1. Introduction
The research in this article aimed to present the possibilities of wastewater treatment coming from the confectionery plant using membrane techniques. The activity of confectionery plants requires a considerable amount of fresh water used in the production, as well as for machines and equipment washing, which generates large amounts of wastewater. Wastewater from the food industry contains a high level of organic compounds. They can be up to 10 times higher compared with municipal wastewater [
1,
2,
3]. Wastewater discharging characterized by a high load of organic compounds can create a severe environmental problem. That is why, before the discharge of such wastewater to environments, it should be cleaned appropriately.
In highly developed countries, closed water circuits and heat recovery systems are used. To be able to use the technological water, the applied methods of wastewater treatment must guarantee the removal of almost total organic mass and compounds that may cause interference in a given industry. For the wastewater treatment in a closed cycle, mainly physicochemical methods are used, such as chemical precipitation, sorption, and membrane filtration. More expensive, more modern technology of wastewater treatment, such as nanofiltration or reverse osmosis, allows more effective purification. The advantage of these methods is that, after the treatment process in the wastewater, there are no semi-finished products of pollutants decomposition and additional chemicals [
4,
5].
The main advantages of membrane processes are low power demand, small device constructions, high flexibility in terms of installation efficiency and effectiveness, the ability to incorporate membrane modules into existing systems of wastewater treatment equipment, removing a whole pollutants range, and not only changing them into forms of occurrence, effective removal of pathogenic microorganisms, effective removal of organic fouling, and obtaining water of better quality than specified in the requirements [
5]. However, as is well known, these processes are accompanied by the inherent phenomena contributing to the reduction of the membrane performance owing to the increase of filtration system resistance. They include the fouling phenomenon. There are many studies reported in literature that aimed at reducing the fouling process by selecting suitable membranes and their properties [
4,
6] and the application of processes mitigating the phenomenon of fouling [
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23]. Among them are methods for using photochemical processes, for example, Fe(II)/UV/chlorine [
7], UV/H
2O
2 [
13], and O
3 [
14]. One of the methods discussed by Zabihi et al. is the surface modification by UV radiation, which improves membrane anti-fouling [
8]. Vatankhah et al. conducted research on the initial ozonation of municipal wastewater to mitigate the formation of nanofiltration membrane fouling. The results indicated that pre-ozonation of membrane bioreactor effluent with a relatively low specific ozone dose (0.2 mgO
3/mg) could effectively mitigate a significant portion of fouling on the membrane compared with filtration without pre-ozonation [
14]. Benito et al. proved that it is possible to use UV to alleviate the fouling of ultrafiltration membranes. The results were that UV pretreatments decreased the transmembrane pressure by 30%–44%, and consequently the membrane fouling. The removal of dissolved organic carbon by the membrane was improved by 31% in the case of UV/H
2O
2 pretreatment [
13]. Further, the use of electrooxidation [
15] before the membrane process can effectively remove organic carbon and reduce the turbidity or color of wastewater. The results showed that the electrochemical pretreatment decreased the transmembrane pressure by36%–67%, and consequently the membrane fouling, with increasing applied current densities. The removal of dissolved organic carbon and turbidity by the membrane process was enhanced 40% and 41%, respectively, using the electro-oxidation pretreatment [
15].
Not only wastewater from the confectionery industry, but all industrial wastewater should be adequately cleaned to protect the environment. Treatment of industrial wastewater for reuse is prevalent among researchers around the world [
24,
25,
26,
27,
28,
29,
30,
31,
32]. This is related to the continuous increase in water shortages and natural environment protection [
6]. Research on recovery and successive reuse of process water obtained from dairy wastewater using reverse osmosis technology was carried out by Stoica et al. [
9]. They tested several RO (reverse osmonics) membranes for their fouling affinity. Briao et al. [
10] also attempted to recover water from washable dairy wastewater using membrane processes, that is, nanofiltration and reverse osmosis. They found that dairy wastewater treated with the reverse osmonics process can be used as cooling water. Thanks to RO, solid milk components can also be recovered to add them to fermented milk drinks and “dulce de leche”. They showed that, in the case of a dairy company that processes 1000 m
3 of milk per day, potential profits of USD 349,000 per year could be obtained. Ceramic membranes can also be successfully used for the treatment of industrial wastewater [
11]. From the literature review made by Samaei et al., it can be concluded that the MF (microfiltration) and UF (ultrafiltration) ceramic membranes have gained much attention in the treatment of wastewater from the food industry owing to their higher durability compared with polymeric membranes [
11]. Therefore, the development of effective industrial wastewater treatment technologies for reuse is one of the research priorities in the scientific communities [
31].
In view of the foregoing, the membrane fouling by confectionery wastewater has not been explored fully to-date, including the impact of pretreated photooxidation. In this paper, we summarize the results of NF membrane tests using wastewater from a confectionery factory in order to investigate, in particular, the following: (1) removal efficiency of organic compounds, color, and turbidity; (2) the use of photooxidation to mitigate membrane fouling; and (3) determining the percentage of reversible fouling and irreversible fouling.
2. Methodology
2.1. Confectionery Wastewater
The research subject was industrial wastewater collected from the confectionery plant in Poland.
Table 1 presents the physicochemical characteristics of the treated wastewater subjected to pressure membrane filtration.
2.2. Nanofiltration Run
The nanofiltration process was carried out in a pressure chamber of the American company Osmonics type GH-100-400 with a capacity of 0.35 L equipped with a magnetic stirrer. The process was carried out in a dead-end one-way filtration system. In the dead-end system, the wastewater was pretreated in the ultrafiltration process. The reason was high turbidity. Then, they were purified by nanofiltration. The first stage of the research was the filtration of distilled water under transmembrane pressure from 0.5 to 2 MPa, and the volume flow of deionized water
Jw was determined. Next, confectionery wastewater filtration was carried out with the value of the filtrate stream
Jv. Four flat nanofiltration membranes were used during the tests. The characteristics of nanofiltration membranes are shown in
Table 2.
The next stage of research was determining the susceptibility of nanofiltration membranes to the fouling phenomenon, and was carried out in a plate-and-frame membrane module SEPA CF-NP manufactured by the American company Osmonics. The experimental installation was operated in a batch mode as a cross-flow system. The permeate was continuously collected from the setup, and thus feed was progressively concentrated. The filtration area of the membrane was 155 cm
2, and the effective filtration area was 144 cm
2. Before each experiment, the clean water flux was determined using ultrapure water. The process was operated at a constant pressure of 1 MPa and a constant temperature of 21 ± 1 °C. Each filtration run consisted of three cycles including 60 min of filtration followed by forward flushing with ultrapure water during 60 s. The schematic diagram of the experimental setup is shown in
Figure 1.
The nanofiltration process was carried out using commercial flat nanofiltration membrane with the symbols NF-270. The membrane was received as a flat sheet and stored under dry conditions at room temperature. According to the manufacturer, the membrane is a thin-film composite with a polyamide active layer on a microporous polysulfone supporting layer. Molecular weight cut-off (MWCO) was 200 Da.
The wettability angle of the membranes was measured, which is a measure of their hydrophilic/hydrophobic nature. The measurement was made using a goniometer. The volume of the measuring drop for deionized water was 0.2 μL and the number of droplets applied in each case was 5.
2.3. Membrane Fouling Characterization
During the processes, the volume of the received filtrate was measured, which allowed calculating the value of the ultrapure water jet (
Jw) and the filtrate flow (
Jv) from the general dependence [
31]:
The volume of permeate was monitored in order to determine the permeability from the following equation:
where
is permeability (L·m
−2·h
−1·bar
−1) in short (LMHB),
is permeate volume (L),
is membrane surface area (m
2),
is permeate time collection (h), and
is transmembrane pressure (bar).
On the basis of the obtained results of the volume of ultrapure water, wastewater flow, and flushing strains after washing, the membrane can be determined of the percentage fouling share. The percentage of fouling (
Rf) is determined as the sum of reversible (
Rrf) and irreversible fouling (
Rif).
where
Jwp is volumetric flux ultrapure water determined from the flux after forward flushing after wastewater filtration, m
3/m
2∙s; and
Jw is volumetric flux of ultrapure water prior to wastewater filtration, m
3/m
2∙s.
Consequently, total hydraulic resistance consists of membrane resistance (Rm) and resistance caused by reversible (Rrf) and irreversible fouling (Rif).
2.4. Photooxidation Process
The next stage of the research involved the use of a photolytic oxidation process to pretreat sugar wastewater. The process of irradiating the prepared solutions with UV rays was carried out in a 1 L glass batch reactor.
Figure 2 shows the diagram of the reactor in which the irradiation process took place. All experiments were carried out at room temperature.
The effluent treatment efficiency was determined for the following irradiation times: 10, 20, 30, and 60 min. The reactor was placed on a magnetic stirrer, which ensured constant mixing of the entire volume of the reaction mixture. All experiments were carried out at room temperature. UV radiation was emitted by a medium pressure mercury UV lamp model TQ 150 V from Heraeus (Hanau, Germany). The radiation emitted by the lamp was in the wavelength range of 313 to 546. The lamp worked in a cooling jacket fed with tap water, which protected the mixture from overheating. The working capacity of the reactor was 700 L. For aeration, an aeration pump was used to provide 0.6 L per minute air flow.
2.5. Quality Analysis
The effectiveness of the process was also evaluated based on the degree of pollutant load removal. The evaluation of the efficiency of a treatment process was based on the change of wastewater quality indicators before and after the membrane process. The following parameters were controlled: color, COD, TOC, absorbance of UV254, nitrate, phosphate, ammonium, conductivity, and pH. Color measurements were performed with a UV–vis Spectroquant® Pharo 300 (Merck, Kenilworth, NJ, USA). COD, nitrate, phosphate, and ammonium concentrations were determined spectrophotometrically with Merck test kits. The absorbance was measured at 254 nm, using a UV–visible light (UV–vis) Cecil 1000 (Analytik Jena AG company, Jena, Germany). TOC was measured using a TOC-L series analyser (Shimadzu, Kioto, Prefektura Kioto, Japan). pH and conductivity were monitored by multifunctional analyzer CX-461 (Elmetron, Zabrze, Poland).
3. Results and Discussion
3.1. Nanofiltration of Wastewater from a Confectionery Factory in the Dead-End System
In the first stage of research, four nanofiltration membranes were tested [
34]. These wastewaters were subjected to initial purification in the ultrafiltration process. An ultrafiltration membrane of the HZ15 symbol from Osmonics was used for this purpose. The ultrafiltration effect of wastewater treatment was the complete removal of the suspension and turbidity. After the initial purification, to separate multivalent ions, bacteria, and organic impurities from wastewater, the filtrate was directed to a selected nanofiltration membrane. In
Figure 3 and
Figure 4, the dependence of the volume ultrapure water jet
Jw and the filtrate
Jv on the transmembrane pressure during the nanofiltration process is shown.
The largest volume water jet was obtained during the process on a flat composite membrane with the polymer skin layer with the NF-270 symbol of the Dow Filmtec company. This value is 27% higher than the volumetric water jet in the case of the Nadir NP010 polysulfone nanofiltration membrane. Meanwhile, the volumetric flow of permeate in the case of the NF-270 membrane was at the level of 3.85·10
−5 m
3/m
2s and was 29% lower than the volume jet of ultrapure water. As suggested by Gündogdu M. et al. [
27], the difference in performance of membranes observed during this work may be owing to differences in properties such as pore size, surface charge, hydrophobic or hydrophilic properties, and the structure or composition of the active layer for the used membranes. Considering the jet of treated wastewater, equal values for the NF-270 and NP010 membranes were observed. The high efficiency of the NF-270 membrane could be dictated by its thickness, probably the lowest among the tested membranes, and the hydrophilic nature of its surface [
21]. In the case of the NP010 membrane, the reason could be the most open structure, as testified by the MWCO value of 1000. Gündoğdu M. et al. [
27] used processes such as NF and RO for the reflux (machine syrup) demineralisation of the MBR (membrane bioreactor). NF membranes usually provide good retention of small organic molecules and inorganic salts, especially if multivalent ions are involved. Wastewater that reached the treatment plant is a mixture of wastewater of both municipal and industrial origin. They investigated the possibility of using NF and RO processes on a mini-pilot scale to reuse treated wastewater as irrigation and process water. They conducted research using the NF270, NF90, and TR 60 membranes. The average value of recovered water was 50.2%, 56.0%, and 51.8% respectively. As in the case of this research, the NF270 membrane was characterised by the highest efficiency, most probably because of the larger pore size [
27].
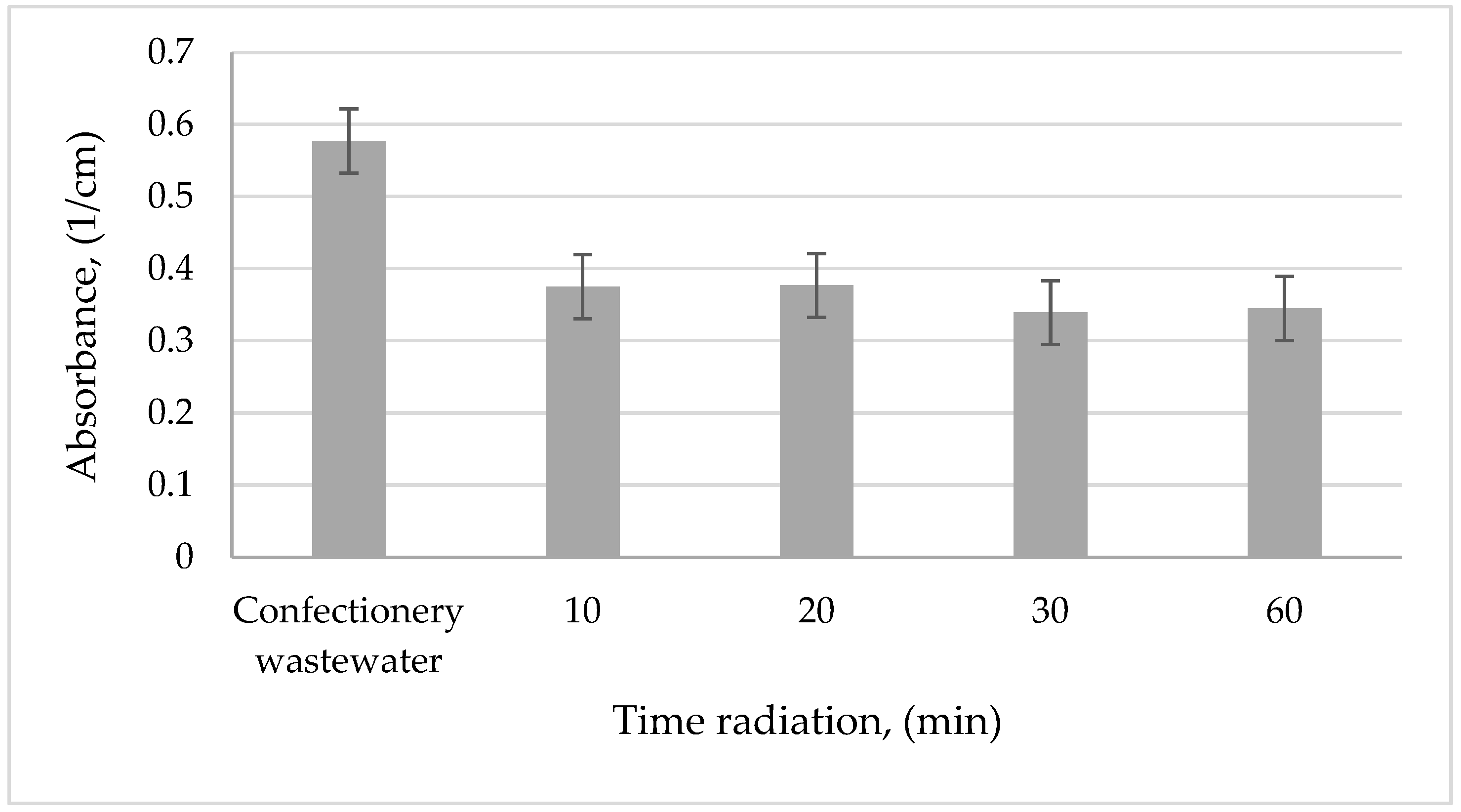
3.2. The Effectiveness of the Process UV
The first stage of the study was to compare the efficiency of confectionery wastewater treatment by photooxidation at different radiation times, that is, 10, 20, 30, and 60 min. The most preferred radiation time is chosen based on the removal of organic compounds and to reduce the color of wastewater. Considering the pH and sewage conductivity, there were no significant changes in value. The photolysis process was intended to reduce the concentration of organic impurities found in confectionery wastewater. The test results obtained are shown in
Figure 5,
Figure 6 and
Figure 7.
In the case of the absorbance value of confectionery sewage, its decrease in the range of 35%–41% along with the increase of UV radiation time was noted. It can be stated that, after 30 min of radiation, satisfactory results were obtained. Meanwhile, the color of sewage was reduced by 59% after the first 10 min of UV radiation. Lower efficiency of the photooxidation process was found when organic carbon was removed. Its concentration remained almost constant. After 30 min of exposure, only a 6% decrease in the concentration of this component was noted. On the basis of these results, it was found that 30 min of exposure will be sufficient for pre-treatment of wastewater from the confectionery industry.
3.3. The Effectiveness of the Process NF and UV + NF
Figure 8 is a photo showing raw wastewater from the confectionery industry, followed by wastewater after filtration through a soft filter and permeate the process of nanofiltration.
Table 3 shows the physicochemical characteristics of treated sewage in the process of nanofiltration and photooxidation of nanofiltration.
On the basis of the conducted research, no effect of initial photooxidation on the effectiveness of the nanofiltration process was found. Although, sewage after UV was characterized by reduced color, absorbance, and concentration of organic carbon.
The color of tested sewage was very high and amounted to 632 mg/L. It was associated with the high turbidity. The color of sewage was reduced to the greatest extent by as much as 98%. The photooxidation process reduced the color by 59%, and then the nanofiltration process by 97% (
Figure 9). Similarly, a high degree of removal was noted for TOC. Its value decreased by 99.2% (NF process) and 99.1% (UV + NF) (
Figure 9). However, the absorbance of the tested wastewater was completely reduced in the nanofiltration process.
Taking into account the content of biogenic compounds, it was noted that the nitrate nitrogen concentration decreased by 64%, ammonia nitrogen by 42%, and phosphate phosphorus by 94%. Samuel Bunanii S. et al. [
29], for the treatment of municipal wastewater for reuse, also used nanofiltration membranes, that is, CK, NF-90 and NF-270. Egea-Corbacho A. et al. [
30], during the wastewater treatment from the Medina Sidonia treatment plant in Spain by nanofiltration, also obtained total phosphorus removal. In the case of ammonia nitrogen, they got a comparable removal rate at the level of 30%. It can be concluded that the high efficiency of the composite membrane with the Dow Filmtec polyamide skin layer of the NF-270 symbol stopped the contaminants contained in industrial wastewater from sugar plants. Also, no improvement in the efficiency of wastewater treatment through the use of pre-oxidation was observed.
3.4. Effect of Photooxidation on Nanofiltration Membrane Fouling
A hydraulic performance of membrane was evaluated by permeability loss as a function of time. As seen in
Figure 10, the permeability of membrane for wastewater decreased significantly compared with the permeability of ultrapure water along the nanofiltration.
As seen in
Figure 10, the permeability of membranes for wastewater decreased significantly compared with the permeability of ultrapure water along the nanofiltration. It was found that the volume stream of treated sewage decreased with the increasing time. A positive effect of membrane washing with ultrapure water was observed. It was observed that the introduction of rinsing over the membrane surface restored the initial volume flow of treated wastewater. In the case of initial photooxidation of confectionery wastewater, nanofiltration efficiency increased by 17%. Similar effects were obtained in studies [
35] where UV/H
2O
2 was used to treat wastewater from an oil refinery prior to the nanofiltration process. The authors noted an increase in the efficiency and lifetime of nanofiltration membranes [
35]. The same applies to improving the efficiency of ultrafiltration membranes in other research [
13], where UV radiation was found to improve the process efficiency ultrafiltration of municipal wastewater.
Moreover, on the basis of Equations (4) and (5), the percentage of reversible and irreversible fouling was determined (
Figure 11).
In the first case, pollutants on the surface can be removed, which allows for the reversion to its initial productivity. If deposition, accumulation of contaminants occurs within pores, the fouling is irreversible. Thus, rinsing purification did not re-form the initial transport properties. On the basis of the results obtained, it was found that, already after the first 15 min of conducting the process, irreversible fouling was the dominant phenomenon. As a result, reduction of the flow was observed. After 60 min of filtration, a 40% decrease in permeate flux was noted. Therefore, more frequent flushing of the membrane should be introduced.
The contact angle of the NF-270 furniture was 32°, while after the nanofiltration process, it increased to 48°. This means that the impurities being absorbed on the membrane surface can often change hydrophilic/hydrophobic properties. This was caused by hydrophobic substances present in the wastewater, which likely affect sorption on the membrane surface. This is because this was the confectionery wastewater from cookie production. Confectionery wastewater is generated mainly in the washing and rinsing processes, including washing dishes and devices, technological equipment, and rinsing floors and pipelines. They contain sugar and fats from the production process as well as washing substances. The fats probably contributed to the creation of irreversible pollution and changed the membrane’s properties to hydrophobic.
However, the photooxidation process influenced the reduction of irreversible fouling by 4% and increased reversible fouling by 0.7%. It may be related to the fact that photooxidation breaks down and ionizes the molecules of all organic compounds.
It was found that the combined process supported that the lower organics content after the oxidation pre-treatment could have contributed to improving the performance of the NF process. Similarly, Li et al. reported that the oxidation pre-treatment contributed to improving the performance of the UF process [
36].
4. Conclusions
However, despite efficient NF retention, membrane contamination is still a major obstacle leading to reduced membrane permeability and a significant increase in operating and maintenance costs. The use of photooxidation methods as pretreatment is one of the potential technologies to minimize membrane contamination. It was found that the use of UV reduced the phenomenon of fouling of nanofiltration membranes. The value of the permeate volumetric flow after the hour of running the process increased by 17%. However, no impact of UV on the efficiency of wastewater treatment was found. However, the NF process provided the required quality of treated wastewater that can be reused in industrial applications. The NF process resulted in a total decrease in absorbance, 99% TOC removal, and 98% color removal.
Studies should be also conducted to determine the economic and environmental implications of applying this technique for wastewater pretreatment. More importantly, further research on the mitigation of fouling, including the use of photochemical methods, is also necessary.