Innovative Feasibility Study for the Reclamation of the Cascajo Wetlands in Peru Utilizing Sustainable Technologies
Abstract
:1. Introduction
2. Materials and Methods
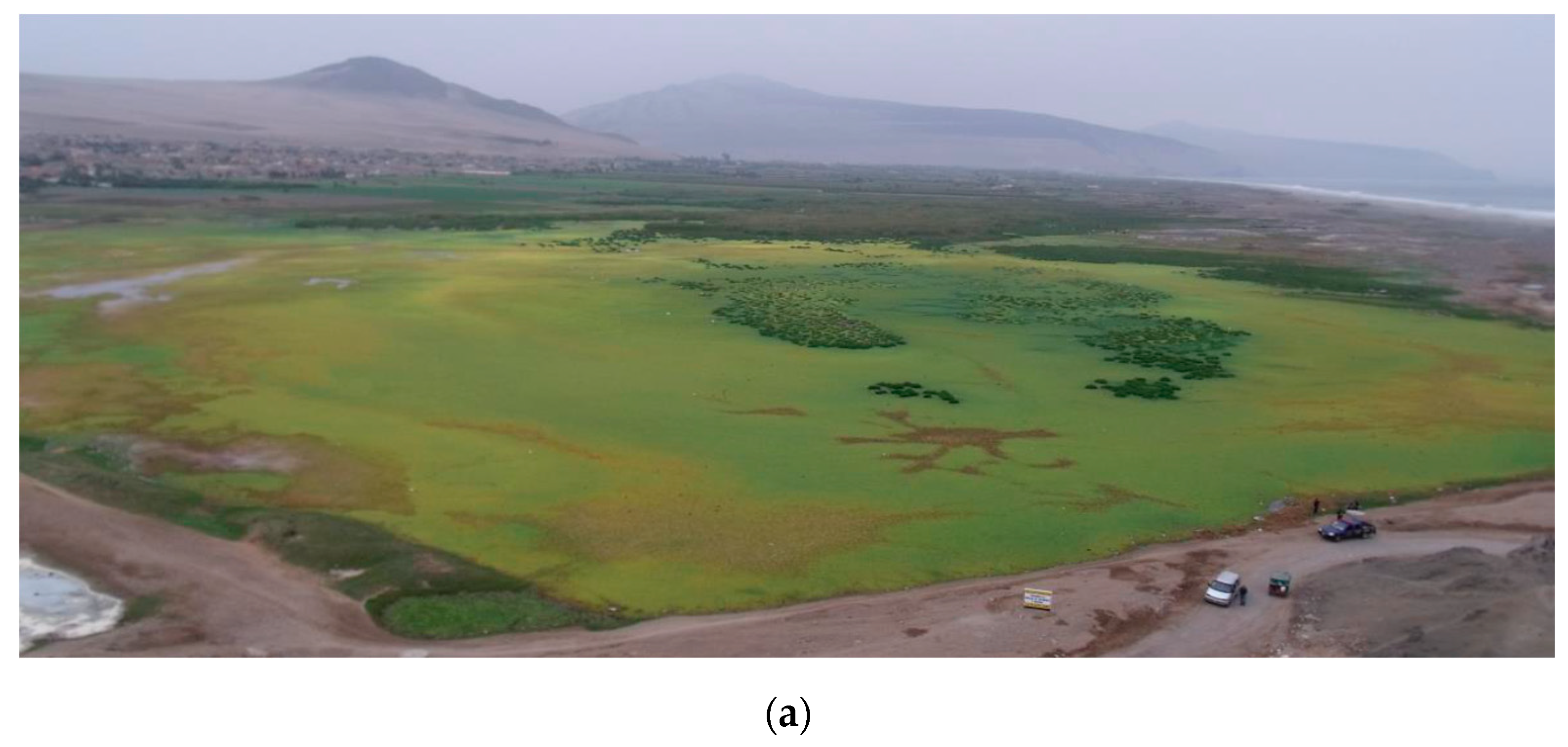
2.1. Study Site
2.2. Removal of Invasive Species from Wetlands
2.3. Micro-Nano Bubbles (MNBs)
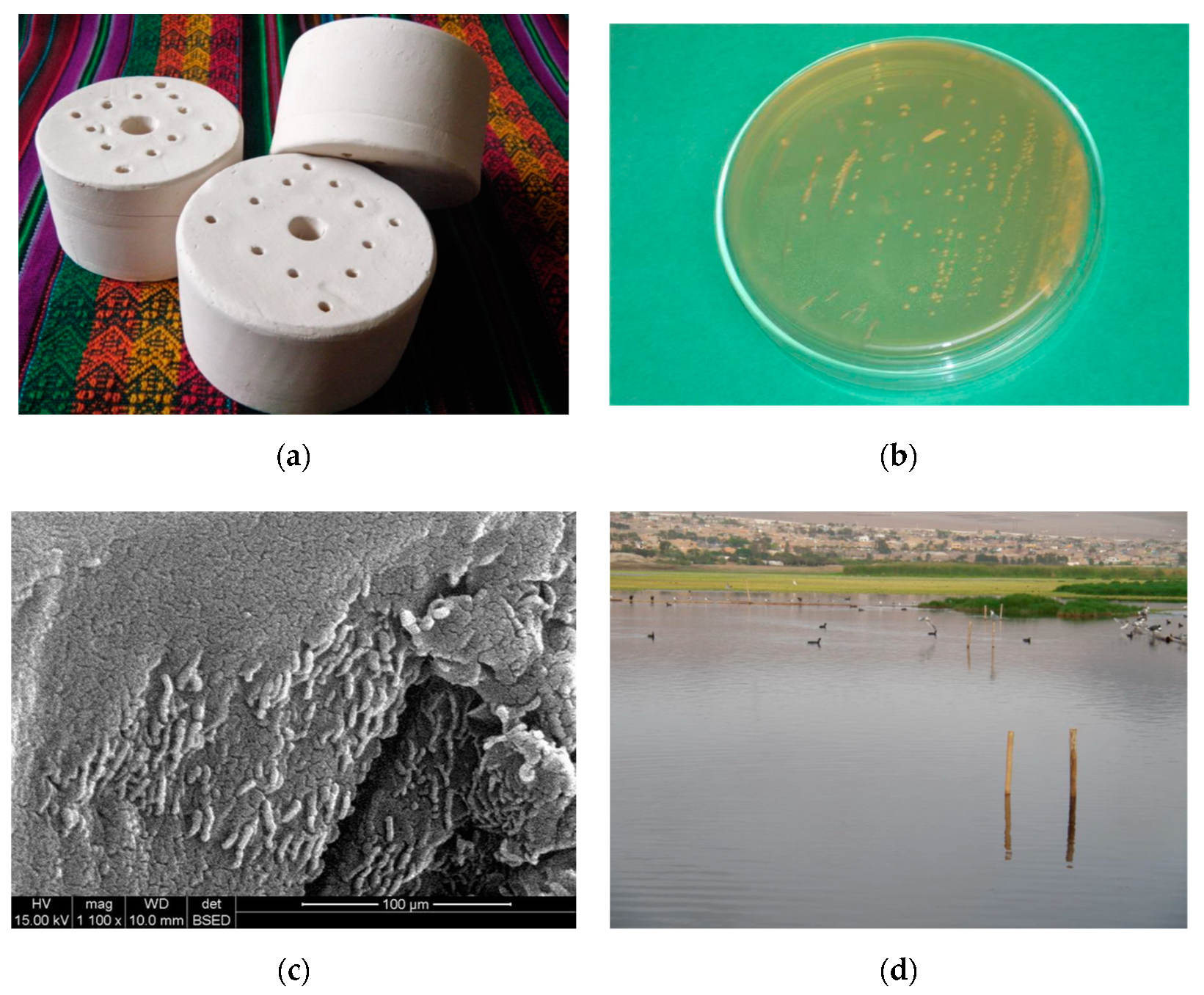
2.4. Ceramic-Based Bio-Filters (CBBFs)
2.5. Bio-Fence Preparation
2.6. Sampling
2.7. Remote Sensing
2.8. Statistical Analysis
3. Results and Discussion
3.1. The Effect of MNBs
3.2. The Effect of CBBFs
3.3. The Effect of Bio-fence
3.4. Remote Sensing and Correlation Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cohen-Shacham, E.; Andrade, A.; Dalton, J.; Dudley, N.; Jones, M.; Kumar, C.; Maginnis, S.; Maynard, S.; Nelson, C.R.; Renaud, F.G.; et al. Core Principles for Successfully Implementing and Upscaling Nature-Based Solutions. Environ. Sci. Policy 2019, 98, 20–29. [Google Scholar] [CrossRef]
- Dushkova, D.; Haase, D. Not Simply Green: Nature-Based Solutions as a Concept and Practical Approach for Sustainability Studies and Planning Agendas in Cities. Land 2020, 9, 19. [Google Scholar] [CrossRef] [Green Version]
- Kabisch, N.; Bosch, M.V.D.; Lafortezza, R. The Health Benefits of Nature-Based Solutions to Urbanization Challenges for Children and the Elderly—A Systematic Review. Environ. Res. 2017, 159, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Nesshöver, C.; Assmuth, T.; Irvine, K.; Rusch, G.; Waylen, K.; Delbaere, B.; Haase, D.; Jones-Walters, L.; Keune, H.; Kovács, E.; et al. The Science, Policy and Practice of Nature-Based Solutions: An Interdisciplinary Perspective. Sci. Total. Environ. 2017, 579, 1215–1227. [Google Scholar] [CrossRef] [PubMed]
- Albert, C.; Schröter, B.; Haase, D.; Brillinger, M.; Henze, J.; Herrmann, S.; Gottwald, S.; Guerrero, P.; Nicolas, C.; Matzdorf, B. Addressing Societal Challenges through Nature-Based Solutions: How Can Landscape Planning and Governance Research Contribute? Landsc. Urban Plan. 2019, 182, 12–21. [Google Scholar] [CrossRef]
- Sang, N. (Ed.) Modelling Nature-Based Solutions: Integrating Computational and Participatory Scenario Modelling for Environmental Management and Planning; Cambridge University Press: Cambridge, UK, 2020. [Google Scholar]
- Hanson, H.I.; Wickenberg, B.; Olsson, J.A. Working on the Boundaries—How Do Science Use and Interpret the Nature-Based Solution Concept? Land Use Policy 2020, 90, 104302. [Google Scholar] [CrossRef]
- Thorslund, J.; Destouni, G.; Jaramillo, F.; Jawitz, J.; Manzoni, S.; Basu, N.B.; Chalov, S.R.; Cohen, M.J.; Creed, I.F.; Goldenberg, R.; et al. Wetlands as Large-Scale Nature-Based Solutions: Status and Challenges for Research, Engineering and Management. Ecol. Eng. 2017, 108, 489–497. [Google Scholar] [CrossRef]
- Pauleit, S.; Zölch, T.; Hansen, R.; Randrup, T.B.; Konijnendijk van den Bosch, C. Nature-Based Solutions and Climate Change—Four Shades of Green. In Nature-Based Solutions to Climate Change Adaptation in Urban Areas. Theory and Practice of Urban Sustainability Transitions; Kabisch, N., Korn, H., Stadler, J., Bonn, A., Eds.; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Hu, T.; Liu, J.; Zheng, G.; Li, Y.; Xie, B. Quantitative Assessment of Urban Wetland Dynamics Using High Spatial Resolution Satellite Imagery between 2000 and 2013. Sci. Rep. 2018, 8, 7409. [Google Scholar] [CrossRef]
- Hook, D.D. Wetlands: History, Current Status, and Future. Environ. Environ. Toxicol. Chem. 1993, 12, 2157–2166. [Google Scholar] [CrossRef]
- Bai, J.; Huang, L.; Gao, H.; Zhang, G. Wetland Biogeochemistry and Ecological Risk Assessment. Phys. Chem. Earth 2017, 97, 1–2. [Google Scholar] [CrossRef]
- Guzy, J.C.; Mccoy, E.D.; Deyle, A.C.; Gonzalez, S.M.; Halstead, N.; Mushinsky, H.R. Urbanization Interferes with the Use of Amphibians as Indicators of Ecological Integrity of Wetlands. J. Appl. Ecol. 2012, 49, 941–952. [Google Scholar] [CrossRef]
- Mutiti, S.; Sadowski, H.; Melvin, C.; Mutiti, C. Effectiveness of Man-Made Wetland Systems in Filtering Contaminants from Urban Runoff in Milledgeville, Georgia. Water Environ. Res. 2015, 87, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.L.; Ficken, C.; Eken, M.; Hendrickson, J.; Beeri, O. Wetland Soil Carbon in a Watershed Context for the Prairie Pothole Region. J. Environ. Qual. 2016, 45, 368–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Withey, P.; van Kooten, G.C. The Effect of Climate Change on Optimal Wetlands and Waterfowl Management in Western Canada. Ecol. Econ. 2011, 70, 798–805. [Google Scholar] [CrossRef]
- Guttery, R.S.; Poe, S.L.; Sirmans, C.F. Federal Wetlands Regulation: Restrictions on the Nationwide Permit Program and the Implications for Residential Property Owners. Am. Bus. Law J. 2000, 37, 299–342. [Google Scholar] [CrossRef]
- Chen, H.; Zou, J.; Cui, J.; Nie, M.; Fang, C. Wetland Drying Increases the Temperature Sensitivity of Soil Respiration. Soil Biol. Biochem. 2018, 120, 24–27. [Google Scholar] [CrossRef]
- Guo, M.; Li, J.; Sheng, C.; Xu, J.; Wu, L. A Review of Wetland Remote Sensing. Sensors (Switzerland) 2017, 17, 777. [Google Scholar] [CrossRef] [Green Version]
- Vos, J.; Vincent, L. Volumetric Water Control in a Large-Scale Open Canal Irrigation System with Many Smallholders: The Case of Chancay-Lambayeque in Peru. Agric. Water Manag. 2011, 98, 705–714. [Google Scholar] [CrossRef]
- Boelens, R.; Vos, J. The Danger of Naturalizing Water Policy Concepts: Water Productivity and Efficiency Discourses from Field Irrigation to Virtual Water Trade. Agric. Water Manag. 2012, 108, 16–26. [Google Scholar] [CrossRef]
- Vera Delgado, J. The Socio-Cultural, Institutional and Gender Aspects of the Water Transfer-Agribusiness Model for Food and Water Security. Lessons Learned from Peru. Food Secur. 2015, 7, 1187–1197. [Google Scholar] [CrossRef]
- The World Bank—WB. Peru: Hydro-Economic Analysis and Prioritization of Water Resource Initiatives; The World Bank: Washington, DC, USA, 2015; Available online: https://www.2030wrg.org/wp-content/uploads/2015/05/2030-WRG_Peru-Final-Report_English.pdf (accessed on 1 December 2019).
- Aponte, H.; Jiménez, R.; Alcántara, B. Challenges for Management and Conservation of Santa Rosa Wetland Peru. Científica 2012, 9, 1–9. [Google Scholar]
- Cano, A. «Top-Down» or «Bottom-Up»? Social Participation, Agriculture and Mining in the Integrated Management of the Chancay-Lambayeque Watershed. Apuntes 2013, 73, 43–76. [Google Scholar] [CrossRef]
- ECLAC/OECD. Environmental Performance Reviews: Peru, 2016, Highlights and recommendations. Available online: https://repositorio.cepal.org/bitstream/handle/11362/40172/1/S1600312_en.pdf (accessed on 11 April 2020).
- Aponte, H.; Ramírez, D. Humedales De La Costa Central Del Perú: Estructura Y Amenazas De Sus Comunidades Vegetales. Ecol. Apl. 2011, 10, 2011. [Google Scholar]
- Ramirez, D.W.; Aponte, H.; Cano, A. Flora Vascular y Vegetación Del Humedal de Santa Rosa (Chancay, Lima). Rev. Peru. Biol. 2011, 17, 105–110. [Google Scholar] [CrossRef]
- Burkett, V.; Kusler, J. Climate Change: Potential Impacts and Interactions in Wetlands of the United States. J. Am. Water Resour. Assoc. 2000, 36, 313–320. [Google Scholar] [CrossRef]
- Park, J.B.K.; Rupert, J.; Craggs, J.; Tanner, C.T. Eco-Friendly and Low-Cost Enhanced Pond and Wetland (EPW) System for the Treatment of Secondary Wastewater Effluent. Ecol. Eng. 2018, 120, 170–179. [Google Scholar] [CrossRef]
- Osland, M.J.; Enwright, N.M.; Day, R.H.; Gabler, C.A.; Camille, L.; Stagg, C.L.; Grace, J.B. Beyond Just Sea-Level Rise: Considering Macroclimatic Drivers within Coastal Wetland Vulnerability Assessments to Climate Change. Glob. Chang. Biol. 2015, 22, 1–11. [Google Scholar] [CrossRef]
- Lavado-Casimiro, W.; Espinoza, J.C. Impactos de El Niño y La Niña En Las Lluvias Del Perú (1965–2007). Rev. Bras. Meteorol. 2014, 29, 171–182. [Google Scholar] [CrossRef] [Green Version]
- Rau, P.; Bourrel, L.; Labat, D.; Frappart, F.; Ruelland, D.; Lavado, W.; Dewitte, B.; Felipe, O. Hydroclimatic Change Disparity of Peruvian Pacific Drainage Catchments. Theor. Appl. Clim. 2017, 134, 139–153. [Google Scholar] [CrossRef]
- Day, J.W.; Alejandro Yañéz, A.; Mitsch William, J.; Ana Laura, L.-D.; Day, J.N.; Jae, Y.K.; Robert, L.; Joel, L.; David, Z.L. Using Ecotechnology to Address Water Quality and Wetland Habitat Loss Problems in the Mississippi Basin: A Hierarchical Approach. Biotechnol. Adv. 2003, 22, 135–159. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, M.-Y.; Zeng, G.-M.; Yu, Z.; Cui, F.; Yang, Z.-Z.; Shen, L.-Q. Active Capping Technology: A New Environmental Remediation of Contaminated Sediment. Environ. Sci. Pollut. Res. 2016, 23, 4370–4386. [Google Scholar] [CrossRef] [PubMed]
- Eckley, C.S.; Gilmour, C.C.; Janssen, S.; Luxton, T.P.; Randall, P.M.; Whalin, L.; Austin, C. The Assessment and Remediation of Mercury Contaminated Sites: A Review of Current Approaches. Sci. Total. Environ. 2020, 707, 136031. [Google Scholar] [CrossRef] [PubMed]
- Gensemer, R.W.; Playle. R.C. Critical Reviews in Environmental Science and Technology: The Bioavailability and Toxicity of Aluminum in Aquatic Environments The Bioavailability and Toxicity of Aluminum in Aquatic Environments. Crit. Rev. Environ. Sci. Technol. 1999, 294, 37–41. [Google Scholar] [CrossRef]
- Ayeni, O.; Kambizi, L.; Laubscher, C.; Fatoki, O.S.; Olatunji, O. Risk Assessment of Wetland under Aluminium and Iron Toxicities: A Review. Aquat. Ecosyst. Heal. Manag. 2014, 17, 122–128. [Google Scholar] [CrossRef]
- Da Silva, M.B.; Abrantes, N.; Nogueira, V.; Gonçalves, F.J.M.; Pereira, R. TiO2 Nanoparticles for the Remediation of Eutrophic Shallow Freshwater Systems: Efficiency and Impacts on Aquatic Biota under a Microcosm Experiment. Aquat. Toxicol. 2016, 178, 58–71. [Google Scholar] [CrossRef]
- Pester, M.; Knorr, K.-H.; Friedrich, M.W.; Wagner, M.; Loy, A. Sulfate-Reducing Microorganisms in Wetlands—Fameless Actors in Carbon Cycling and Climate Change. Front. Microbiol. 2012, 3, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Lamia, A.; Hamdi, M. Fermentative Decolorization of Olive Mill Wastewater by Lactobacillus Plantarum. Process. Biochem. 2003, 39, 59–65. [Google Scholar] [CrossRef]
- Golalikhani, M.; Razavi, S.H. An Efficient Biological Treatment on Dairy Wastewater by Lactobacillus Plantarum: Mathematical Modeling and Process Parameters Optimization. Int. J. Food Eng. 2016, 12, 63–73. [Google Scholar] [CrossRef]
- Guerra, F.D.; Attia, M.F.; Whitehead, D.C.; Alexis, F. Nanotechnology for Environmental Remediation: Materials and Applications. Molecules (Basel Switzerland) 2018, 23, 1760. [Google Scholar] [CrossRef] [Green Version]
- Dewidar, K.; Khedr, A. Water Quality Assessment with Simultaneous Landsat-5 TM at Manzala Lagoon, Egypt. Hydrobiologia 2001, 457, 49–58. [Google Scholar] [CrossRef]
- Xu, P.; Niu, Z.; Tang, P. Comparison and Assessment of NDVI Time Series for Seasonal Wetland Classification. Int. J. Digit. Earth 2017, 11, 1103–1131. [Google Scholar] [CrossRef]
- Arana, C. Relaciones Fitogeograficas de La Flora Vascular de Los Pantanos de Villa. In Los Pantanos de Villa: Biología y Conservación; Cano, A., Young, K.R., Eds.; Museo de Historia Natural-UNMSM: Lima, Peru, 2016; Volume 11, pp. 163–179. (In Spanish) [Google Scholar]
- Sridhar, M.K.C. Trace Elements. Acts Hydrochim. Hydrobiol. 1998, 16, 293–297. [Google Scholar] [CrossRef]
- Chan, K.S.; Page, R.A. Creep Damage Development in Structural Ceramics. J. Am. Ceram. Soc. 1993, 76, 803–826. [Google Scholar] [CrossRef]
- Bovea, M.D.; Saura, Ú.; Ferrero, J.L.; Giner, J. Cradle-to-Gate Study of Red Clay for Use in the Ceramic Industry. Int. J. Life Cycle Assess. 2006, 12, 439–447. [Google Scholar] [CrossRef]
- Raj, R. Fundamental Research in Structural Ceramics for Service Near 2000 °C. J. Am. Ceram. Soc. 1993, 76, 2147–2174. [Google Scholar] [CrossRef]
- Fadda, S.; Sanz, Y.; Vignolo, G.; Aristoy, M.C.; Oliver, G.; Toldrá, F. Hydrolysis of Pork Muscle Sarcoplasmic Proteins by Lactobacillus Curvatus and Lactobacillus Sake. Appl. Environ. Microbiol. 1999, 65, 578–584. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.L.; Deletic, A.; Alcazar, L.; Bratieres, K.; Fletcher, T.D.; McCarthy, D.T. Removal of Clostridium Perfringens, Escherichia Coli and F-RNA Coliphages by Stormwater Biofilters. Ecol. Eng. 2012, 49, 137–145. [Google Scholar] [CrossRef]
- Briggiler, M.M.; Reinheimer, J.; Quiberoni, A. Phage Adsorption and Lytic Propagation in Lactobacillus Plantarum: Could Host Cell Starvation Affect Them? BMC Microbiol. 2015, 15, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Carranzo, I.V.; APHA; AWWA; WEF. Standard Methods for Examination of Water and Wastewater. An. Hidrol. Médica 2012, 5, 185–186. [Google Scholar] [CrossRef]
- Landsat Algorithms. Available online: https://developers.google.com/earth-engine/landsat (accessed on 1 December 2019).
- Chander, G.; Markham, B.L.; Helder, D.L. Summary of Current Radiometric Calibration Coefficients for Landsat MSS, TM, ETM+, and EO-1 ALI Sensors. Remote. Sens. Environ. 2009, 113, 893–903. [Google Scholar] [CrossRef]
- Huete, A.R.; Didan, K.; Miura, T.; Rodriguez, E.; Gao, X.; Ferreira, L. Overview of the Radiometric and Biophysical Performance of the MODIS Vegetation Indices. Remote. Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- Landsat 7 Collection 1 Tier 1 8-Day EVI Composite. Available online: https://developers.google.com/earth-engine/datasets/catalog/LANDSAT_LE07_C01_T1_8DAY_EVI (accessed on 1 December 2019).
- Wang, X.; Ma, T. Application of Remote Sensing Techniques in Monitoring and Assessing the Water Quality of Taihu Lake. Bull. Environ. Contam. Toxicol. 2001, 67, 863–870. [Google Scholar] [CrossRef]
- El-Zeiny, A.; El-Kafrawy, S. Assessment of water pollution induced by human activities in Burullus Lake using Landsat 8 operational land imager and GIS. Egypt. J. Remote Sens. Space Sci. 2017, 20, S49–S56. [Google Scholar] [CrossRef] [Green Version]
- World Bank. Peru—Integrated Water Resources Management in Ten Basins Project; World Bank: Washington, DC, USA, 2014. [Google Scholar]
- Verones, F.; Bartl, K.; Pfister, S.; Jiménez Vílchez, R.; Hellweg, S. Modeling the local biodiversity impacts of agricultural water use: case study of a wetland in the coastal arid area of Peru. Environ. Sci. Technol. 2012, 1, 4966–4974. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Wang, J.; Chen, J.; Guo, J.; Yang, H.; Chen, Q. Successful Control of Phosphorus Release from Sediments Using Oxygen Nano-Bubble-Modified Minerals. Sci. Total. Environ. 2019, 663, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Meegoda, J.N.; Aluthgun Hewage, S.; Batagoda, J.H. Stability of Nanobubbles. Environ. Eng. Sci. 2018, 35, 1216–1227. [Google Scholar] [CrossRef]
- Siracusa, G.; La Rosa, A.D. Design of a Constructed Wetland for Wastewater Treatment in a Sicilian Town and Environmental Evaluation Using the Emergy Analysis. Ecol. Model. 2006, 197, 490–497. [Google Scholar] [CrossRef]
- Davidson, J.; Helwig, N.; Summerfelt, S.T. Fluidized Sand Biofilters Used to Remove Ammonia, Biochemical Oxygen Demand, Total Coliform Bacteria, and Suspended Solids from an Intensive Aquaculture Effluent. Aquac. Eng. 2008, 39, 6–15. [Google Scholar] [CrossRef] [Green Version]
- Verma, M.; Brar, S.K.; Tyagi, R.D.; Surampalli, R.Y.; Valéro, J.R. Starch Industry Wastewater as a Substrate for Antagonist, Trichoderma Viride Production. Bioresour. Technol. 2007, 98, 2154–2162. [Google Scholar] [CrossRef]
- Yildiz, B.S. Water and Wastewater Treatment: Biological Processes. Metrop. Sustain. Underst. Improv. Urban Environ. 2012, 406–428. [Google Scholar] [CrossRef]
- Mahlangu, T.O.; Mamba, B.B.; Momba, M.N.B. A Comparative Assessment of Chemical Contaminant Removal by Three Household Water Treatment Filters. Water SA 2012, 38, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Tahsin, S.; Medeiros, S.C.; Singh, A. Assessing the Resilience of Coastalwetlands to Extreme Hydrologic Events Using Vegetation Indices: A Review. Remote. Sens. 2018, 10, 1390. [Google Scholar] [CrossRef] [Green Version]
- Walter, M.; Mondal, P. A Rapidly Assessedwetland Stress Index (RAWSI) Using Landsat 8 and Sentinel-1 Radar Data. Remote. Sens. 2019, 11, 2549. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, R.; Sahana, M.; Sajjad, H. Preparing Turbidity and Aquatic Vegetation Inventory for Waterlogged Wetlands in Lower Barpani Sub-Watersheds (Assam), India Using Geospatial Technology. Egypt. J. Remote Sens. Sp. Sci. 2017, 20, 243–249. [Google Scholar] [CrossRef] [Green Version]
- Dong, Z.; Wang, Z.; Liu, D.; Song, K.; Li, L.; Jia, M.; Ding, Z. Mapping Wetland Areas Using Landsat-Derived NDVI and LSWI: A Case Study of West Songnen Plain, Northeast China. J. Indian Soc. Remote. Sens. 2014, 42, 569–576. [Google Scholar] [CrossRef]
- Michishita, R.; Gong, P.; Xu, B. Spectral mixture analysis for bi-sensor wetland mapping using Landsat TM and Terra MODIS data. Int. J. Remote. Sens. 2011, 33, 3373–3401. [Google Scholar] [CrossRef]
- Barrett, D.C.; Frazier, A.E. Automated method for monitoring water quality using landsat imagery. Water (Switzerland) 2016, 8, 257. [Google Scholar] [CrossRef] [Green Version]
- Alka, S.; Sushma, P.; Singh, T.S.; Patel, J.G.; Tanwar, H. Wetland Information System Using Remote Sensing and GIS in Himachal Pradesh, India. Asian J. Geoinf. 2014, 14, 13–22. [Google Scholar]
- Morsy, S.; Shaker, A.; El-Rabbany, A. Using multispectral airborne LiDAR data for land/water discrimination: A case study at Lake Ontario, Canada. Appl. Sci. 2018, 8, 349. [Google Scholar] [CrossRef] [Green Version]
- Gray, P.C.; Ridge, J.T.; Poulin, S.; Seymour, A.C.; Schwantes, A.M.; Swenson, J.J.; Johnston, D.W. Integrating drone imagery into high resolution satellite remote sensing assessments of estuarine environments. Remote Sens. 2018, 10, 1257. [Google Scholar] [CrossRef] [Green Version]
- Japitana, M.V.; Demetillo, A.T.; Burce, M.E.C.; Taboada, E.B. Catchment characterization to support water monitoring and management decisions using remote sensing. Sustain. Environ. Res. 2019, 29, 8. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, F.J.A.; De Souza, R.O.; Araújo, A.L.C. Revisiting the Influence of Loading on Organic Material Removal in Primary Facultative Ponds. Braz. J. Chem. Eng. 2010, 27, 63–69. [Google Scholar] [CrossRef]
- Jasper, J.T.; Nguyen, M.T.; Jones, Z.L.; Ismail, N.S.; Sedlak, D.L.; Sharp, J.O.; Luthy, R.G.; Horne, A.J.; Nelson, K.L. Unit Process Wetlands for Removal of Trace Organic Contaminants and Pathogens from Municipal Wastewater Effluents. Environ. Eng. Sci. 2013, 30, 421–436. [Google Scholar] [CrossRef] [PubMed]



| Temperature (°C) | pH | CODm(mg/L) | BODm(mg/L) | TN (mg/L) | TP (mg/L) |
|---|---|---|---|---|---|
| 18 (0.1) *1 | 9.2 (0.3) | 1300.0 (113.1) | 494.4 (79.6) | 155.0 (17.0) | 10.2 (0.5) |
| Time | Temperature (°C)*1 | pH | COD (mg/L) | BOD (mg/L) | TN (mg/L) | TP (mg/L) |
|---|---|---|---|---|---|---|
| 23/July/2011 | 17.6 (0.8) | 8.9 (0.1) | 1295.3 (63.4) | 547.8 (4.1) | 161.6 (4.0) | 10.3 (0.2) |
| 30/July/2011 | 17.9 (0.6) | 8.7 (0.1) | 1131.0 (65.3) | 535.3 (4.2) | 154.4 (2.1) | 9.7 (0.1) |
| 23/Nov/2011 | 21.3 (0.5) | 7.6 (0.4) | 428.4 (38.5) | 168.2 (17.7) | 89.5 (4.2) | 5.1 (0.1) |
| 30/Nov/2011 | 21.0 (0.8) | 7.6 (0.1) | 304.6 (42.5) | 103.1 (18.9) | 75.6 (4.0) | 4.8 (0.1) |
| 23/Mar/2012 | 27.3 (1.0) | 7.5 (0.0) | 70.8 (1.1) | 31.7 (0.8) | 18.6 (0.7) | 2.1 (0.1) |
| 30/Mar/2012 | 24.3 (1.0) | 7.4 (0.1) | 66.1 (1.9) | 29.3 (0.5) | 16.2 (0.9) | 1.9 (0.1) |
| Time | Temperature (°C)*1 | pH | COD (mg/L) | BOD (mg/L) | TN (mg/L) | TP (mg/L) |
|---|---|---|---|---|---|---|
| Jun/2012 | 17.5 (0.7) | 7.4 (0.1) | 40.4 (8.4) | 19.0 (2.3) | 10.0 (0.5) | 1.0 (0.3) |
| Jul | 16.5 (0.7) | 7.3 (0.1) | 29.4 (8.1) | 16.5 (2.5) | 7.7 (0.6) | 0.8 (0.2) |
| Aug | 16.5 (0.7) | 7.3 (0.0) | 19.5 (1.1) | 12.3 (0.9) | 2.9 (0.2) | 0.6 (0.2) |
| Time | Temperature (°C)*1 | pH | COD (mg/L) | BOD (mg/L) | TN (mg/L) | TP (mg/L) |
|---|---|---|---|---|---|---|
| Jan/2012 | 22.8 (0.5) | 8.0 (0.3) | 72.6 (6.9) | 34.6 (7.4) | 31.7 (7.3) | 2.3 (0.9) |
| Feb | 25.0 (0.8) | 7.6 (0.2) | 68.7 (6.2) | 30.3 (4.9) | 26.2 (5.9) | 2.0 (0.7) |
| Mar | 28.3 (0.5) | 7.7 (0.2) | 64.3 (7.1) | 27.2 (5.0) | 21.9 (4.0) | 1.7 (0.5) |
| Apr | 24.5 (1.3) | 7.6 (0.1) | 58.4 (6.2) | 24.2 (3.9) | 18.6 (3.2) | 1.5 (0.4) |
| May | 20.0 (1.4) | 7.6 (0.1) | 49.7 (9.7) | 19.7 (1.6) | 15.7 (3.2) | 1.3 (0.4) |
| Jun | 17.3 (0.5) | 7.6 (0.1) | 36.8 (6.1) | 16.7 (0.7) | 12.4 (2.9) | 1.0 (0.2) |
| Jul | 17.5 (0.6) | 7.5 (0.1) | 30.0 (4.9) | 15.0 (0.8) | 9.7 (1.6) | 0.8 (0.2) |
| Aug | 16.5 (0.6) | 7.6 (0.1) | 23.4 (3.0) | 13.9 (1.3) | 7.6 (1.1) | 0.7 (0.1) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jindo, K.; Morikawa Sakura, M.S. Innovative Feasibility Study for the Reclamation of the Cascajo Wetlands in Peru Utilizing Sustainable Technologies. Water 2020, 12, 1097. https://doi.org/10.3390/w12041097
Jindo K, Morikawa Sakura MS. Innovative Feasibility Study for the Reclamation of the Cascajo Wetlands in Peru Utilizing Sustainable Technologies. Water. 2020; 12(4):1097. https://doi.org/10.3390/w12041097
Chicago/Turabian StyleJindo, Keiji, and Marino S. Morikawa Sakura. 2020. "Innovative Feasibility Study for the Reclamation of the Cascajo Wetlands in Peru Utilizing Sustainable Technologies" Water 12, no. 4: 1097. https://doi.org/10.3390/w12041097





