Factors Influencing the Concentration of Fecal Coliforms in Oysters in the River Blackwater Estuary, UK
Abstract
:1. Introduction
2. Methods
2.1. Description of the Sampling Area
2.2. The Effect of Tidal Regime on E. coli and FS Numbers in Salcott Creek
2.3. The Effect of Distance from the Main Sewage Source on the Numbers of E. coli and FS in Salcott Creek
2.4. Twelve-Month Microbiological Study
2.5. Bacterial Analysis of Water
2.6. Bacterial Analysis of Oysters
2.7. Abiotic Measurements
2.8. Statistical Analysis
3. Results and Discussion
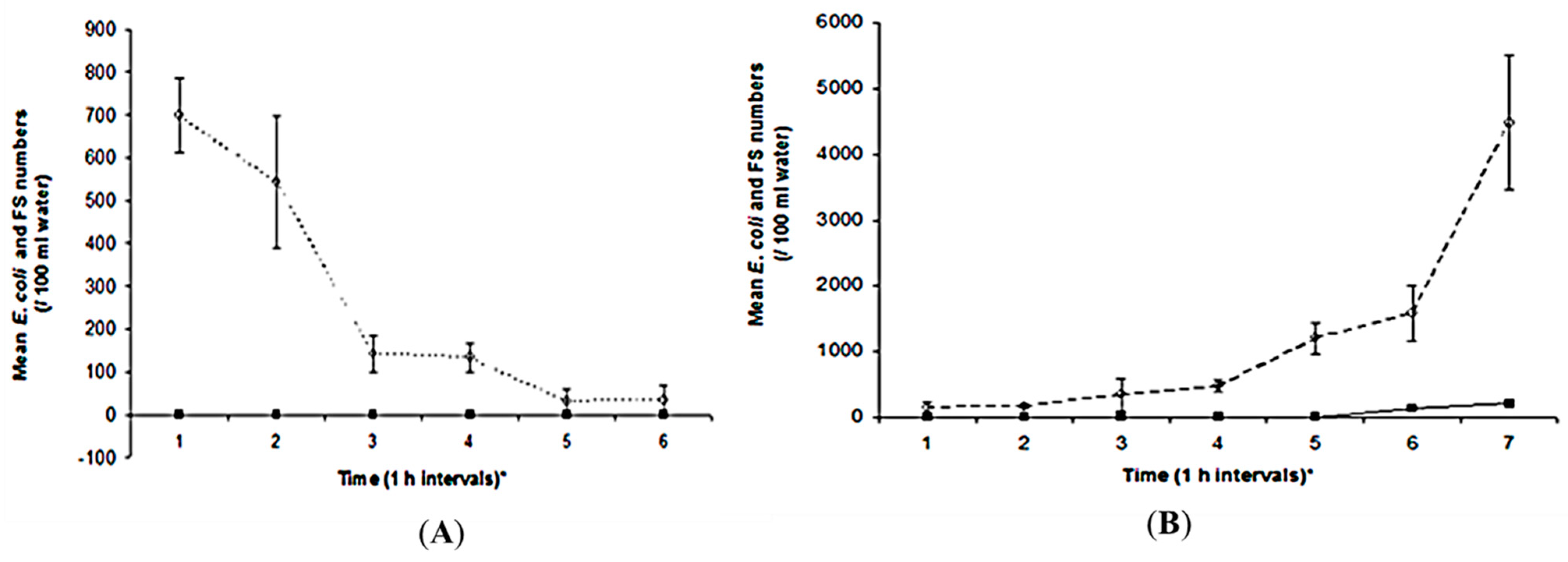
3.1. Assessment of the Tidal Effects on E. coli and FS Numbers in Waters Overlying the Oyster Bed (OB)
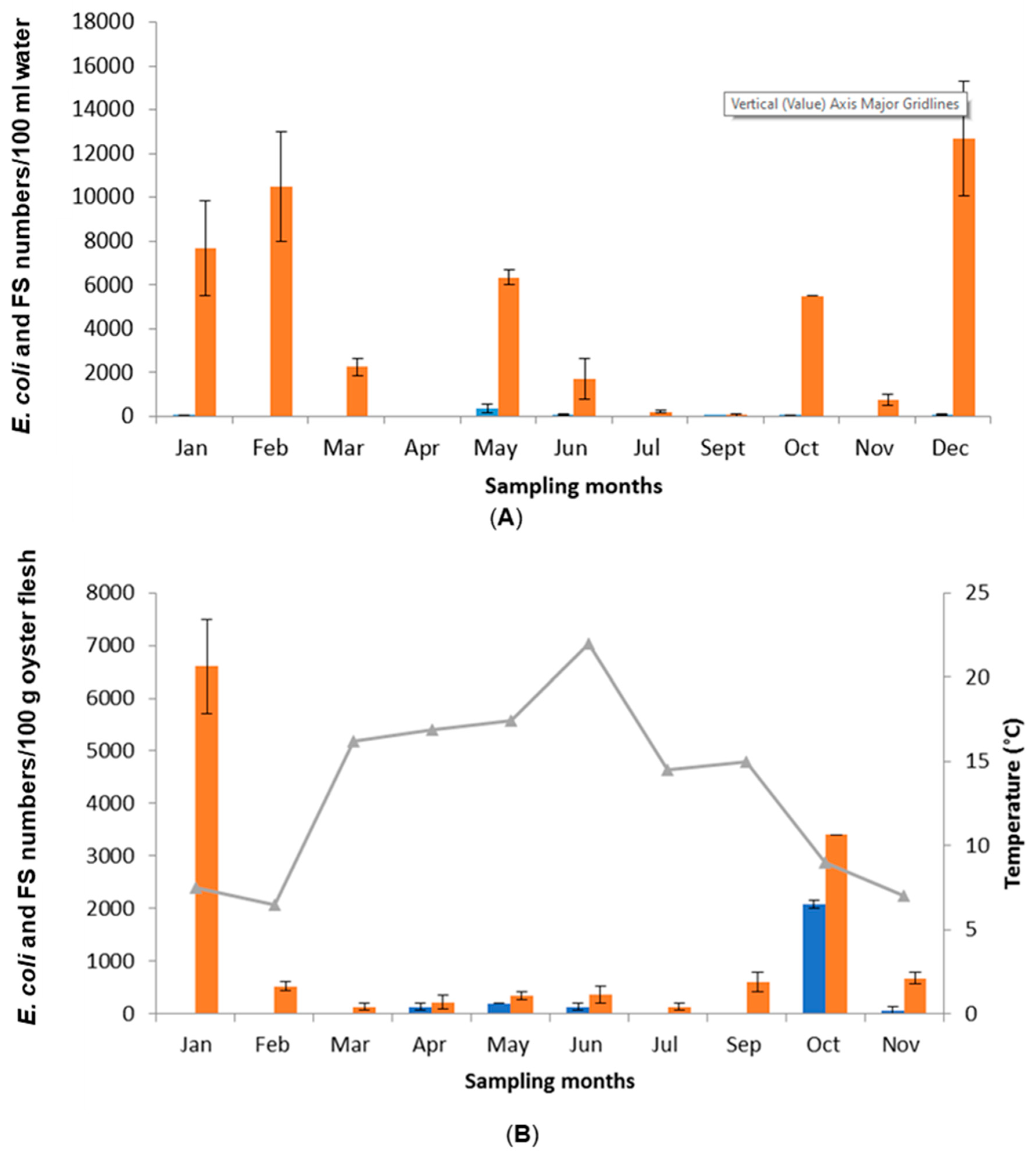
3.2. Seasonal Variations in Microbiological Quality of Water and Oyster Samples Collected from the Oyster Beds
3.3. Comparison of Fecal Indicator Numbers between the Different Inputs
3.4. The Effect of Distance from the Sewage Source on the Numbers of E. coli and FS in Salcott Creek
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sobsey, M.; Handzel, T.; Venczel, L. Chlorination and safe storage of household drinking water in developing countries to reduce waterborne disease. Water Sci. Technol. 2003, 47, 221–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riou, P.; Le Saux, J.; Dumas, F.; Caprais, M.; Le Guyader, S.; Pommepuy, M. Microbial impact of small tributaries on water and shellfish quality in shallow coastal areas. Water Res. 2007, 41, 2774–2786. [Google Scholar] [CrossRef] [Green Version]
- Scott, T.M.; Rose, J.B.; Jenkins, T.M.; Farrah, S.R.; Lukasik, J. Microbial Source Tracking: Current Methodology and Future Directions†. Appl. Environ. Microbiol. 2002, 68, 5796–5803. [Google Scholar] [CrossRef] [Green Version]
- Zimmer-Faust, A.G.; Brown, C.A.; Manderson, A. Statistical models of fecal coliform levels in Pacific Northwest estuaries for improved shellfish harvest area closure decision making. Mar. Pollut. Bull. 2018, 137, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Gallard, J.; Wu, B.; Harwood, V.J.; Sadowsky, M.J.; Hamilton, K.A.; Ahmed, W. Synergy between quantitative microbial source tracking (qMST) and quantitative microbial risk assessment (QMRA): A review and prospectus. Environ. Int. 2019, 130, 104703. [Google Scholar] [CrossRef] [PubMed]
- Jagals, P.; Grabow, W.O.K.; de Villiers, J.C. Evaluation of indicators for assessment of human and animal faecal pollution of surface run-off. Water Sci. Technol. 1995, 31, 235–241. [Google Scholar] [CrossRef]
- Donnison, A.M.; Ross, C.M. Animal and human faecal pollution in New Zealand rivers. N. Zeal. J. Mar. Freshw. Res. 1999, 33, 119–128. [Google Scholar] [CrossRef]
- Stelma, G.N.; McCabe, L.J. Nonpoint Pollution from Animal Sources and Shellfish Sanitation. J. Food Prot. 1992, 55, 649–656. [Google Scholar] [CrossRef]
- Blanch, A.R.; Belanche-Muñoz, L.; Bonjoch, X.; Ebdon, J.; Gantzer, C.; Lucena, F.; Ottoson, J.; Kourtis, C.; Iversen, A.; Kühn, I.; et al. Tracking the origin of faecal pollution in surface water: An ongoing project within the European Union research programme. J. Water Health 2004, 2, 249–260. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-Y.; Panicker, G.; Bej, A.K. Detection of pathogenic bacteria in shellfish using multiplex PCR followed by CovaLink™ NH microwell plate sandwich hybridization. J. Microbiol. Methods 2003, 53, 199–209. [Google Scholar] [CrossRef]
- Oliveira, J.; Cunha, Â.; Castilho, F.; Romalde, J.L.; Pereira, M.J. Microbial contamination and purification of bivalve shellfish: Crucial aspects in monitoring and future perspectives—A mini-review. Food Control. 2011, 22, 805–816. [Google Scholar] [CrossRef]
- Godfree, A.F.; Kay, D.; Wyer, M. Faecal streptococci as indicators of faecal contamination in water. J. Appl. Microbiol. 1997, 83, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, C.M.; Wyer, M.D.; Kay, D.; Crowther, J.; McDonald, A.T.; Walters, M.; Gawler, A.; Hindle, T. Microbial source tracking: a forensic technique for microbial source identification? J. Environ. Monit. 2007, 9, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Teplitski, M.; Wright, A.C.; Lorca, G. Biological approaches for controlling shellfish-associated pathogens. Curr. Opin. Biotechnol. 2009, 20, 185–190. [Google Scholar] [CrossRef]
- European Communities. Commission Regulation (EC) No 2074/2005 of 5 December 2005 laying down implementing measures for certain products under Regulation (EC) No 853/2004 of the European Parliament and of the Council and for the organisation of official controls under Regulation. Off. J. Eur. Communities 2004, L338, 27–59. [Google Scholar]
- Lee, R.; Morgan, O. Environmental factors influencing the microbiological contamination of commercially harvested shellfish. Water Sci. Technol. 2003, 47, 65–70. [Google Scholar] [CrossRef]
- Almeida, A.; Soares, F. Microbiological monitoring of bivalves from the Ria Formosa Lagoon (south coast of Portugal): A 20years of sanitary survey. Mar. Pollut. Bull. 2012, 64, 252–262. [Google Scholar] [CrossRef]
- Maldon District Council and Colchester Borough Council. Blackwater Estuary Management Plan; Maldon District Council: Maldon, UK; Colchester Borough Council: Colchester, UK, 1996. [Google Scholar]
- Erkenbrecher, C.W. Sediment bacterial indicators in an urban shellfishing subestuary of the lower Chesapeake Bay. Appl. Environ. Microbiol. 1981, 42, 484–492. [Google Scholar] [CrossRef] [Green Version]
- Hughes, K.A. Influence of Seasonal Environmental Variables on the Distribution of Presumptive Fecal Coliforms around an Antarctic Research Station. Appl. Environ. Microbiol. 2003, 69, 4884–4891. [Google Scholar] [CrossRef] [Green Version]
- Zaccone, R.; Mancuso, M.; Modica, A.; Zampino, D.C. Microbiological indicators for aquaculture impact in Mar Piccolo (Taranto, Italy). Aquac. Int. 2005, 13, 167–173. [Google Scholar] [CrossRef]
- Chandran, A.; Hatha, A.M. Relative survival of Escherichia coli and Salmonella typhimurium in a tropical estuary. Water Res. 2005, 39, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Kucuksezgin, F.; Kacar, A.; Kucuksezgin, G.; Uluturhan, E. Monitoring metal contamination levels and fecal pollution in clam (Tapes decussatus) collected from Izmir Bay (Turkey). Environ. Monit. Assess. 2009, 162, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Sasikumar, G.; Krishnamoorthy, M. Faecal indicators and sanitary water quality of shellfish-harvesting environment: influences of seasonal monsoon and river-runoff. Indian J. Mar. Sci. 2010, 39, 434–444. [Google Scholar]
- Chinnadurai, S.; Mohamed, K.; Sharma, J.; Venkatesan, V.; Kripa, V. Assessment of bio-accumulation of bacteria in oysters from shellfish growing waters in Ashtamudi Lake (Kerala, India): A RAMSAR wetland. Reg. Stud. Mar. Sci. 2016, 7, 118–122. [Google Scholar] [CrossRef] [Green Version]
- Kay, D.; Kershaw, S.; Lee, R.; Wyer, M.D.; Watkins, J.; Francis, C. Results of field investigations into the impact of intermittent sewage discharges on the microbiological quality of wild mussels (Mytilus edulis) in a tidal estuary. Water Res. 2008, 42, 3033–3046. [Google Scholar] [CrossRef]
- Clements, K.; Quilliam, R.; Jone, D.; Wilson, J.; Malham, S. Spatial and temporal heterogeneity of bacteria across an intertidal shellfish bed: Implications for regulatory monitoring of faecal indicator organisms. Sci. Total. Environ. 2015, 506, 1–9. [Google Scholar] [CrossRef]
- Pourcher, A.-M.; Devriese, L.; Hernandez, J.; Delattre, J. Enumeration by a miniaturized method ofEscherichia coli, Streptococcus bovisand enterococci as indicators of the origin of faecal pollution of waters. J. Appl. Bacteriol. 1991, 70, 525–530. [Google Scholar] [CrossRef]
- Sinton, L.W.; Finlay, R.K.; Hannah, D.J. Distinguishing human from animal faecal contamination in water: A review. N. Z. J. Mar. Freshw. Res. 1998, 32, 323–348. [Google Scholar] [CrossRef] [Green Version]
- Geldreich, E.E.; Kenner, B.A. Concepts of fecal streptococci in stream pollution. J. Water Pollut. Control. Fed. 1969, 41, 336. [Google Scholar]
- Geldreich, E.E.; Litsky, W. Fecal coliform and fecal streptococcus density relationships in waste discharges and receiving waters. CRC Crit. Rev. Environ. Control. 1976, 6, 349–369. [Google Scholar] [CrossRef]
- Wu, J.-Y. Linking landscape patterns to sources of water contamination: Implications for tracking fecal contaminants with geospatial and Bayesian approaches. Sci. Total. Environ. 2019, 650, 1149–1157. [Google Scholar] [CrossRef] [PubMed]



| Category | Criteria | Treatment |
| Class A | Mollusks must contain less than 230 E. coli per 100 grams of flesh | Can be harvested for direct human consumption |
| Class B | 90% of sampled mollusks must contain less than 4600 E. coli per 100 grams of flesh; 10% of samples must not exceed 46,000 E. coli per 100 grams of flesh | Can go for human consumption after purification in an approved plant or after relaying in an approved Class A relaying area or after an EC approved heat treatment process |
| Class C | Mollusks must contain less than 46,000 E. coli per 100 grams of flesh | Can go for human consumption only after relaying for at least two months in an approved relaying area followed, where necessary, by treatment in a purification center or after an EC approved heat treatment process |
| Prohibited Area | Above 46,000 E. coli per 100 grams of flesh | Must not be harvested or offered for human consumption |
| SITE NO | SITE NAME | SOURCE | pH | Salinity | COMMENTS |
|---|---|---|---|---|---|
| 1 | Main Channel | Water | 7.2–8.5 | 0 | |
| 2 | Quinces Corner input (QC) | Water | 7.2–8.5 | 10–30 ppt on low tide and between 30 and 32 on high tide | Water originates from adjacent farmland (grazed by sheep) and also from surrounding fields where large flocks of Brent geese rest, especially during the winter period |
| 3 | Salcott STW (outlet pipe) | Water | 7.2–8.5 | 0 | Salcott Sewage treatment work (STW) outlet pipe. Salcott STW receives secondary treatment (conventional primary tanks with trickling filter and settlement tank as secondary treatment). |
| 4 | Stream | Water | 7.2–8.5 | 0 | Water from pastureland drains here via a tidal sluice |
| 5 | Virley (Tiptree STW) | Water | 7.2–8.5 | 0 | Water at this site originates from Tiptree STW. Sewage effluent from Tiptree STW is piped for approximately 3.5 km and then discharged at Virley Brook. |
| 6 | Oyster Beds (OB) | Water, oysters | 7.2–8.5 | 22–34 ppt during low tide and from 32–35 ppt during high tide |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florini, S.; Shahsavari, E.; Ngo, T.; Aburto-Medina, A.; Smith, D.J.; Ball, A.S. Factors Influencing the Concentration of Fecal Coliforms in Oysters in the River Blackwater Estuary, UK. Water 2020, 12, 1086. https://doi.org/10.3390/w12041086
Florini S, Shahsavari E, Ngo T, Aburto-Medina A, Smith DJ, Ball AS. Factors Influencing the Concentration of Fecal Coliforms in Oysters in the River Blackwater Estuary, UK. Water. 2020; 12(4):1086. https://doi.org/10.3390/w12041086
Chicago/Turabian StyleFlorini, Styliani, Esmaeil Shahsavari, Tien Ngo, Arturo Aburto-Medina, David J. Smith, and Andrew S Ball. 2020. "Factors Influencing the Concentration of Fecal Coliforms in Oysters in the River Blackwater Estuary, UK" Water 12, no. 4: 1086. https://doi.org/10.3390/w12041086
APA StyleFlorini, S., Shahsavari, E., Ngo, T., Aburto-Medina, A., Smith, D. J., & Ball, A. S. (2020). Factors Influencing the Concentration of Fecal Coliforms in Oysters in the River Blackwater Estuary, UK. Water, 12(4), 1086. https://doi.org/10.3390/w12041086





