Distribution of Heavy Metals in Vegetative Biofiltration Columns
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
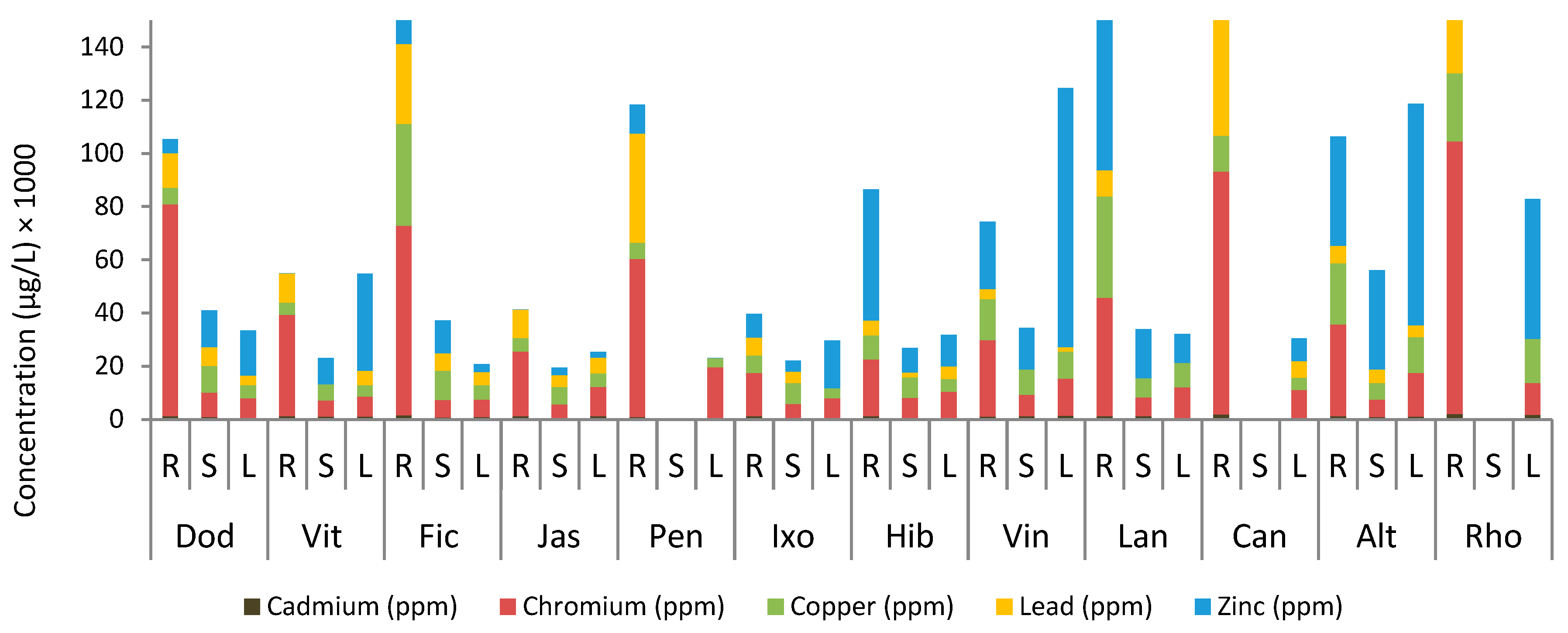
3.1. Heavy Metals in Effluent Water
3.2. Heavy Metals in Plant Samples
3.3. Heavy Metals in Soil Samples
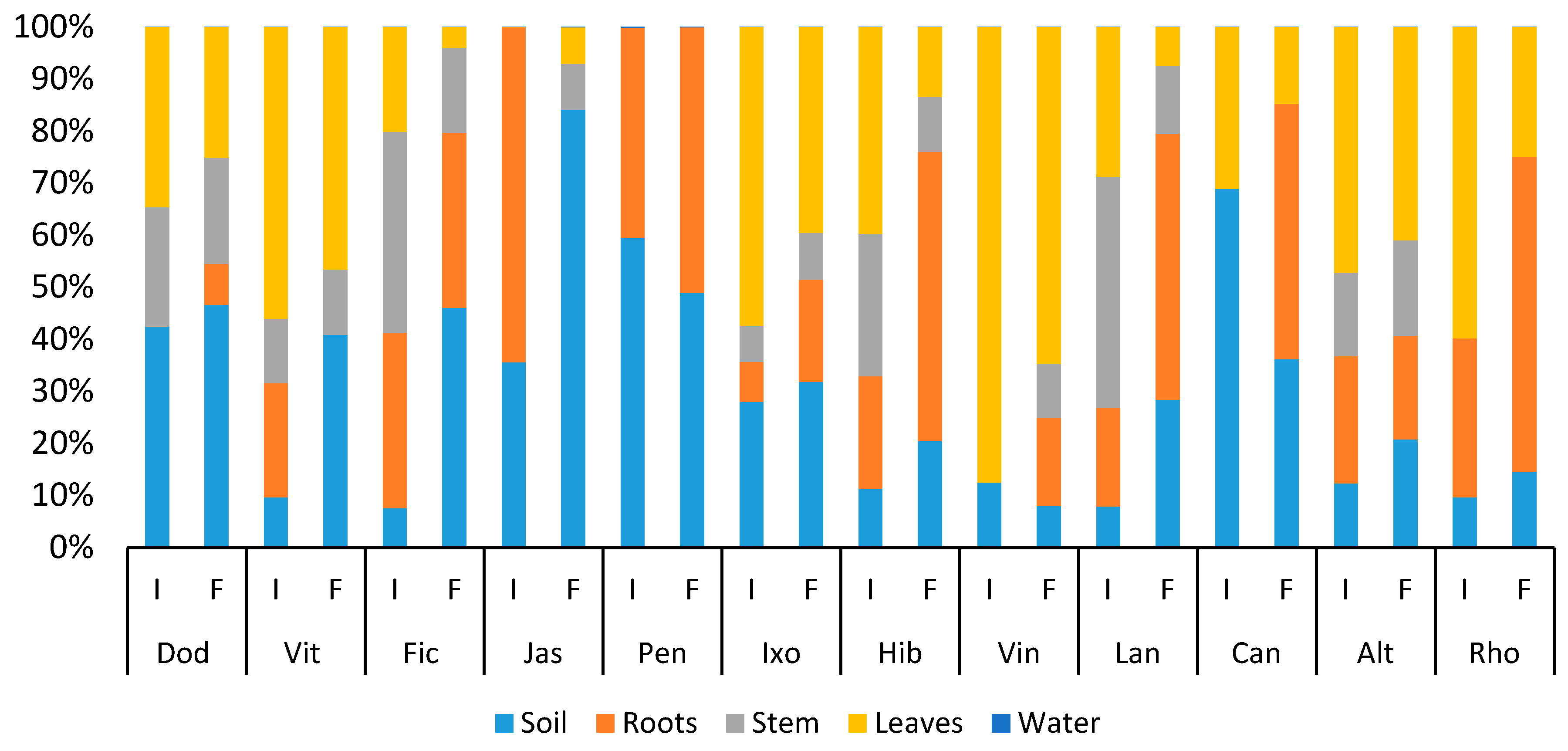
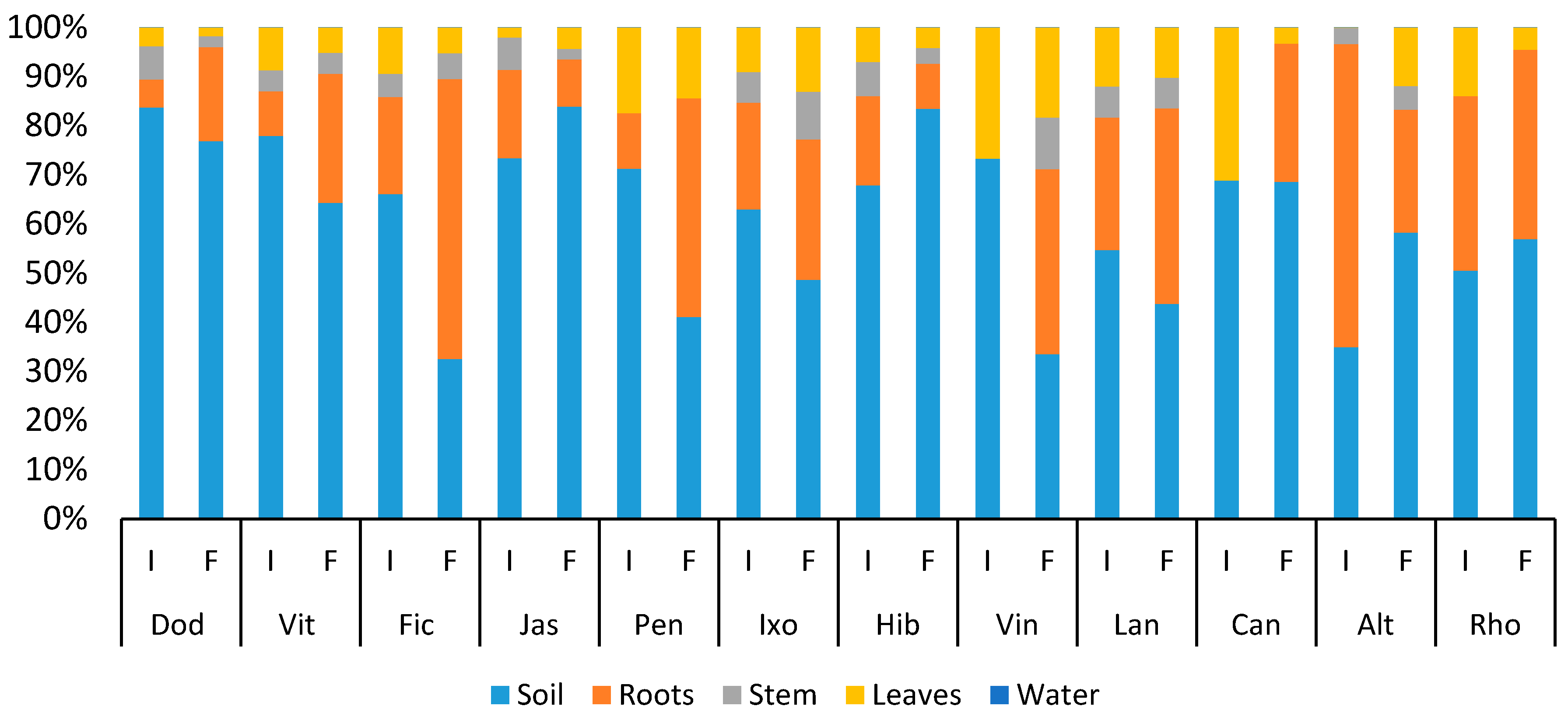
3.4. Distribution of Heavy Metals
3.4.1. Copper
3.4.2. Lead
3.4.3. Zinc
3.4.4. Chromium
3.4.5. Cadmium
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| Alt | Alternanthera ficondea |
| Can | Canna indica |
| Dod | Dodonaea viscose |
| Fic | Ficus nitida |
| Hib | Hibiscus rosa-sinensis |
| Ixo | Ixora coccinaea |
| Jas | Jasmine sambac |
| Lan | Lantana camara |
| Pen | Pennisetum seateceum |
| Rho | Rhoeo discolor |
| Vin | Vinca rosea |
| Vit | Vitex agnus |
References
- Woods-Ballard, B.; Kellagher, R.; Martin, P.; Jefferies, C.; Bray, R.; Shaffer, P. The SUDS Manual; CIRIA: London, UK, 2007; Volume 697. [Google Scholar]
- Henderson, C.; Greenway, M.; Phillips, I. Sorption behaviour of nutrients in loamy-sand biofiltration media subject to different conditions (vegetation, enrichment and incubation time). In Proceedings of the 13th International Rainwater Catchment Systems Conference and 5th International Water Sensitive Urban Design Conference, Sydney, Australia, 21–23 August 2007. [Google Scholar]
- Davis, A.P.; Shokouhian, M.; Sharma, H.; Minami, C. Water quality improvement through biofiltration media: Nitrogen and phosphorus removal. Water Environ. Res. 2006, 78, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Blick, S.A.; Kelly, F.; Skupien, J.J. New Jersey Stormwater Best Management Practices Manual; Chapter 9; New Jersey Department of Environmental Protection, Division of Watershed Management: Trenton, NJ, USA, 2004.
- Davis, A.P.; Shokouhian, M.; Sharma, H.; Minami, C.; Winogradoff, D. Water quality improvement through biofiltration: Lead, copper, and zinc removal. Water Environ. Res. 2003, 75, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Geronimo, F.K.F.; Maniquiz-Redillas, M.C.; Kim, L.H. Fate and removal of nutrients in biofiltration systems. Desalin. Water Treat. 2015, 53, 3072–3079. [Google Scholar] [CrossRef]
- Hatt, B.; Deletic, A.; Fletcher, T. Stormwater reuse: Designing biofiltration systems for reliable treatment. Water Sci. Technol. 2007, 55, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.P. Field performance of biofiltration: Water quality. Environ. Eng. Sci. 2007, 24, 1048–1064. [Google Scholar] [CrossRef]
- Trowsdale, S.A.; Simcock, R. Urban stormwater treatment using biofiltration. J. Hydrol. 2011, 397, 167–174. [Google Scholar] [CrossRef]
- Li, H.; Davis, A.P. Heavy metal capture and accumulation in biofiltration media. Environ. Sci. Technol. 2008, 42, 5247–5253. [Google Scholar] [CrossRef]
- Strawn, D.G.; Scheidegger, A.M.; Sparks, D.L. Kinetics and mechanisms of Pb (II) sorption and desorption at the aluminum oxide—Water interface. Environ. Sci. Technol. 1998, 32, 2596–2601. [Google Scholar] [CrossRef]
- Elbana, T.A.; Ramadan, M.A.; Gaber, H.M.; Bahnassy, M.H.; Kishk, F.M.; Selim, H.M. Heavy metals accumulation and spatial distribution in long term wastewater irrigated soils. J. Environ. Chem. Eng. 2013, 1, 925–933. [Google Scholar] [CrossRef]
- Turer, D.; Maynard, J.B.; Sansalone, J.J. Heavy metal contamination in soils of urban highways: Comparison between runoff and soil concentrations at Cincinnati, Ohio. Water Air Soil Pollut. 2001, 132, 293–314. [Google Scholar] [CrossRef]
- Sun, X.; Davis, A.P. Heavy metal fates in laboratory biofiltration systems. Chemosphere 2007, 66, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Sarret, G.; Vangronsveld, J.; Manceau, A.; Musso, M.; D’Haen, J.; Menthonnex, J.; Hazemann, J. Accumulation forms of Zn andPb in Phaseolus vulgaris in the presence and Absence of EDTA. Environ. Sci. Technol. 2001, 35, 2854–2859. [Google Scholar] [CrossRef] [PubMed]
- Madyiwa, S.; Chimbari, M.J.; Schutte, C.F.; Nyamangara, N. Greenhouse studies on the phyto-extraction capacity of Cynodont Nlemfuensis for lead and cadmium under irrigation with treated wastewater. Phys. Chem. Earth 2003, 28, 859–867. [Google Scholar] [CrossRef]
- Kim, I.S.; Kang, K.H.; Perry, J.G. Investigation of heavy metal accumulation in polygonum thunbergii for phytoextraction. Environ. Pollut. 2003, 126, 235–243. [Google Scholar] [CrossRef]
- Poschenrieder, C.; Bech, J.; Llugany, M.; Pace, A.; Fenés, E.; Barcelό, J. Copper in plant species in a copper gradient in Catalonia (North East Spain) and their potential for phytoremediation. Plant Soil 2001, 230, 247–256. [Google Scholar] [CrossRef]
- Chowdhury, R.K. Greywater reuse through a biofiltration system prototype in the arid region. Water Sci. Technol. 2015, 72, 2201–2211. [Google Scholar] [CrossRef]
- Chowdhury, R.; Abaya, J.; Ksiksi, T.; Mohamed, M.M.A. Removal of heavy metals in vegetative greywater biofiltration columns. J. Environ. Eng. 2018, 144, 1–8. [Google Scholar] [CrossRef]
- Fowdar, H.S.; Hatt, B.E.; Cresswell, T.; Harrison, J.J.; Cook, P.L.M.; Deletic, A. Phosphorus fate and dynamics in greywater biofiltration systems. Environ. Sci. Technol. 2017, 51, 2280–2287. [Google Scholar] [CrossRef]
- Prathapar, S.A.; Jamrah, A.; Ahmed, M.; Al Adawi, S.; Al Sidairi, S.; Al Harassi, A. Overcoming constraints in treated greywater reuse in Oman. Desalination 2005, 186, 177–186. [Google Scholar] [CrossRef]
- Eriksson, E.; Donner, E. Metals in greywater: Sources, presence and removal efficiencies. Desalination 2009, 248, 271–278. [Google Scholar] [CrossRef]
- Rodda, N.; Salukazana, L.; Jackson, S.A.F.; Smith, M.T. Use of domestic greywater for small-scale irrigation of food crops: Effects on plants and soil. Phys. Chem. Earth 2011, 36, 1051–1062. [Google Scholar] [CrossRef]
- Ghaitidak, D.M.; Yadav, K.D. Characteristics and treatment of greywater—A review. Environ. Sci. Pollut. Res. 2013, 20, 2795–2809. [Google Scholar] [CrossRef]
- Turner, R.D.; Warne, M.S.J.; Dawes, L.A.; Vardy, S.; Will, G.D. Irrigated greywater in an urban sub-division as a potential source of metals to soil, groundwater and surface water. J. Environ. Manag. 2016, 18, 806–817. [Google Scholar] [CrossRef]
- Al-Isawi, R.H.; Almuktar, S.A.; Scholz, M. Monitoring and assessment of treated river, rain, gully pot and grey waters for irrigation of Capsicum annuum. Environ. Monit. Assess. 2016, 188, 188–287. [Google Scholar] [CrossRef] [Green Version]
- Diaper, C.; Toifl, M.; Storey, M. CSIRO: Water for a Healthy Country National Research Flagship in Greywater Technology Testing Protocol; CSIRO: Canberra, Australia, 2008. [Google Scholar]
- Eriksson, E.; Auffarth, K.; Henze, M.; Ledin, A. Characteristics of grey wastewater. Urban Water 2002, 4, 85–104. [Google Scholar] [CrossRef]
- EPA. Hazardous Waste Test Methods/SW-846; Environmental Protection Agency (EPA): Washington, DC, USA, 2014. Available online: https://www.epa.gov/hw-sw846 (accessed on 17 May 2016).
- Bradl, H.B. Adsorption of heavy metal ions on soils and soils constituents. J. Colloid Interface Sci. 2004, 277, 1–18. [Google Scholar] [CrossRef]
- Laurenson, G.; Laurenson, S.; Bolan, N.; Beecham, S.; Clark, I. The role of bioretention systems in the treatment of stormwater. Adv. Agron. 2013, 120, 223–274. [Google Scholar]
- Crawford, R.J.; Harding, I.H.; Mainwaring, D.E. Adsorption and coprecipitation of single heavy metal ions onto the hydrated oxides of iron and chromium. Langmuir 1993, 9, 3050–3056. [Google Scholar] [CrossRef]
- McGrath, S.P.; Sanders, J.R.; Shalaby, M.H. The effects of soil organic matter levels on soil solution concentrations and extractabilities of manganese, zinc and copper. Geoderma 1988, 42, 177–188. [Google Scholar] [CrossRef]
- Deng, H.; Ye, Z.H.; Wong, M.H. Accumulation of lead, zinc, copper and cadmium by 12 wetland plant species thriving in metal-contaminated sites in China. Environ. Pollut. 2004, 132, 29–40. [Google Scholar] [CrossRef]
- Khan, M.A.; Ahmad, I.; Rahman, I.U. Effect of environmental pollution on heavy metals content of Withania Somnifera. J. Chin. Chem. Soc. 2007, 54, 339–343. [Google Scholar] [CrossRef]
- Jenne, E.A. Controls on Mn, Fe, Co, Ni, Cu, and Zn concentrations in soils and water: The significant role of hydrous Mn and Fe oxides. In Trace Inorganics in Water; Baker, R.A., Ed.; American Chemical Society: Washington, WA, USA, 1968; Volume 73, pp. 337–387. [Google Scholar]
- McBride, M.; Sauve, S.; Hendershot, W. Solubility control of Cu, Zn, Cd and Pb in contaminated soils. Eur. J. Soil Sci. 1997, 48, 337–346. [Google Scholar] [CrossRef]
- Kuo, S.; McNeal, B.L. Effects of pH and phosphate on cadmium sorption by a hydrous ferric oxide. Soil Sci. Soc. Am. J. 1984, 48, 1040–1044. [Google Scholar] [CrossRef]
- Christensen, T.H. Cadmium soil sorption at low concentrations: I. Effect of time, cadmium load, pH, and calcium. Water Air Soil Pollut. 1984, 21, 105–114. [Google Scholar] [CrossRef]
- Santillan-Medrano, J.; Jurinak, J.J. The chemistry of lead and cadmium in soil: Solid phase formation. Soil Sci. Soc. Am. J. 1975, 39, 851–856. [Google Scholar] [CrossRef]
- Street, J.J.; Sabey, B.R.; Lindsay, W.L. Influence of pH, phosphorus, cadmium, sewage sludge, and incubation time on the solubility and plant uptake of cadmium. J. Environ. Qual. 1978, 7, 286–290. [Google Scholar] [CrossRef]
- Li, Y.; Wen, M.; Li, J.; Chai, B.; Jiang, C. Reduction and accumulative characteristics of dissolved heavy metals in modified bioretention media. Water 2018, 10, 1488. [Google Scholar] [CrossRef] [Green Version]
- Vatanpour, N.; Feizy, J.; Talouki, H.H.; Es’haghi, Z.; Scesi, L.; Malvandi, A.M. The high levels of heavy metal accumulation in cultivated rice from the Tajan river basin: Health and ecological risk assessment. Chemosphere 2020, 245, 125639. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chowdhury, R.; Abaya, J.; Ksiksi, T.; Mohamed, M.; Beecham, S.; Rahman, A. Distribution of Heavy Metals in Vegetative Biofiltration Columns. Water 2020, 12, 747. https://doi.org/10.3390/w12030747
Chowdhury R, Abaya J, Ksiksi T, Mohamed M, Beecham S, Rahman A. Distribution of Heavy Metals in Vegetative Biofiltration Columns. Water. 2020; 12(3):747. https://doi.org/10.3390/w12030747
Chicago/Turabian StyleChowdhury, Rezaul, Jameelu Abaya, Taoufik Ksiksi, Mohamed Mohamed, Simon Beecham, and Ataur Rahman. 2020. "Distribution of Heavy Metals in Vegetative Biofiltration Columns" Water 12, no. 3: 747. https://doi.org/10.3390/w12030747
APA StyleChowdhury, R., Abaya, J., Ksiksi, T., Mohamed, M., Beecham, S., & Rahman, A. (2020). Distribution of Heavy Metals in Vegetative Biofiltration Columns. Water, 12(3), 747. https://doi.org/10.3390/w12030747







