 0.05 | 0.20 | 2.13 | 0.06 | 0.14 | 0.38 | 0.79 |
| 3 | 100 | 0.13 | 8.60 | 0.36 | 0.02 | 0.21 | 2.58 | 0.11 | 0.29 | 0.38 | 1.57 |
| 3 | 200 | 4.01 | 15.5 | 0.87 | 0.12 | 0.43 | 75.4 | 0.91 | 0.59 | 0.38 | 1.98 |
| 3 | 350 | 0.47 | 8.04 | 0.31 | 0.02 | 0.17 | 1.90 | 0.11 | 0.07 | 0.38 | 0.66 |
| 6 | 20 | 0.96 | 5.83 | 0.09 | 0.03 | 0.49 | 1.25 | 0.04 | 0.07 | 0.37 | 0.54 |
| 6 | 100 | 0.13 | 5.99 | 0.09 | 0.01 | 0.04 | 0.51 | 0.04 | 0.07 | 0.37 | 0.10 |
| 6 | 240 | 1.80 | 9.45 | 0.16 | 0.16 | 0.23 | 21.0 | 0.20 | 0.42 | 2.04 | 0.97 |
| 6 | 320 | 0.52 | 10.9 | 0.62 | 0.08 | 0.15 | 3.17 | 0.04 | 0.19 | 0.95 | 1.25 |
| 6 | 440 | 0.01 | 34.2 | 0.42 | 0.01 | 0.07 | 11.9 | 0.23 | 0.07 | 0.37 | 0.28 |
| 7 | 20 | 1.09 | 9.38 | 0.22 | 0.12 | 0.21 | 2.69 | 0.04 | 0.41 | 0.37 | 2.11 |
| 7 | 100 | 0.25 | 2.87 | 0.44 | 0.01 | 0.14 | 2.99 | 0.09 | 0.07 | 0.37 | 0.38 |
| 7 | 442 | 0.05 | 8.65 | 0.51 | 0.01 | 0.04 | 4.35 | 0.16 | 0.07 | 3.97 | 0.39 |
| 8 | 212 | 0.09 | 4.39 | 0.15 | 0.02 | 0.08 | 1.29 | 0.03 | 0.07 | 3.38 | 0.21 |
| 8 | 482 | 0.04 | 6.06 | 0.09 | 0.02 | 0.19 | 2.62 | 0.11 | 0.23 | 2.05 | 0.10 |
| 9 | 10 | 1.56 | 4.47 | 0.19 | 0.16 | 0.24 | 2.16 | 0.25 | 0.41 | 0.78 | 2.45 |
| 9 | 220 | 1.48 | 2.34 | 0.09 | 0.02 | 0.09 | 1.55 | 0.10 | 0.07 | 0.38 | 0.23 |
| 9 | 513 | 0.09 | 16.9 | 0.44 | 0.01 | 0.12 | 7.71 | 0.38 | 0.19 | 1.53 | 0.22 |
| 10 | 169 | 0.14 | 8.51 | 0.16 | 0.02 | 0.19 | 4.51 | 0.21 | 0.20 | 1.07 | 0.30 |
| 10 | 242 | 0.12 | 7.19 | 0.17 | 0.02 | 0.15 | 3.75 | 0.24 | 0.23 | 0.38 | 0.84 |
| 10 | 500 | 0.09 | 15.8 | 0.33 | 0.01 | 0.17 | 6.02 | 0.65 | 0.19 | 1.64 | 0.33 |
| 11 | 200 | 0.56 | 8.22 | 0.18 | 0.02 | 0.27 | 4.51 | 0.46 | 0.07 | 1.27 | 0.28 |
| 11 | 240 | 0.10 | 11.4 | 0.23 | 0.01 | 0.18 | 5.28 | 0.44 | 0.16 | 1.15 | 0.27 |
| 11 | 389 | 0.14 | 20.4 | 0.51 | 0.02 | 0.23 | 6.09 | 0.46 | 0.13 | 9.86 | 0.38 |
| 12 | 35 | 0.96 | 7.34 | 0.30 | 0.17 | 0.35 | 2.10 | 0.27 | 0.34 | 24.6 | 1.86 |
| 12 | 280 | 0.29 | 4.30 | 0.09 | 0.09 | 0.30 | 12.0 | 0.29 | 0.34 | 0.38 | 1.13 |
| 12 | 394 | 1.79 | 14.0 | 0.24 | 0.04 | 0.08 | 5.88 | 0.19 | 0.07 | 6.90 | 0.57 |
| 14 | 20 | 1.75 | - | 4.95 | 0.02 | 0.60 | - | 7.55 | 0.57 | 66.2 | 2.19 |
| 14 | 205 | 0.06 | 13.7 | 0.19 | 0.01 | 0.06 | 6.03 | 0.12 | 0.12 | 0.37 | 0.10 |
| 14 | 320 | 1.08 | 4.32 | 0.28 | 0.04 | 0.11 | 0.51 | 0.12 | 0.19 | 0.37 | 0.90 |
| 15 | 40 | 0.71 | 7.72 | 0.09 | 0.07 | 0.09 | 0.51 | 0.04 | 0.31 | 0.37 | 0.56 |
| 15 | 200 | 0.29 | 178 | 0.79 | 0.02 | 0.09 | 56.2 | 0.77 | 0.07 | 8.28 | 0.65 |
| 15 | 390 | 0.13 | 28.1 | 0.26 | 0.01 | 0.04 | 10.6 | 0.20 | 0.07 | 1.52 | 0.10 |
| 16 | 20 | 0.92 | 9.73 | 0.09 | 0.14 | 0.14 | 1.40 | 0.04 | 0.33 | 0.37 | 1.18 |
| 16 | 300 | 0.18 | 88.2 | 0.41 | 0.02 | 0.10 | 33.3 | 0.51 | 0.07 | 3.55 | 0.22 |
| 16 | 379 | 0.06 | 12.4 | 0.31 | 0.01 | 0.04 | 5.56 | 0.19 | 0.07 | 0.37 | 0.23 |
| 17 | 20 | 0.99 | 7.16 | 0.28 | 0.13 | 0.32 | 2.32 | 0.22 | 0.41 | 0.38 | 1.55 |
| 17 | 240 | 0.10 | 57.0 | 0.29 | 0.01 | 0.04 | 20.1 | 0.31 | 0.07 | 1.92 | 0.10 |
| 17 | 300 | 0.05 | 10.5 | 0.09 | 0.01 | 0.04 | 0.51 | 0.04 | 0.07 | 0.37 | 0.10 |
| 19 | 20 | 1.15 | 6.16 | 0.18 | 0.14 | 0.32 | 2.63 | 0.22 | 0.39 | 0.82 | 1.36 |
| 19 | 191 | 0.40 | 31.6 | 0.33 | 0.02 | 0.04 | 10.4 | 0.22 | 0.22 | 0.37 | 0.45 |
| 19 | 400 | 0.14 | 17.3 | 0.36 | 0.01 | 0.11 | 5.28 | 0.33 | 0.11 | 2.60 | 0.28 |
| 20 | 20 | 1.35 | 6.30 | 0.27 | 0.19 | 0.35 | 1.41 | 0.28 | 0.60 | 0.38 | 1.70 |
| 20 | 288 | 0.42 | 14.4 | 0.23 | 0.07 | 0.24 | 7.42 | 1.01 | 0.28 | 0.91 | 1.08 |
| 20 | 330 | 0.02 | 21.3 | 0.16 | 0.02 | 0.04 | 6.83 | 0.18 | 0.07 | 0.37 | 0.10 |
| 21 | 30 | 1.11 | 15.2 | 0.69 | 0.18 | 0.42 | 8.70 | 0.29 | 0.47 | 1.08 | 3.27 |
| 21 | 300 | 0.09 | 33.7 | 0.57 | 0.02 | 0.14 | 11.1 | 0.65 | 0.18 | 3.05 | 0.22 |
| 22 | 15 | 1.32 | 11.3 | 0.22 | 0.14 | 0.38 | 4.46 | 0.33 | 0.46 | 0.38 | 1.58 |
| 23 | 15 | 1.97 | 4.82 | 0.09 | 0.13 | 0.37 | 2.32 | 3.57 | 0.43 | 0.38 | 3.35 |
| 23 | 252 | 0.09 | 10.2 | 0.28 | 0.01 | 0.11 | 3.05 | 0.25 | 0.07 | 0.38 | 0.25 |
| Depth | S | T | F | δ18O | O2 | SPM | Al | Ba | Cd | Cu | Fe | Mn | Ni | Ti | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | 0.848 | ||||||||||||||
| T | −0.782 | −0.729 | |||||||||||||
| F | −0.751 | −0.632 | 0.666 | ||||||||||||
| δ18O | −0.658 | −0.538 | 0.704 | 0.628 | |||||||||||
| O2 | −0.555 | −0.384 | 0.379 | 0.588 | 0.448 | ||||||||||
| SPM | −0.517 | −0.603 | 0.468 | 0.213 | 0.286 | 0.216 | |||||||||
| Al | 0.455 | 0.448 | −0.468 | −0.355 | −0.317 | −0.154 | −0.295 | ||||||||
| Ba | 0.234 | 0.264 | −0.067 | −0.226 | −0.098 | −0.184 | −0.109 | 0.49 | |||||||
| Cd | −0.583 | −0.59 | 0.527 | 0.447 | 0.401 | 0.443 | 0.739 | −0.335 | −0.194 | ||||||
| Cu | −0.530 | −0.543 | 0.572 | 0.360 | 0.466 | 0.147 | 0.572 | −0.333 | 0.014 | 0.648 | |||||
| Fe | 0.398 | 0.36 | −0.506 | −0.439 | −0.326 | −0.336 | −0.099 | 0.742 | 0.409 | −0.156 | −0.07 | ||||
| Mn | 0.149 | 0.124 | −0.144 | −0.177 | −0.074 | −0.176 | 0.159 | 0.468 | 0.315 | 0.064 | 0.29 | 0.652 | |||
| Ni | −0.464 | −0.502 | 0.533 | 0.337 | 0.373 | 0.312 | 0.579 | −0.182 | −0.085 | 0.727 | 0.641 | −0.012 | 0.258 | ||
| Ti | 0.288 | 0.245 | −0.161 | −0.249 | −0.284 | −0.271 | −0.002 | 0.277 | 0.317 | −0.004 | 0.158 | 0.384 | 0.447 | −0.067 | |
| Zn | −0.565 | −0.602 | 0.644 | 0.385 | 0.443 | 0.268 | 0.748 | −0.283 | 0.132 | 0.760 | 0.739 | −0.120 | 0.158 | 0.762 | −0.023 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivaro, P.; Ardini, F.; Vivado, D.; Cabella, R.; Castagno, P.; Mangoni, O.; Falco, P. Potential Sources of Particulate Iron in Surface and Deep Waters of the Terra Nova Bay (Ross Sea, Antarctica). Water 2020, 12, 3517. https://doi.org/10.3390/w12123517
Rivaro P, Ardini F, Vivado D, Cabella R, Castagno P, Mangoni O, Falco P. Potential Sources of Particulate Iron in Surface and Deep Waters of the Terra Nova Bay (Ross Sea, Antarctica). Water. 2020; 12(12):3517. https://doi.org/10.3390/w12123517
Chicago/Turabian StyleRivaro, Paola, Francisco Ardini, Davide Vivado, Roberto Cabella, Pasquale Castagno, Olga Mangoni, and Pierpaolo Falco. 2020. "Potential Sources of Particulate Iron in Surface and Deep Waters of the Terra Nova Bay (Ross Sea, Antarctica)" Water 12, no. 12: 3517. https://doi.org/10.3390/w12123517
APA StyleRivaro, P., Ardini, F., Vivado, D., Cabella, R., Castagno, P., Mangoni, O., & Falco, P. (2020). Potential Sources of Particulate Iron in Surface and Deep Waters of the Terra Nova Bay (Ross Sea, Antarctica). Water, 12(12), 3517. https://doi.org/10.3390/w12123517






