Effect of Heavy Metal Ions on Steroid Estrogen Removal and Transport in SAT Using DLLME as a Detection Method of Steroid Estrogen
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Study Area
2.2. DLLME Method and Optimization
2.2.1. Detection Operation
2.2.2. Optimization of DLLME
2.3. Isothermal Adsorption
2.4. Laboratory Simulation of SAT System
2.4.1. Soil Column Description
2.4.2. SAT Simulation Scenarios
2.5. Retardation Factor
3. Results and Discussion
3.1. Optimized Effect of DLLME
3.2. Steroid Estrogens Concentration of Varied Water Sources in SAT Site
3.3. 17β-E2 Adsorption Isotherm Comparison in Solutions with and Without Cu
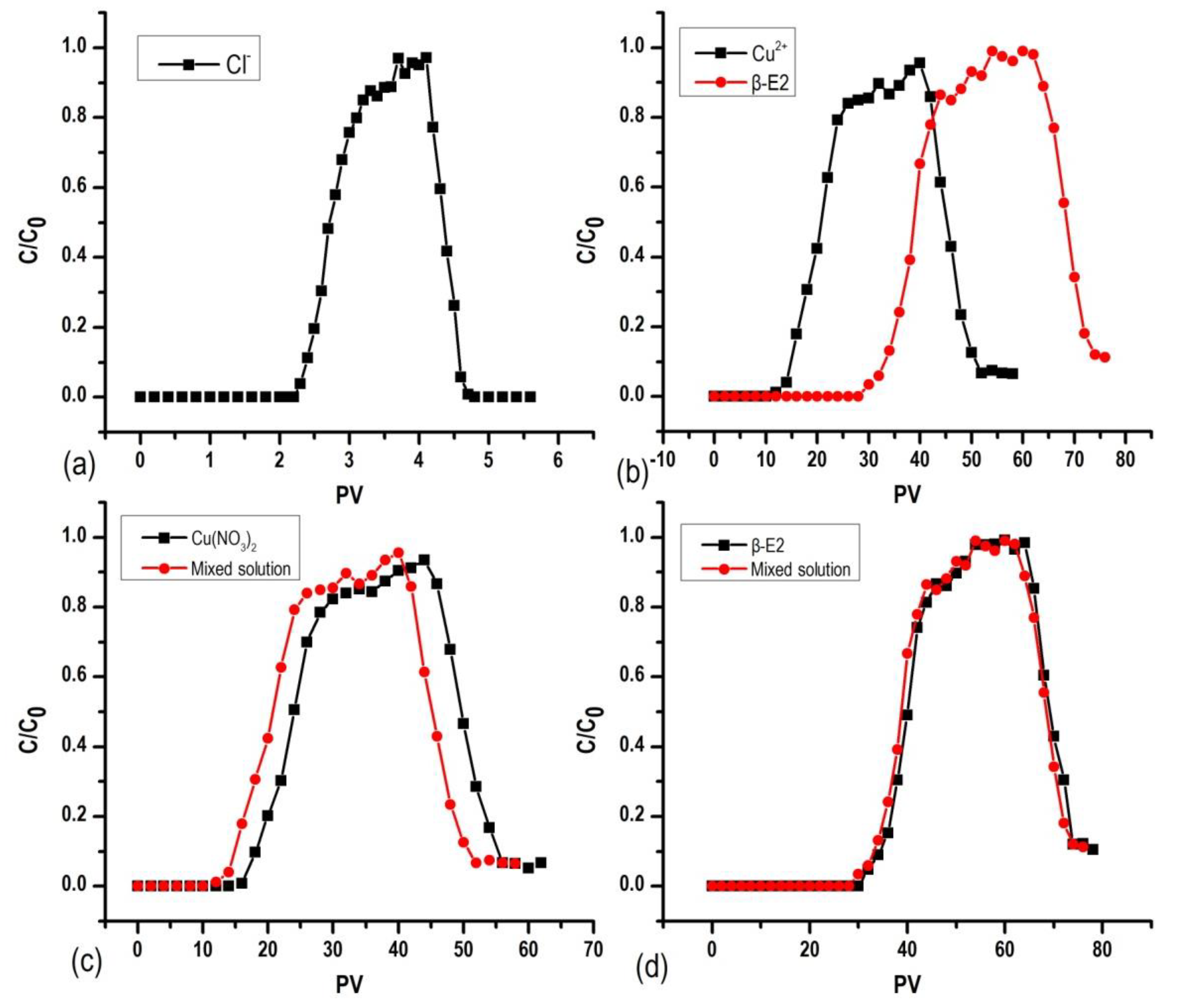
3.4. Co-Transport of 17β-E2 and Cu2+ in SAT
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, G.; Yang, Y.; Lu, Y.; Zhang, X.; Wu, Y.; Chen, Y. Design of an enhanced SAT using the graphene-MAR mixture for the removal of 17β-E2 at a demonstration site of Qianjin farm in China. Environ. Sci. Pollut. Res 2018, 25, 28120–28128. [Google Scholar] [CrossRef]
- Steiner, L.D.; Bidwell, V.J.; Di, H.J.; Cameron, K.C.; Northcott, G.L. Transport and modeling of estrogenic hormones in a dairy farm effluent through undisturbed soil lysimeters. Environ. Sci. Technol. 2010, 44, 2341–2347. [Google Scholar]
- Caupos, E.; Mazellier, P.; Croue, J.P. Photodegradation of estrone enhanced by dissolved organic matter under simulated sunlight. Water Res. 2011, 45, 3341–3350. [Google Scholar] [CrossRef]
- Wagner, M.; Oehlmann, J. Endocrine disruptors in bottled mineral water: Total estrogenic burden and migration from plastic bottles. Environ. Sci. Pollut. Res. 2009, 16, 278–286. [Google Scholar] [CrossRef] [Green Version]
- Nie, M.; Yan, C.; Dong, W.; Liu, M.; Zhou, J.; Yang, Y. Dong Occurrence, distribution and risk assessment of estrogens in surface water, suspended particulate matter, and sediments of the Yangtze Estuary. Chemosphere 2015, 127, 109–116. [Google Scholar] [CrossRef]
- Lei, B.; Wen, Y.; Wang, X.; Zha, J.; Li, W.; Wang, Z.; Sun, Y.; Kang, J.; Wang, Y. Effects of estrone on the early life stages and expression of vitellogenin and estrogen receptor genes of Japanese medaka (Oryzias latipes). Chemosphere 2013, 93, 1104–1110. [Google Scholar] [CrossRef]
- Li, J.; Fu, J.; Xiang, X.; Wu, M.; Liu, X. Kinetics, equilibrium, and mechanisms of sorption and desorption of 17α-ethinyl estradiol in two natural soils and their organic fractions. Sci. Total Environ. 2013, 452, 404–410. [Google Scholar]
- Feng, X.; Ding, S.; Tu, J.; Wu, F.; Deng, N. Degradation of estrone in aqueous solution by Photo-Fenton system. Sci. Total Environ. 2005, 345, 229–237. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, L.; Wu, F.; Deng, N.S. Photodegradation of 17α-Ethynylestradiol in Water by Fe (Ⅲ)/Oxalate System. Res. Environ. Sci. 2005, 18, 56–58. (In Chinese) [Google Scholar]
- Petrie, B.; McAdam, E.J.; Hassard, F.; Stephenson, T.; Lester, J.N.; Cartmell, E. Diagnostic investigation of steroid estrogen removal by activated sludge at varying solids retention time. Chemosphere 2014, 113, 101–108. [Google Scholar]
- Bai, X.; Casey, F.X.; Hakk, H.; DeSutter, T.M.; Oduor, P.G.; Khan, E. Sorption and degradation of 17β-estradiol-17-sulfate in sterilized soil–water systems. Chemosphere 2015, 119, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, A.; Khansary, M.A.; Marjani, A.; Shirazian, S. Using quantum chemical modeling and calculations for evaluation of cellulose potential for estrogen micropollutants removal from water effluents. Chemosphere 2017, 178, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Mansell, J.; Drewes, J.E. Fate of Steroidal Hormones During Soil-Aquifer Treatment. Groundw. Monit. Remediat. 2004, 24, 94–101. [Google Scholar] [CrossRef]
- Karnjanapiboonwong, A.; Morse, A.N.; Maul, J.D.; Anderson, T.A. Sorption of estrogens, triclosan, and caffeine in a sandy loam and a silt loam soil. J. Soils Sediments 2010, 10, 1300–1307. [Google Scholar] [CrossRef]
- Grover, D.P.; Zhou, J.L.; Frickers, P.E.; Readman, J.W. Improved removal of estrogenic and pharmaceutical compounds in sewage effluent by full scale granular activated carbon: Impact on receiving river water. J. Hazard. Mater. 2011, 185, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Liu, Y.; Liu, S.; Jiang, L.; Tan, X.; Zeng, G.; Li, M.; Liu, S.; Tian, S.; Fang, Y. Activated magnetic biochar by one-step synthesis: Enhanced adsorption and coadsorption for 17β-estradiol and copper. Sci. Total Environ. 2018, 639, 1530–1542. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Wang, F.; Yan, S.; Wang, Z. Effect of heavy metal ions on environmental estrogens adsorption by biochar. Environ. Pollut. Control 2019, 41, 896–900. (In Chinese) [Google Scholar]
- Capriotti, A.L.; Cavaliere, C.; Colapicchioni, V.; Piovesana, S.; Samperi, R.; Laganà, A. Analytical strategies based on chromatography–mass spectrometry for the determination of estrogen-mimicking compounds in food. J. Chromatogr. A 2013, 1313, 62–77. [Google Scholar] [CrossRef]
- Kapelewska, J.; Kotowska, U.; Wiśniewska, K. Determination of personal care products and hormones in leachate and groundwater from Polish MSW landfills by ultrasound-assisted emulsification microextraction and GC-MS. Environ. Sci. Pollut. Res. 2016, 23, 1642–1652. [Google Scholar] [CrossRef] [Green Version]
- Pozo, O.J.; Van Eenoo, P.; Van Thuyne, W.; Deventer, K.; Delbeke, F.T. Direct quantification of steroid glucuronides in human urine by liquid chromatography–electrospray tandem mass spectrometry. J. Chromatogr. A 2008, 1183, 108–118. [Google Scholar] [CrossRef]
- Ding, J.; Gao, Q.; Li, X.S.; Huang, W.; Shi, Z.G.; Feng, Y.Q. Magnetic solid-phase extraction based on magnetic carbon nanotube for the determination of estrogens in milk. J. Sep. Sci. 2011, 34, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Farajzadeh, M.A.; Mogaddam, M.R.A.; Aghdam, A.A. Comparison of air-agitated liquid–liquid microextraction technique and conventional dispersive liquid–liquid micro-extraction for determination of triazole pesticides in aqueous samples by gas chromatography with flame ionization detection. J. Chromatogr. A 2013, 1300, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.; Santos, J.L.; Aparicio, I.; Alonso, E. Determination of hormones, a plasticizer, preservatives, perfluoroalkylated compounds, and a flame retardant in water samples by ultrasound-assisted dispersive liquid–liquid microextraction based on the solidification of a floating organic drop. Talanta 2015, 143, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Jiang, Z.; Sun, Z. Distribution and formation environment of Fe-Mn nodules in soil derived from Quaternary loess in north China. Acta Pedol. Sinica 2019, 56, 288–293. (In Chinese) [Google Scholar]
- Rykowska, I.; Ziemblińska, J.; Nowak, I. Modern approaches in dispersive liquid–liquid microextraction (DLLME) based on ionic liquids: A review. J. Mol. Liq. 2018, 259, 319–339. [Google Scholar] [CrossRef]
- Han, Y.; Jia, X.; Liu, X.; Duan, T.; Chen, H. DLLME combined with GC–MS for the determination of methylparaben, ethylparaben, propylparaben and butylparaben in beverage samples. Chromatographia 2010, 72, 351–355. [Google Scholar] [CrossRef]
- Almeida, C.; Fernandes, J.O.; Cunha, S.C. A novel dispersive liquid–liquid microextraction (DLLME) gas chromatography-mass spectrometry (GC–MS) method for the determination of eighteen biogenic amines in beer. Food Control 2012, 25, 380–388. [Google Scholar] [CrossRef]
- Andruch, V.; Burdel, M.; Kocúrová, L.; Šandrejová, J.; Balogh, I.S. Application of ultrasonic irradiation and vortex agitation in solvent microextraction. TrAC 2013, 49, 1–19. [Google Scholar] [CrossRef]
- Wei, N.; Zhao, X.E.; Zhu, S.; He, Y.; Zheng, L.; Chen, G.; You, J.; Liu, S.; Liu, Z. Determination of dopamine, serotonin, biosynthesis precursors and metabolites in rat brain microdialysates by ultrasonic-assisted in situ derivatization–dispersive liquid–liquid microextraction coupled with UHPLC-MS/MS. Talanta 2016, 161, 253–264. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.G.; Zeng, G.M.; Hu, X.J.; Hu, X.; Li, T.T.; Li, H.Y.; Wang, Y.Q.; Jiang, L.H. Grafting of β-cyclodextrin to magnetic graphene oxide via ethylenediamine and application for Cr (VI) removal. Carbohydr. Polym. 2014, 113, 166–173. [Google Scholar] [CrossRef]
- Relyea, J.F. Theoretical and experimental considerations for the use of the column method for determining retardation factors. Radioact. Waste Manage. Nucl. Fuel Cycle 1982, 3, 151–166. [Google Scholar]
- Socas-Rodríguez, B.; Herrera-Herrera, A.V.; Asensio-Ramos, M.; Hernández-Borges, J. Recent applications of carbon nanotube sorbents in analytical chemistry. J. Chromatogr. A 2014, 1357, 110–146. [Google Scholar] [CrossRef] [PubMed]
- Lucas, S.D.; Jones, D.L. Biodegradation of estrone and 17 β-estradiol in grassland soils amended with animal wastes. Soil Biolog. Biochem 2006, 38, 2803–2815. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, D.; Sun, C. Effect of heavy metals on the sorption of hydrophobic organic compounds to wood charcoal. Environ. Sci. Technol 2007, 41, 2536–2541. [Google Scholar] [CrossRef] [PubMed]
- Arye, G.; Dror, I.; Berkowitz, B. Fate and transport of carbamazepine in soil aquifer treatment (SAT) infiltration basin soils. Chemosphere 2011, 82, 244–252. [Google Scholar] [CrossRef]





| Metal | Maximal Allowed Amount a (µg L−1) | Concentration(µg L−1) | |||||
|---|---|---|---|---|---|---|---|
| QJJ01 | QJJ02 | QJJ03 | QJJ04 | QJJ05 | XQ | ||
| Al | 200 | 44.31 | 31.70 | 40.42 | 33.07 | ||
| Mn | 100 | 1852 | 2009 | 1662 | 2149 | 2202 | |
| Cu | 1000 | 4.98 | 3.02 | 2.45 | 2.12 | 2.66 | 5370 |
| Zn | 1000 | 61.18 | 46.85 | 76.52 | 39.80 | 41.13 | 4725 |
| Fe | 300 | 5027 | 7070 | 8898 | 4221 | 1894 | 5231 |
| Cr | 50 | 1.86 | 0.65 | 2.68 | 1.83 | 3.06 | 628.3 |
| Cd | 5 | 0.03 | 0.04 | 0.02 | 0.01 | 0.01 | 18.17 |
| Mo | 70 | 6.33 | 7.14 | 2.44 | 1.24 | 2.17 | |
| Ni | 20 | 4.50 | 4.58 | 4.06 | 4.15 | 4.25 | 210.5 |
| Pb | 10 | 0.90 | 0.50 | 0.53 | 0.32 | 0.24 | 90.21 |
| Parameter | Units | Average Value |
|---|---|---|
| Structure | Medium sand | |
| Hydraulic conductivity | m d−1 | 7.543 |
| Porosity | 0.44 | |
| Dry density | g cm−3 | 1.77 |
| pH | 7.07 | |
| 17β-Estradiol concentration | mg L−1 | 0 |
| Cu2+ concentration | mg L−1 | 0 |
| Median diameter | mm | 0.409 |
| Average diameter | mm | 0.479 |
| Specific surface area | m2 g−1 | 0.003 |
| Water Sample | Object | Original (μg L−1) | Spiked (μg L−1) | Detected (μg L−1) | RR (%) | RSD (%) |
|---|---|---|---|---|---|---|
| SPSE I | 17β-E2 | 1.02 | 200.00 | 182.19 | 90.59 | 4.19 |
| E3 | 9.32 | 200.00 | 181.39 | 86.04 | 3.57 | |
| SPSE II | 17β-E2 | 3.99 | 200.00 | 181.46 | 88.73 | 3.14 |
| E3 | 12.81 | 200.00 | 184.86 | 86.03 | 3.25 | |
| Fishpond I | 17β-E2 | 0.02 | 200.00 | 182.71 | 91.34 | 3.85 |
| E3 | 0.15 | 200.00 | 182.83 | 91.34 | 3.56 | |
| Fishpond II | 17β-E2 | 0.03 | 200.00 | 182.96 | 91.46 | 3.94 |
| E3 | 0.12 | 200.00 | 183.08 | 91.48 | 3.97 | |
| River | 17β-E2 | 0.01 | 200.00 | 183.21 | 91.60 | 3.08 |
| E3 | 0.07 | 200.00 | 183.34 | 91.63 | 4.27 | |
| Groundwater | 17β-E2 | ND a | 200.00 | 181.11 | 90.55 | 3.03 |
| E3 | ND | 200.00 | 182.57 | 91.29 | 4.20 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Yang, Y.; Lu, Y.; Chen, Y.; Li, W.; Wang, S. Effect of Heavy Metal Ions on Steroid Estrogen Removal and Transport in SAT Using DLLME as a Detection Method of Steroid Estrogen. Water 2020, 12, 589. https://doi.org/10.3390/w12020589
Zhang G, Yang Y, Lu Y, Chen Y, Li W, Wang S. Effect of Heavy Metal Ions on Steroid Estrogen Removal and Transport in SAT Using DLLME as a Detection Method of Steroid Estrogen. Water. 2020; 12(2):589. https://doi.org/10.3390/w12020589
Chicago/Turabian StyleZhang, Ge, Yuesuo Yang, Ying Lu, Yu Chen, Wenbo Li, and Siyuan Wang. 2020. "Effect of Heavy Metal Ions on Steroid Estrogen Removal and Transport in SAT Using DLLME as a Detection Method of Steroid Estrogen" Water 12, no. 2: 589. https://doi.org/10.3390/w12020589
APA StyleZhang, G., Yang, Y., Lu, Y., Chen, Y., Li, W., & Wang, S. (2020). Effect of Heavy Metal Ions on Steroid Estrogen Removal and Transport in SAT Using DLLME as a Detection Method of Steroid Estrogen. Water, 12(2), 589. https://doi.org/10.3390/w12020589






