Comparative Analysis of Bacterial and Archaeal Community Structure in Microwave Pretreated Thermophilic and Mesophilic Anaerobic Digesters Utilizing Mixed Sludge under Organic Overloading
Abstract
1. Introduction
2. Materials and Methods
2.1. Sludge Sample Collection
2.2. Experimental Approach
2.3. Analytical Procedures
2.4. Microbial Community Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Sludge Solubilization by Microwave Pretreatment
3.2. Performance of Bench-Scale Anaerobic Digesters by Microwave Pretreatment
3.2.1. The Relationship between Process Stability and Volatile Fatty Acids Accumulation
3.2.2. The Relationship between Process Stability and Microbial Community Structure
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ariunbaatar, J.; Panico, A.; Esposito, G.; Pirozzi, F.; Lens, P.N.L. Pretreatment methods to enhance anaerobic digestion of organic solid waste. Appl. Energy 2014, 123, 143–156. [Google Scholar] [CrossRef]
- Oladejo, J.; Shi, K.; Luo, X.; Yang, G.; Wu, T. A Review of Sludge-to-Energy Recovery Methods. Energies 2019, 12, 60. [Google Scholar] [CrossRef]
- Hamid, H.; Eskicioglu, C. Effect of microwave hydrolysis on transformation of steroidal hormones during anaerobic digestion of municipal sludge cake. Water Res. 2013, 47, 4966–4977. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Demirel, B.; Scherer, P. The roles of acetotrophic and hydrogenotrophic methanogens during anaerobic conversion of biomass to methane: A review. Rev. Environ. Sci. Biotechnol. 2008, 7, 173–190. [Google Scholar] [CrossRef]
- Appels, L.; Baeyens, J.; Degrève, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- Wacławek, S.; Grübel, K.; Silvestri, D.; Padil, V.V.T.; Wacławek, M.; Černík, M.; Varma, R.S. Disintegration of Wastewater Activated Sludge (WAS) for Improved Biogas Production. Energies 2019, 12, 21. [Google Scholar] [CrossRef]
- Kor-Bicakci, G.; Eskicioglu, C. Recent developments on thermal municipal sludge pretreatment technologies for enhanced anaerobic digestion. Renew. Sust. Energy Rev. 2019, 110, 423–443. [Google Scholar] [CrossRef]
- Hosseini Koupaie, E.; Eskicioglu, C. Conventional heating vs. microwave sludge pretreatment comparison under identical heating/cooling profiles for thermophilic advanced anaerobic digestion. Waste Manag. 2016, 53, 182–195. [Google Scholar] [CrossRef]
- Mehdizadeh, S.N.; Eskicioglu, C.; Bobowski, J.; Johnson, T. Conductive heating and microwave hydrolysis under identical heating profiles for advanced anaerobic digestion of municipal sludge. Water Res. 2013, 47, 5040–5051. [Google Scholar] [CrossRef]
- Kor-Bicakci, G.; Ubay-Cokgor, E.; Eskicioglu, C. Effect of dewatered sludge microwave pretreatment temperature and duration on net energy generation and biosolids quality from anaerobic digestion. Energy 2019, 168, 782–795. [Google Scholar] [CrossRef]
- Toreci, I.; Kennedy, K.J.; Droste, R.L. Effect of High-Temperature Microwave Irradiation on Municipal Thickened Waste Activated Sludge Solubilization. Heat Transf. Eng. 2010, 31, 766–773. [Google Scholar] [CrossRef]
- Park, W.J.; Ahn, J.H.; Hwang, S.; Lee, C.K. Effect of output power, target temperature, and solid concentration on the solubilization of waste activated sludge using microwave irradiation. Bioresour. Technol. 2010, 101, S13–S16. [Google Scholar] [CrossRef] [PubMed]
- Cella, M.A.; Akgul, D.; Eskicioglu, C. Assessment of microbial viability in municipal sludge following ultrasound and microwave pretreatments and resulting impacts on the efficiency of anaerobic sludge digestion. Appl. Microbiol. Biotechnol. 2016, 100, 2855–2868. [Google Scholar] [CrossRef] [PubMed]
- Świątczak, P.; Cydzik-Kwiatkowska, A.; Rusanowska, P. Microbiota of anaerobic digesters in a full-scale wastewater treatment plant. Arch. Environ. Prot. 2017, 43, 53–60. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef]
- Guo, J.; Peng, Y.; Ni, B.J.; Han, X.; Fan, L.; Yuan, Z. Dissecting microbial community structure and methane-producing pathways of a full-scale anaerobic reactor digesting activated sludge from wastewater treatment by metagenomic sequencing. Microb. Cell Fact. 2015, 14, 33. [Google Scholar] [CrossRef]
- Mei, R.; Narihiro, T.; Nobu, M.K.; Kuroda, K.; Liu, W.T. Evaluating digestion efficiency in full-scale anaerobic digesters by identifying active microbial populations through the lens of microbial activity. Sci. Rep. 2016, 6, 34090. [Google Scholar] [CrossRef]
- Westerholm, M.; Crauwels, S.; Van Geel, M.; Dewil, R.; Lievens, B.; Appels, L. Microwave and ultrasound pre-treatments influence microbial community structure and digester performance in anaerobic digestion of waste activated sludge. Appl. Microbiol. Biotechnol. 2016, 100, 5339–5352. [Google Scholar] [CrossRef]
- Zhang, J.; Lv, C.; Tong, J.; Liu, J.; Liu, J.; Yu, D.; Wang, Y.; Chen, M.; Wei, Y. Optimization and microbial community analysis of anaerobic co-digestion of food waste and sewage sludge based on microwave pretreatment. Bioresour. Technol. 2016, 200, 253–261. [Google Scholar] [CrossRef]
- American Public Health Association/American Water Works Association/Water Environment Federation. Standard Methods for the Examination of Water and Wastewater, Standard Methods, 20th ed.; American Public Health Association/American Water Works Association/Water Environment Federation: Washington, DC, USA, 2005. [Google Scholar]
- Frølund, B.; Griebe, T.; Nielsen, P.H. Enzymatic activity in the activated-sludge floc matrix. Appl. Microbiol. Biotechnol. 1995, 43, 755–761. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Ackman, R.G. Porous polymer bead packings and formic acid vapor in the GLC of volatile free fatty acids. J. Chromatogr. Sci. 1972, 10, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Van Huyssteen, J.J. Gas chromatographic separation of anaerobic digester gases using porous polymers. Water Res. 1967, 1, 237–242. [Google Scholar] [CrossRef]
- Parada, A.E.; Needham, D.M.; Fuhrman, J.A. Every base matters: Assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples. Environ. Microbiol. 2016, 18, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.R.; Wang, Q.; Cardenas, E.; Fish, J.; Chai, B.; Farris, R.J.; Kulam-Syed-Mohideen, A.S.; McGarrell, D.M.; Marsh, T.; Garrity, G.M.; et al. The Ribosomal Database Project: Improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009, 37, D141–D145. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Kor-Bicakci, G. Effect of Microwave Pretreatment on Fate of Antimicrobials and Conventional Pollutants during Anaerobic Sludge Digestion and Biosolids Quality for Land Application. Ph.D. Thesis, Istanbul Technical University, Istanbul, Turkey, 2018. [Google Scholar]
- Parkin, G.F.; Owen, W.F. Fundamentals of anaerobic digestion of wastewater sludges. J. Environ. Eng. 1986, 112, 867–920. [Google Scholar] [CrossRef]
- Batstone, D.J.; Pind, P.F.; Angelidaki, I. Kinetics of thermophilic, anaerobic oxidation of straight and branched chain butyrate and valerate. Biotechnol. Bioeng. 2003, 84, 195–204. [Google Scholar] [CrossRef]
- Hobson, P.N.; Shaw, B.G. Inhibition of methane production by Methanobacterium formicicum. Water Res. 1976, 10, 849–852. [Google Scholar] [CrossRef]
- Ramirez, I.; Mottet, A.; Carrère, H.; Déléris, S.; Vedrenne, F.; Steyer, J.P. Modified ADM1 disintegration/hydrolysis structures for modeling batch thermophilic anaerobic digestion of thermally pretreated waste activated sludge. Water Res. 2009, 43, 3479–3492. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Ahn, Y.H.; Speece, R.E. Comparative process stability and efficiency of anaerobic digestion; mesophilic vs. thermophilic. Water Res. 2002, 36, 4369–4385. [Google Scholar] [CrossRef]
- Speece, R.E.; Boonyakitsombut, S.; Kim, M.; Azbar, N.; Ursillo, P. Overview of anaerobic treatment: Thermophilic and propionate implications. Water Environ. Res. 2006, 78, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Fukuzaki, S.; Nishio, N.; Shobayashi, M.; Nagai, S. Inhibition of the Fermentation of Propionate to Methane by Hydrogen, Acetate, and Propionate. Appl. Environ. Microbiol. 1990, 56, 719–723. [Google Scholar] [CrossRef]
- Aitken, M.D.; Walters, G.W.; Crunk, P.L.; Willis, J.L.; Farrell, J.B.; Schafer, P.L.; Arnett, C.; Turner, B.G. Laboratory evaluation of thermophilic-anaerobic digestion to produce Class A biosolids. 1. Stabilization performance of a continuous-flow reactor at low residence time. Water Environ. Res. 2005, 77, 3019–3027. [Google Scholar] [CrossRef]
- Marchaim, U.; Krause, C. Propionic to acetic acid ratios in overloaded anaerobic digestion. Bioresour. Technol. 1993, 43, 195–203. [Google Scholar] [CrossRef]
- Hill, D.T.; Cobb, S.A.; Bolte, J.P. Using volatile fatty acid relationships to predict anaerobic digester failure. Trans. ASAE 1987, 30, 496–501. [Google Scholar] [CrossRef]
- Chouari, R.; Le Paslier, D.; Dauga, C.; Daegelen, P.; Weissenbach, J.; Sghir, A. Novel major bacterial candidate division within a municipal anaerobic sludge digester. J. Appl. Environ. Microbiol. 2005, 71, 2145–2153. [Google Scholar] [CrossRef]
- Pelletier, E.; Kreimeyer, A.; Bocs, S.; Rouy, Z.; Gyapay, G.; Chouari, R.; Riviere, D.; Ganesan, A.; Daegelen, P.; Sghir, A.; et al. “Candidatus Cloacamonas Acidaminovorans”: Genome sequence reconstruction provides a first glimpse of a new bacterial division. J. Bacteriol. 2008, 190, 2572–2579. [Google Scholar] [CrossRef]
- Riviere, D.; Desvignes, V.; Pelletier, E.; Chaussonnerie, S.; Guermazi, S.; Weissenbach, J.; Li, T.; Camacho, P.; Sghir, A. Towards the definition of a core of microorganisms involved in anaerobic digestion of sludge. ISME J. 2009, 3, 700–714. [Google Scholar] [CrossRef] [PubMed]
- González, L.; Ortiz-Cornejo, N.L.; Luna-Guido, M.; Dendooven, L.; Navarro-Noya, Y.E. Archaeal and Bacterial Community Structure in an Anaerobic Digestion Reactor (Lagoon Type) Used for Biogas Production at a Pig Farm. J. Mol. Microbiol. Biotechnol. 2017, 27, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Goux, X.; Calusinska, M.; Lemaigre, S.; Marynowska, M.; Klocke, M.; Udelhoven, T.; Benizri, E.; Delfosse, P. Microbial community dynamics in replicate anaerobic digesters exposed sequentially to increasing organic loading rate, acidosis, and process recovery. Biotechnol. Biofuels 2015, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.M.; Han, S.K.; Lee, C.Y. Enhancement of methane production in anaerobic digestion of sewage sludge by thermal hydrolysis pretreatment. Bioresour. Technol. 2018, 259, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Leven, L.; Eriksson, A.R.; Schnurer, A. Effect of process temperature on bacterial and archaeal communities in two methanogenic bioreactors treating organic household waste. FEMS Microbiol. Ecol. 2007, 59, 683–693. [Google Scholar] [CrossRef]
- Pervin, H.M.; Dennis, P.G.; Lim, H.J.; Tyson, G.W.; Batstone, D.J.; Bond, P.L. Drivers of microbial community composition in mesophilic and thermophilic temperature-phased anaerobic digestion pre-treatment reactors. Water Res. 2013, 47, 7098–7108. [Google Scholar] [CrossRef]
- Godon, J.J.; Moriniere, J.; Moletta, M.; Gaillac, M.; Bru, V.; Delgenes, J.P. Rarity associated with specific ecological niches in the bacterial world: The ‘Synergistes’ example. Environ. Microbiol. 2005, 7, 213–224. [Google Scholar] [CrossRef]
- Gagliano, M.C.; Braguglia, C.M.; Gianico, A.; Mininni, G.; Nakamura, K.; Rossetti, S. Thermophilic anaerobic digestion of thermal pretreated sludge: Role of microbial community structure and correlation with process performances. Water Res. 2015, 68, 498–509. [Google Scholar] [CrossRef]
- Luo, G.; Wang, W.; Angelidaki, I. Anaerobic digestion for simultaneous sewage sludge treatment and CO biomethanation: Process performance and microbial ecology. Environ. Sci. Technol. 2013, 47, 10685–10693. [Google Scholar] [CrossRef]
- Hori, T.; Haruta, S.; Ueno, Y.; Ishii, M.; Igarashi, Y. Dynamic transition of a methanogenic population in response to the concentration of volatile fatty acids in a thermophilic anaerobic digester. Appl. Environ. Microbiol. 2006, 72, 1623–1630. [Google Scholar] [CrossRef]
- Hao, L.P.; Lü, F.; He, P.J.; Li, L.; Shao, L.M. Predominant contribution of syntrophic acetate oxidation to thermophilic methane formation at high acetate concentrations. Environ. Sci. Technol. 2011, 45, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Karakashev, D.; Batstone, D.J.; Angelidaki, I. Influence of environmental conditions on methanogenic compositions in anaerobic biogas reactors. Appl. Environ. Microbiol. 2005, 71, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.P.; Jensen, P.D.; Batstone, D.J. Methanosarcinaceae and acetate-oxidizing pathways dominate in high-rate thermophilic anaerobic digestion of waste-activated sludge. Appl. Environ. Microbiol. 2013, 79, 6491–6500. [Google Scholar] [CrossRef] [PubMed]
- Iino, T.; Tamaki, H.; Tamazawa, S.; Ueno, Y.; Ohkuma, M.; Suzuki, K.; Igarashi, Y.; Haruta, S. Candidatus Methanogranum caenicola: A novel methanogen from the anaerobic digested sludge, and proposal of Methanomassiliicoccaceae fam. nov. and Methanomassiliicoccales ord. nov., for a methanogenic lineage of the class Thermoplasmata. Microbes Environ. 2013, 28, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Karakashev, D.; Batstone, D.J.; Trably, E.; Angelidaki, I. Acetate oxidation Is the dominant methanogenic pathway from acetate in the absence of Methanosaetaceae. Appl. Environ. Microbiol. 2006, 72, 5138–5141. [Google Scholar] [CrossRef] [PubMed]
- McMahon, K.D.; Stroot, P.G.; Mackie, R.I.; Raskin, L. Anaerobic codigestion of municipal solid waste and biosolids under various mixing conditions—II: Microbial population dynamics. Water Res. 2001, 35, 1817–1827. [Google Scholar] [CrossRef]




| Parameters | Mixed Sludge Digester Feed (1FPS:TWAS = 33:67% by Volume) | ||
|---|---|---|---|
| Non-irradiated | 2MW-irradiated | ||
| Control (Raw Sludge) | MW 1—80 °C | MW 2—160 °C | |
| 3TS (% by wt.) | 43.94 ± 0.24 (16) | 3.40 ± 0.35 (16) | 3.37 ± 0.25 (16) |
| 5VS (% by wt.) | 3.38 ± 0.20 (16) | 2.90 ± 0.33 (16) | 2.87 ± 0.24 (16) |
| 6Ammonia (mg N/L/%VS by wt.) | 207 ± 51 (3) | 259 ± 54 (3) | 219 ± 18 (3) |
| pH (-) | 5.63 ± 0.13 (5) | 5.64 ± 0.05 (5) | 5.52 ± 0.20 (5) |
| 6Alkalinity (mg as CaCO3/L/%VS by wt.) | 502 ± 116 (3) | 590 ± 38 (3) | 531 ± 106 (3) |
| Volatile Fatty Acids (VFAs) | |||
| 6,7Acetic acid (mg/L/%VS by wt.) | 316 ± 79 (3) | 415 ± 104 (3) | 451 ± 104 (3) |
| 6,7Propionic acid (mg/L/%VS by wt.) | 241 ± 80 (3) | 337 ± 103 (3) | 322 ± 93 (3) |
| 6,7Butyric acid (mg/L/%VS by wt.) | 174 ± 64 (3) | 208 ± 86 (3) | 203 ± 66 (3) |
| 6,7Total VFAs (mg/L/%VS by wt.) | 731 ± 223 (3) | 960 ± 293 (3) | 976 ± 264 (3) |
| 1MW Pretreatment Conditions | Anaerobic Digester Conditions | ||||
|---|---|---|---|---|---|
| 2Final Temp. (°C) | Holding Time (min) | Mixed Sludge Feed | Digester | Temperature (°C) | 3SRT (days) |
| - | - | Control(non-irradiated) | 4T1—Control 5M1—Control | 55 ± 1 35 ± 1 | 6 |
| 80 | 30 | MW 1—80 °C | T2—80 °C M2—80 °C | 55 ± 1 35 ± 1 | 6 |
| 160 | 30 | MW 2—160 °C | T3—160 °C M3—160 °C | 55 ± 1 35 ± 1 | 6 |
| Sample | Parameter | Frequency |
|---|---|---|
| Digester influent | ||
| Mixed sludge feed | 1TS, 2VS, total & soluble 3COD, pH, alkalinity, ammonia, and total 4VFAs | Upon preparation of feed with fresh substrates (bi-weekly) |
| Biopolymers (protein, humic acids, and sugar) | 5Minimum three sets of data | |
| Digester Effluent | ||
| Digestate | pH | Daily |
| TS, VS and total & soluble COD | Every three days | |
| Alkalinity, ammonia, and total VFAs | Once a week | |
| Biopolymers (protein, humic acids, and sugar) | 5Minimum three sets of data | |
| Digester biogas | Biogas volume | Daily |
| Biogas composition | Once a week | |
| Parameters | Mixed Sludge Digester Feed | ||
|---|---|---|---|
| Non-irradiated | 1MW-irradiated | ||
| Control(Raw Sludge) | MW1—80 °C | MW2—160 °C | |
| 2COD: | |||
| CODsoluble (mg/L/%3VS by wt.) | 42107 ± 444 (9) | 2917 ± 349 (9) | 4028 ± 688 (9) |
| 5The solubilization ratio (%) | 13 ± 0.4 (9) | 19 ± 1.3 (9) | 27 ± 0.8 (9) |
| 6The fold increase in solubilization (-) | - | 1.5 | 2.1 |
| Protein: | |||
| Proteinsoluble (mg/L/%VS by wt.) | 30 ± 0.41 (2) | 94 ± 2.1 (2) | 199 ± 1.9 (2) |
| The solubilization ratio (%) | 4 ± 0.1 (2) | 20 ± 1.1 (2) | 39 ± 0.1 (2) |
| The fold increase in solubilization (-) | - | 4.8 | 9.4 |
| Humic acids: | |||
| Humic acidssoluble (mg/L/%VS by wt.) | 61 ± 1.3 (2) | 131 ± 8.5 (2) | 276 ± 9.6 (2) |
| The solubilization ratio (%) | 8 ± 0.2 (2) | 23 ± 1.2 (2) | 51 ± 1.7 (2) |
| The fold increase in solubilization (-) | - | 2.8 | 6.2 |
| Sugar: | |||
| Sugarsoluble (mg/L/%VS by wt.) | 4.6 ± 0.2 (2) | 8.4 ± 0.6 (2) | 38.4 ± 1.3 (2) |
| The solubilization ratio (%) | 0.9 ± 0.1 (2) | 2.1 ± 0.1 (2) | 9.2 ± 0.8 (2) |
| The fold increase in solubilization (-) | - | 2.2 | 10.4 |
| Parameters | Thermophilic | Mesophilic | ||||
|---|---|---|---|---|---|---|
| 1T1 Control | T2 80 °C | T3 160 °C | 2M1 Control | M2 80 °C | M3 160 °C | |
| Retention Time and Loading Conditions | ||||||
| 3SRT (days) | 6 | 6 | 6 | 6 | 6 | 6 |
| 4OLR (g 5VS/L/d) | 5.72 ± 0.32 (5)b | 5.12 ± 0.15 (5) | 4.91 ± 0.28 (5) | 5.72 ± 0.32 (5) | 5.12 ± 0.15 (5) | 4.91 ± 0.28 (5) |
| OLR (g 6COD/L/d) | 8.71 ± 1.28 (3) | 7.81 ± 0.48 (3) | 7.56 ± 0.33 (3) | 8.71 ± 1.28 (3) | 7.81 ± 0.48 (3) | 7.56 ± 0.33 (3) |
| Removal Efficiencies | ||||||
| 3TS (% by wt.) | 445 ± 2.5 (9) | 44 ± 2 (9) | 46 ± 4 (9) | 38 ± 3.7 (9) | 40 ± 2.1 (9) | 45 ± 2.4 (9) |
| 5VS (% by wt.) | 52 ± 3.0 (9) | 52 ± 2.4 (9) | 54 ± 3.8 (9) | 45 ± 3.6 (9) | 47 ± 1.8 (9) | 53 ± 2.4 (9) |
| Methane Production | ||||||
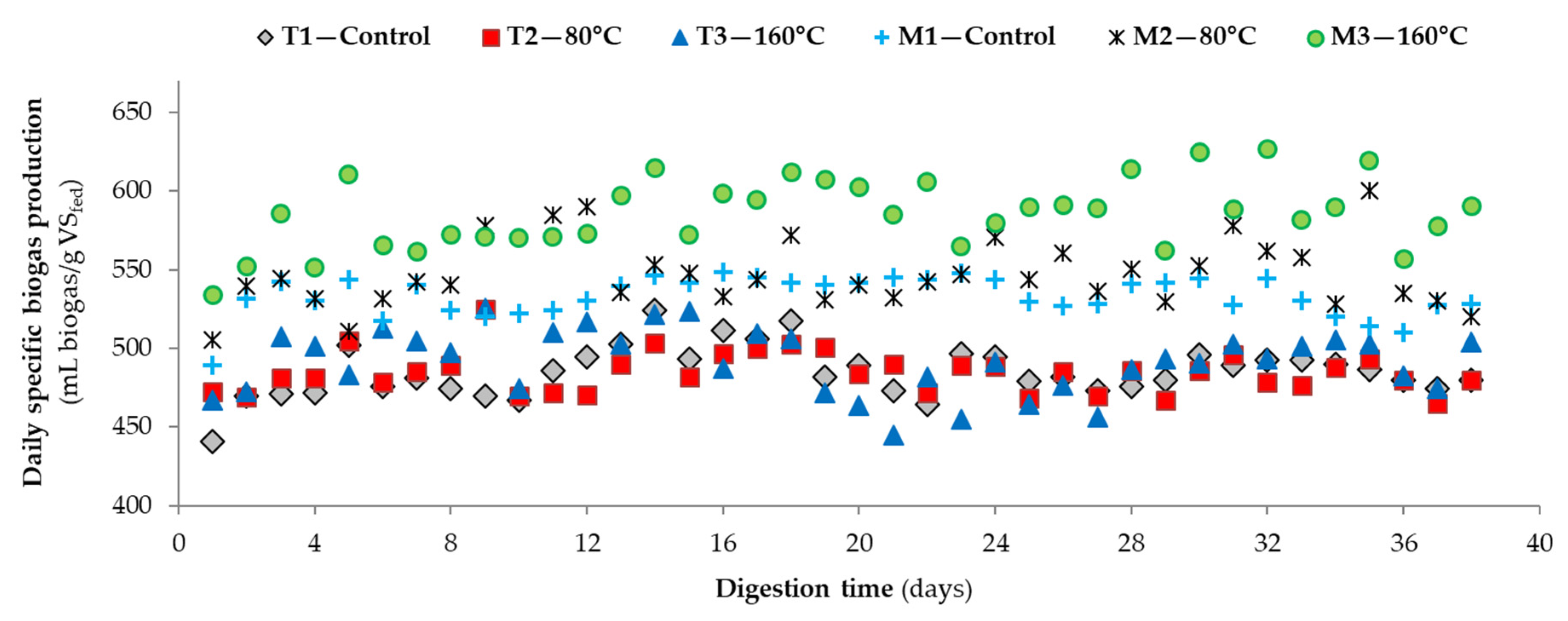
| 6Daily specific methane yield (mL CH4/g VSfed) | 319 ± 17 (32) | 317 ± 16 (32) | 318 ± 17 (32) | 346 ± 18 (32) | 369 ± 16 (32) | 384 ± 25 (32) |
| CH4 content in the biogas (%) | 65.3 ± 0.8 (4) | 65.4 ± 1.1 (4) | 64.9 ± 0.7 (4) | 65.8 ± 0.3 (4) | 66.9 ± 0.4 (4) | 65.1 ± 1.0 (4) |
| Digestate (Effluent) Characteristics | ||||||
| pH (-) | 8.0 ± 0.04 (32) | 7.8 ± 0.03 (32) | 7.7 ± 0.07 (32) | 7.4 ± 0.02 (32) | 7.3 ± 0.03 (32) | 7.4 ± 0.04 (32) |
| VS (% by wt.) | 1.64 ± 0.1 (9) | 1.46 ± 0.1 (9) | 1.37 ± 0.1 (9) | 1.87 ± 0.1 (9) | 1.63 ± 0.1 (9) | 1.45 ± 0.1 (9) |
| Digestate Supernatant Characteristics | ||||||
| Alkalinity (mg/L/% VS by wt. as CaCO3) | 2888 ± 109 (3) | 2440 ± 192 (3) | 2589 ± 392 (3) | 1990 ± 164 (3) | 1850 ± 84 (3) | 2220 ± 275 (3) |
| Ammonia (mg N/L/% VS by wt.) | 781 ± 32 (3) | 709 ± 107 (3) | 798 ± 158 (3) | 538 ± 2.3 (3) | 504 ± 44 (3) | 636 ± 85 (3) |
| 7CODsoluble (mg/L/% VS by wt.) | 2474 ± 263 (5) | 2906 ± 175 (5) | 4535 ± 687 (5) | 515 ± 74 (5) | 488 ± 50 (5) | 1452 ± 179 (5) |
| 8Total 9VFAs (mg/L/%VS by wt.) | 607 ± 219 (5) | 1031 ± 253 (5) | 1341 ± 297 (5) | 27 ± 4 (5) | 25 ± 3 (5) | 104 ± 13 (5) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kor-Bicakci, G.; Ubay-Cokgor, E.; Eskicioglu, C. Comparative Analysis of Bacterial and Archaeal Community Structure in Microwave Pretreated Thermophilic and Mesophilic Anaerobic Digesters Utilizing Mixed Sludge under Organic Overloading. Water 2020, 12, 887. https://doi.org/10.3390/w12030887
Kor-Bicakci G, Ubay-Cokgor E, Eskicioglu C. Comparative Analysis of Bacterial and Archaeal Community Structure in Microwave Pretreated Thermophilic and Mesophilic Anaerobic Digesters Utilizing Mixed Sludge under Organic Overloading. Water. 2020; 12(3):887. https://doi.org/10.3390/w12030887
Chicago/Turabian StyleKor-Bicakci, Gokce, Emine Ubay-Cokgor, and Cigdem Eskicioglu. 2020. "Comparative Analysis of Bacterial and Archaeal Community Structure in Microwave Pretreated Thermophilic and Mesophilic Anaerobic Digesters Utilizing Mixed Sludge under Organic Overloading" Water 12, no. 3: 887. https://doi.org/10.3390/w12030887
APA StyleKor-Bicakci, G., Ubay-Cokgor, E., & Eskicioglu, C. (2020). Comparative Analysis of Bacterial and Archaeal Community Structure in Microwave Pretreated Thermophilic and Mesophilic Anaerobic Digesters Utilizing Mixed Sludge under Organic Overloading. Water, 12(3), 887. https://doi.org/10.3390/w12030887




