Population Characteristics of the Limpet Patella caerulea (Linnaeus, 1758) in Eastern Mediterranean (Central Greece)
Abstract
1. Introduction
2. Materials and Methods
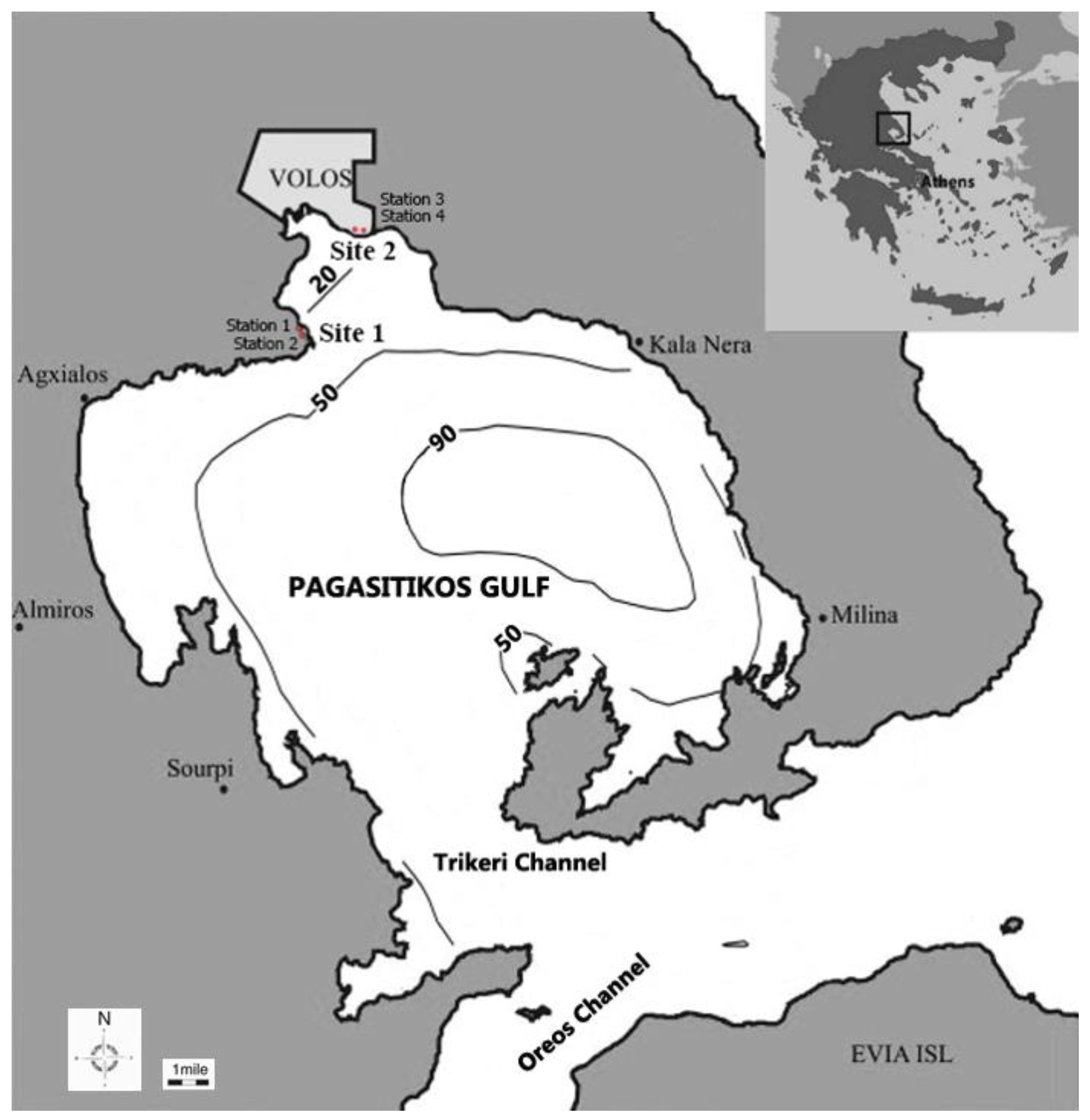
2.1. Study Area
2.2. Field Sampling
2.3. Data Analysis
3. Results
3.1. Physio-Chemical Measurements
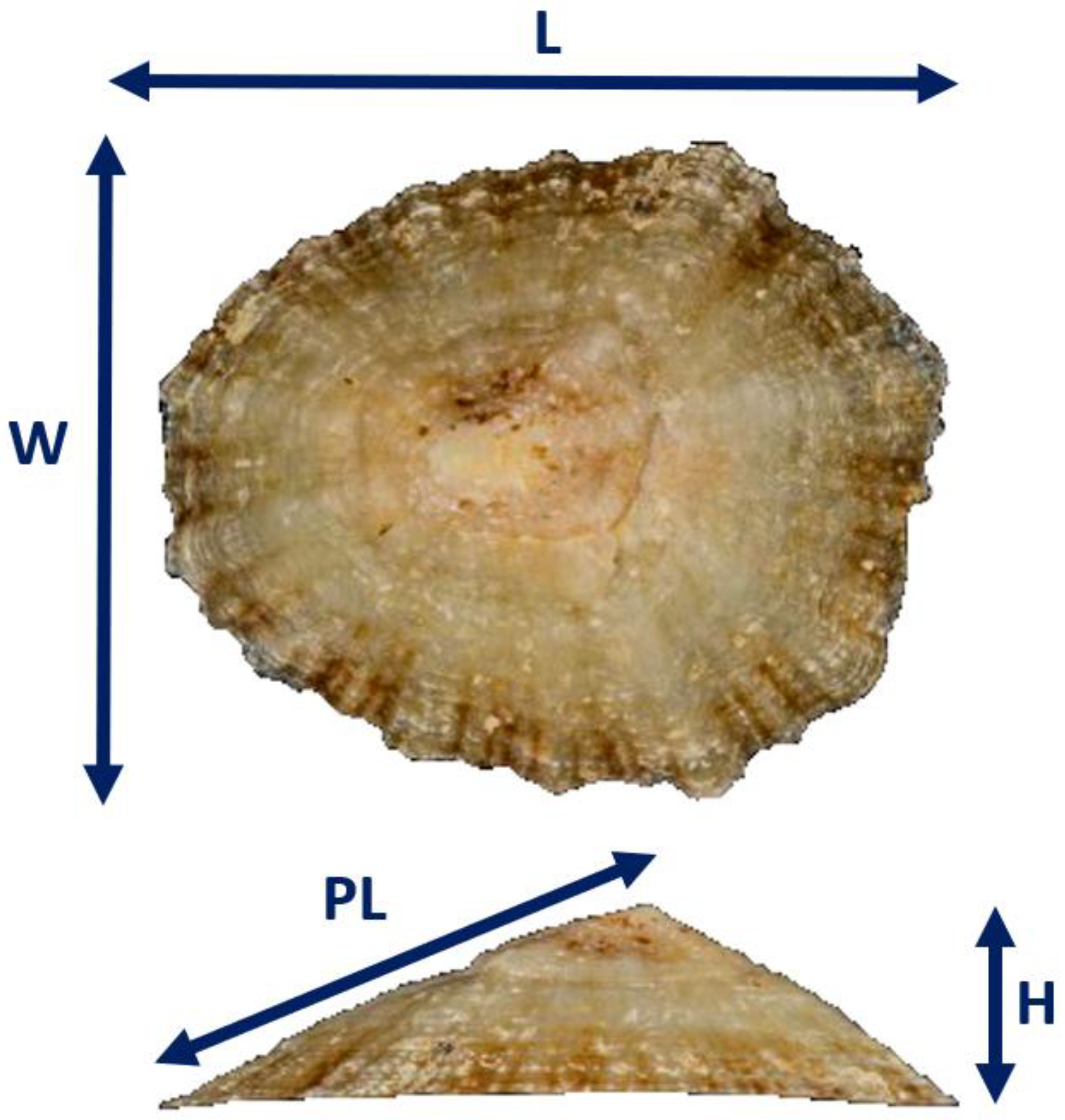
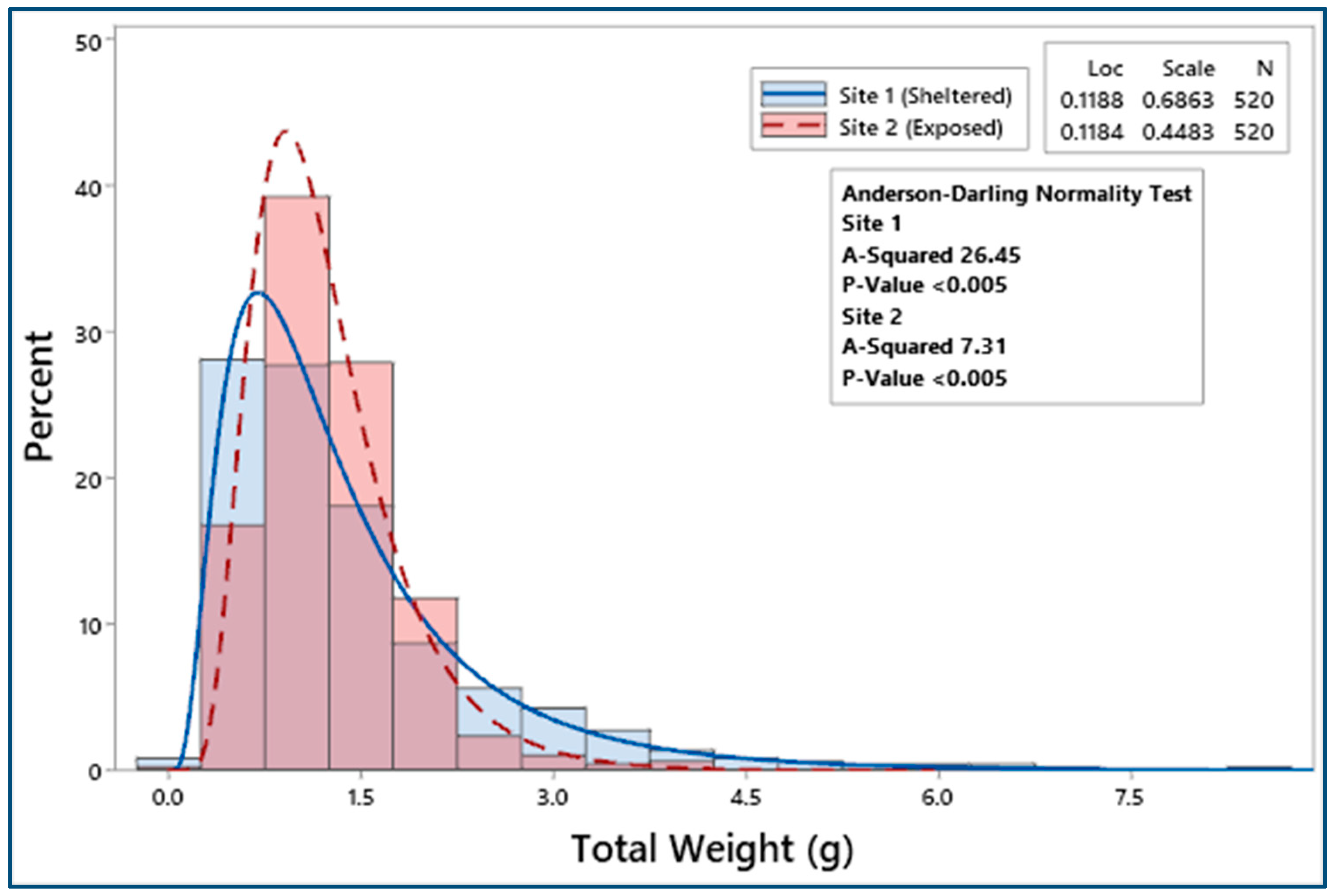
3.2. Biometric Relationships
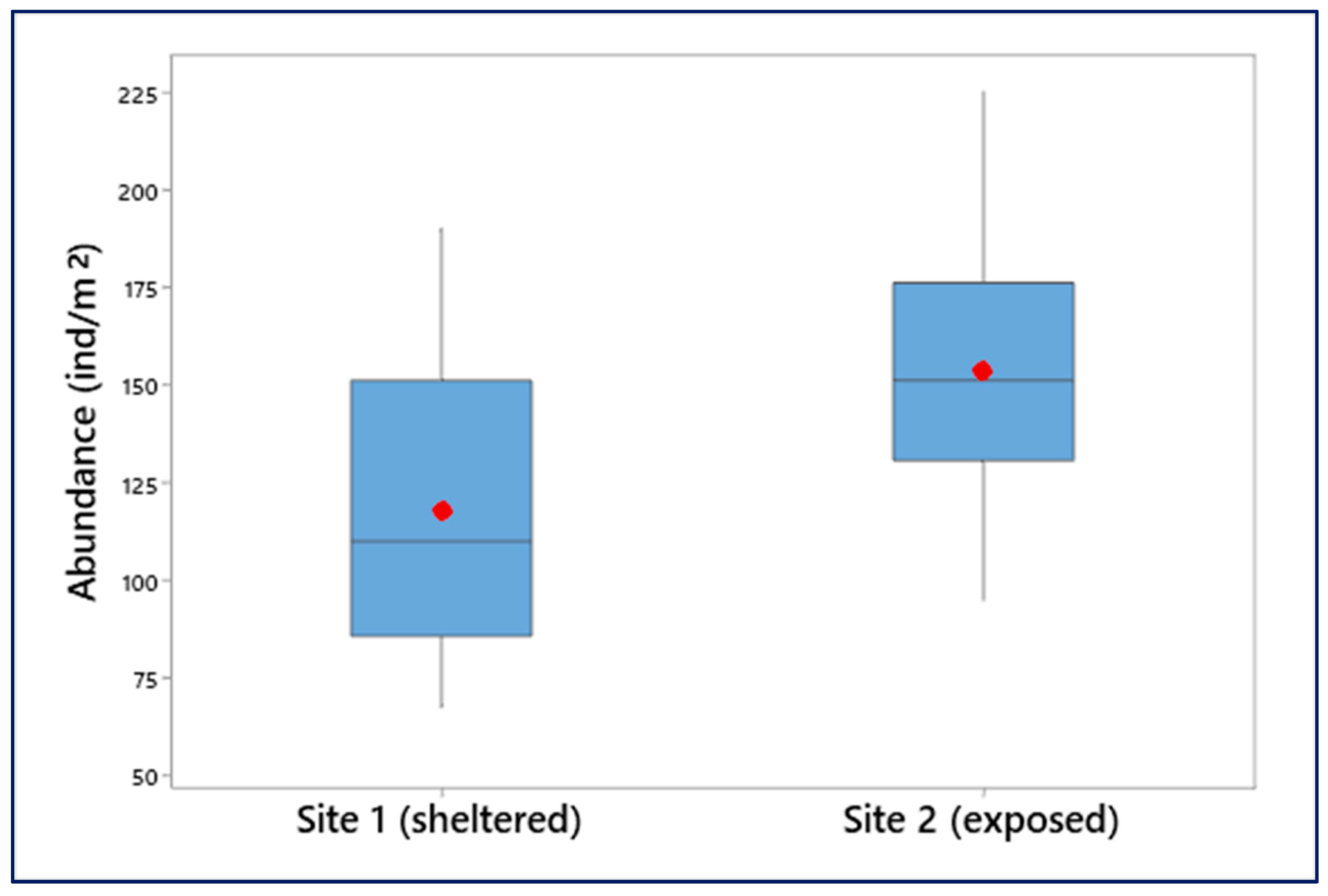
3.3. Population Density
3.4. Distribution Pattern
3.5. Condition Index (C.I.)
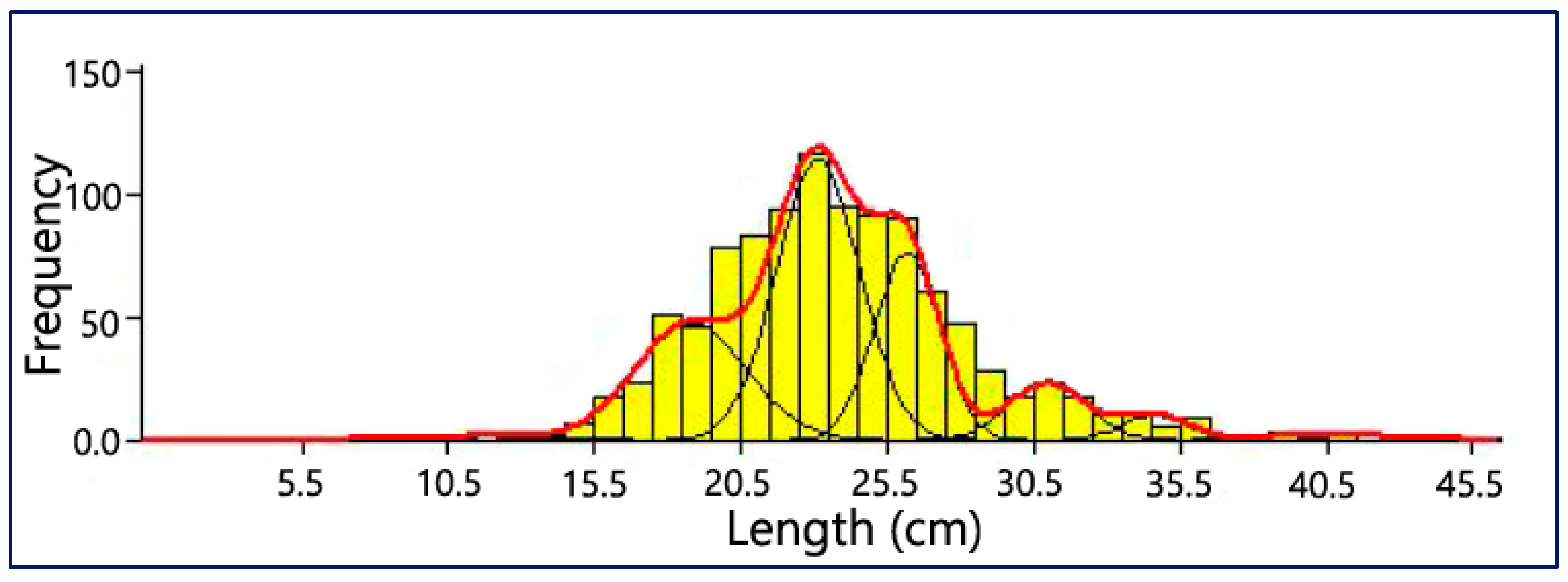
3.6. Age Composition
3.7. Allometric Relationships
4. Discussion
4.1. Biometric Relationships
4.2. Population Density
4.3. Distribution Pattern
4.4. Condition Index
4.5. Age Composition
4.6. Allometric Relationships
Author Contributions
Funding
Conflicts of Interest
References
- Niu, C.; Nakao, S.; Goshima, S. Growth, population age structure and mortality of the limpet Collisella heroldi (DUNKER, 1861) (gastropoda: acmaeidae) in an intertidal rocky shore, in Southern Hokkaido. Bull. Jpn. Soc. Sci. Fish. 1992, 58, 1405–1410. [Google Scholar] [CrossRef][Green Version]
- Casal, G.; Aceña-Matarranz, S.; Fernández-Márquez, D.; Fernández, N. Distribution and abundance patterns of three coexisting species of Patella (Mollusca Gastropoda) in the intertidal areas of the NW Iberian Peninsula: Implications for management. Fish. Res. 2018, 198, 86–98. [Google Scholar] [CrossRef]
- Jenkins, S.R.; Coleman, R.A.; Della Santina, P.; Hawkins, S.J.; Burrows, M.T.; Hartnoll, R.G. Regional scale differences in the determinism of grazing effects in the rocky intertidal. Mar. Ecol. Prog. Ser. 2005, 287, 77–86. [Google Scholar] [CrossRef]
- Coleman, R.; Hawkins, S.; Wood, H. Testing the reproductive benefits of aggregation: The limpet Patella vulgata shows no evidence of synchrony in gonad development. Mar. Ecol. Prog. Ser. 2006, 306, 201–207. [Google Scholar] [CrossRef]
- Coleman, R.A.; Underwood, A.J.; Benedetti-Cecchi, L.; Aberg, P.; Arenas, F.; Arrontes, J.; Castro, J.; Hartnoll, R.G.; Jenkins, S.R.; Paula, J.; et al. A continental scale evaluation of the role of limpet grazing on rocky shores. Oecologia 2006, 147, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Moore, P.; Thompson, R.C.; Hawkins, S.J. Effects of grazer identity on the probability of escapes by a canopy-forming macroalga. J. Exp. Mar. Biol. Ecol. 2007, 344, 170–180. [Google Scholar] [CrossRef]
- Prusina, I.; Peharda, M.; Ezgeta-Balić, D.; Puljas, S.; Glamuzina, B.; Golubić, S. Life-history trait of the Mediterranean keystone species Patella rustica: Growth and microbial bioerosion. Mediterr. Mar. Sci. 2015, 16, 393–401. [Google Scholar] [CrossRef]
- Branch, G.M. The biology of limpets: Physical factors, energy flow, and ecological interactions. Oceanogr. Mar. Biol. Annu. Rev. 1981, 19, 235–380. [Google Scholar]
- Branch, G.M. Limpets: Their role in littoral and sublittoral community dynamics. In The Ecology of Rocky Coast; Moore, P.G., Seed, R., Eds.; Columbia University Press: New York, NY, USA, 1985; pp. 97–116. [Google Scholar]
- Hawkins, S.; Hartnoll, R. Grazing of intertidal algae by marine invertebrates. Oceanogr. Mar. Biol. Annu. Rev. 1983, 21, 195–282. [Google Scholar]
- Menge, B.A.; Berlow, E.L.; Blanchette, C.A.; Na-varrete, S.A.; Yamada, S.B. The keystone species concept: Variation in interaction strength in a rocky intertidal habitat. Ecol. Monogr. 1994, 64, 249–286. [Google Scholar] [CrossRef]
- Menge, B.A. Top-down and bottom-up community regulation in marine rocky intertidal habitats. J. Exp. Mar. Biol. Ecol. 2000, 250, 257–289. [Google Scholar] [CrossRef]
- Arrontes, J.; Arenas, F.; Fernández, C.; Rico, J.M.; Oliveros, J.; Martínez, M.; Viejo, R.M.; Alvarez, D. Effect of grazing by limpets on mid-shore species assemblages in northern Spain. Mar. Ecol. Prog. Ser. 2004, 277, 117–133. [Google Scholar] [CrossRef]
- Bouzaza, Z.; Mezali, K. Discriminant-based study of the shell morphometric relationships of Patella caerulea (Gastropoda: Prosobranchia) of the western Mediterranean Sea. Turk. J. Zool. 2018, 42, 513–522. [Google Scholar] [CrossRef]
- Bannister, J.V. Shell parameters in relation to zonation in Mediterranean limpets. Mar. Biol. 1975, 31, 63–67. [Google Scholar] [CrossRef]
- Davies, P.S. Effect of environment on metabolic activity and morphology of Mediterranean and British species of Patella. Pubbl. Stn. Zool. Napoli 1969, 37, 641–656. [Google Scholar]
- Šimunović, A. Ecological study of Prosobranchiata (Gastropoda) in the eastern part of the Adriatic Sea and their relationship to benthic biocenosis. Acta Adriat. 1995, 36, 3–162. [Google Scholar]
- Mauro, A.; Arculeo, M.; Parrinello, N. Morphological and molecular tools in identifying the Mediterranean limpets Patella caerulea, Patella aspera and Patella rustica. J. Exp. Mar. Biol. Ecol. 2003, 295, 131–143. [Google Scholar] [CrossRef]
- Christiaens, J. Révision du genre Patella (Mollusca, Gastropoda). Bull. Mus. Hist. Nat. 1973, 182, 1305–1392. (In French) [Google Scholar]
- Frenkiel, L. Contribution à l’étude des cycles de reproduction des Patellidae en Algérie. Pubbl. Staz. Zool. Napoli 1975, 39, 153–189. [Google Scholar]
- Beaumont, A.R.; Wei, J.H.C. Morphological and genetic variation in the antartic limpet Nacella concinna (Strebel, 1908). J. Molluscan Stud. 1991, 57, 443–450. [Google Scholar] [CrossRef]
- Nolan, C.P. Size, shape, and shell morphology in the Antarctic limpet Nacella concinna at Signy Island, South Orkney Islands. J. Molluscan Stud. 1991, 57, 225–238. [Google Scholar] [CrossRef]
- Corte-Real, H.B.S.M.; Hawkins, S.J.; Thorpe, J.P. Population differentiation and taxonomic status of the exploited limpet Patella candei in the Macaronesian islands (Azores, Madeira, Canaries). Mar. Biol. 1996, 125, 141–152. [Google Scholar] [CrossRef]
- Christofoletti, R.A.; Takahashi, C.K.; Oliveira, D.N.; Flores, A.A.V. Abundance of sedentary consumers and sessile organisms along the wave exposure gradient of subtropical rocky shores of the south-west Atlantic. J. Mar. Biol. Assoc. UK 2011, 91, 961–967. [Google Scholar] [CrossRef]
- Prusina, I.; Sarà, G.; De Pirro, M.; Dong, J.W.; Han, G.D.; Glamuzina, B.; Williams, G.A. Variations in physiological responses to thermal stress in congeneric limpets in the Mediterranean Sea. J. Exp. Mar. Biol. Ecol. 2014, 456, 34–40. [Google Scholar] [CrossRef]
- Battelli, C. Morphometric characteristics, vertical distribution and density of the limpet Patella caerulea L. in relation to different substrata of the bay of Koper (Gulf of Trieste, northern Adriatic). Ann. Ser. Hist. Nat. Koper 2016, 26, 145–156. [Google Scholar]
- Coleman, R.A. Limpet aggregation does not alter desiccation in the limpet Cellana tramoserica. J. Exp. Mar. Biol. Ecol. 2010, 386, 113–118. [Google Scholar] [CrossRef]
- Rilov, G.; Schiel, D.R. Trophic linkages across seascapes: Subtidal predators limit effective mussel recruitment in rocky intertidal communities. Mar. Ecol. Prog. Ser. 2006, 327, 83–93. [Google Scholar] [CrossRef]
- Griffin, J.N.; De la Haye, K.L.; Hawkins, S.J.; Thompson, R.C.; Jenkins, S.R. Predator diversity and ecosystem functioning: Density modifies the effect of resource partitioning. Ecology 2008, 89, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Bacci, G.; Sella, G. Correlations between characters and environmental conditions in Patella of the caerulea group. Pubbl. Staz. Zool. Napoli 1970, 38, 1–24. [Google Scholar]
- Guerra, M.T.; Gaudencio, M.J. Aspects of the ecology of Patella spp. on the Portuguese coast. Hidrobiología 1986, 142, 57–69. [Google Scholar] [CrossRef]
- Della Santina, P.; Sonni, C.; Sartoni, G.; Chelazzi, G. Food availability and diet composition of three coexisting Mediterranean limpets (Patella spp.). Mar. Biol. 1993, 116, 87–95. [Google Scholar] [CrossRef]
- Navarro, P.G.; Ramirez, R.; Tuya, F.; Fernandez-Gil, C.; Sanchez-Jerez, P.; Haroun, R.J. Hierarchical analysis of spatial distribution patterns of patellid limpets in the Canary Islands. J. Molluscan Stud. 2005, 71, 67–73. [Google Scholar] [CrossRef]
- Espinosa, F.; Guerra-García, J.M.; Fa, D.; García-Gómez, J.C. Effects of competition on an endangered limpet Patella ferruginea (Gastropoda: Patellidae): Implications for conservation. J. Exp. Mar. Biol. Ecol. 2006, 330, 482–492. [Google Scholar] [CrossRef]
- Cabral, J. Shape and growth in European Atlantic Patella limpets (Gastropoda, Mollusca). Ecological implications for survival. Web Ecol. 2007, 7, 11–21. [Google Scholar] [CrossRef]
- Prusina, I.; Ezgeta-Balić, D.; Ljubimir, S.; Dobroslavić, T.; Glamuzina, B. On the reproduction of the Mediterranean keystone limpet Patella rustica: Histological overview. JMBA 2014, 94, 1651–1660. [Google Scholar] [CrossRef]
- Ocana, T.M.J. An Investigation into the Ecology and Life History Dynamics of the Pulmonate Limpet Siphonaria pectinata (L.) at Gibraltar. Unpublished Ph.D. Thesis, King’s College, London, UK, 1997. [Google Scholar]
- Ruitton, S.; Francour, P.; Boudouresque, C.F. Relationships between algae, benthic herbivorous invertebrates and fishes in rocky sublittoral communities of a temperate sea (Mediterranean). Estuar. Coast. Shelf Sci. 2000, 50, 217–230. [Google Scholar] [CrossRef][Green Version]
- Espinosa, F.; Guerra-García, J.M.; Fa, D.; García-Gómez, J.C. Aspects of reproduction and their implications for the conservation of the endangered limpet, Patella ferruginea. Invertebr. Reprod. Dev. 2006, 49, 85–92. [Google Scholar] [CrossRef]
- Belkhodja, H.; Romdhane, M.S. Etude morphométrique du mollusque gastéropode “Patella caerulea Linnaeus”, 1758 des côtes nord de la Tunisie. Bulletin de l’Institut National des Sciences et Technologies de la Mer 2012, 39, 15–23. [Google Scholar]
- Küçükdermenci, A.; Aynur, L.Ö.K.; Kirtik, A.; Kurtay, E. The meat yield variations of Patella caerulea (Linnaeus, 1758) in Urla, Izmir Bay. Acta Biol. Turc. 2017, 30, 174–177. [Google Scholar]
- Triantafyllou, G.; Petihakis, G.; Dounas, C.; Theodorou, A. Assessing marine ecosystem response to nutrient inputs. Mar. Pollut. Bull. 2001, 43, 175–186. [Google Scholar]
- Friligos, N. Eutrophication assessment in Greek coastal waters. Toxicol. Environ. Chem. 1987, 15, 185–196. [Google Scholar] [CrossRef]
- Korres, G.; Triantafyllou, G.; Petihakis, G.; Raitsos, D.E.; Hoteit, I.; Pollani, A.; Tsiaras, K. A data assimilation tool for the Pagasitikos Gulf ecosystem dynamics: Methods and benefits. J. Mar. Syst. 2012, 94, 102–117. [Google Scholar] [CrossRef]
- Petihakis, G. Triantafyllou, G.; Pollani, A.; Koliou, A.; Theodorou, A. Field data analysis and application of a complex water column biogeochemical model in different areas of a semi enclosed basin: Towards the development of an ecosystem management tool. Mar. Environ. Res. 2005, 59, 493–518. [Google Scholar] [CrossRef]
- Petihakis, G.; Triantafyllou, G.; Korres, G.; Pollani, A.; Theodorou, A. Ecosystem modeling: Towards the development of a management tool for a marine coastal system: Part I: General circulation, hydrological and dynamical structure. J. Mar. Syst. 2012, 94, 34–48. [Google Scholar] [CrossRef]
- Bakus, G.J. Quantitative Ecology and Marine Biology; Balkema: Rotterdam, The Netherlands, 1990; 157p. [Google Scholar]
- Underwood, A.J. Experiments in Ecology. Their Logical Design and Interpretation Using Analysis of Variance; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Morisita, M. Measuring of the Dispersion of individuals and analysis of the distributional patterns. Mem. Fac. Sci. Kyushu Univ. Ser. 1959, 3, 65–80. [Google Scholar]
- Elliott, J.M. Some Methods for the Statistical Analysis of Samples of Benthic Invertebrates, 2nd ed.; Freshwater Biological Association: Toulouse, France, 1977; 156p. [Google Scholar]
- Morisita, M. Iδ-Index, a measure of dispersion of individuals. Res. Popul. Ecol. 1962, 4, 1–7. [Google Scholar] [CrossRef]
- Dale, M.R.T.; Dixon, P.; Fortin, M.J.; Legendre, P.; Myers, D.E.; Rosenberg, M.S. Conceptual and mathematical relationships among methods for spatial analysis. Ecography 2002, 25, 558–577. [Google Scholar] [CrossRef]
- Nakhlé, K.F.; Cossa, D.; Khalaf, G.; Beliaeff, B. Brachidontes variabilis and Patella sp. as quantitative biological indicators for cadmium, lead and mercury in the Lebanese coastal waters. Environ. Pollut. 2006, 142, 73–82. [Google Scholar]
- Bhattacharya, C.G. A simple method of resolution of a distribution into Gaussian components. Biometrics 1967, 23, 115–134. [Google Scholar] [CrossRef]
- Gayanilo, F.; Sparre, P.; Pauly, D. The FiSAT User’s Guide, FAO Computerized Information Series Fisheries, 99; ICLARM, DIFMAR: Rome, Italy, 1995. [Google Scholar]
- Pauly, D.; Caddy, J.F. A Modification of Bhattacharya’s Method for the Analysis of Mixtures of Normal Distributions. FAO Fisheries Circular; Food and Agricultural Organization of the United Nations: Rome, Italy, 1985; 16p. [Google Scholar]
- Power, M.E.; Tilman, D.; Estes, J.A.; Menge, B.A.; Bond, W.J.; Mills, L.S.; Daily, G.; Castilla, J.C.; Lubchenco, J.; Paine, R.T. Challenges in the quest for keystones. Bioscience 1996, 46, 609–620. [Google Scholar] [CrossRef]
- Lindberg, D.R.; Estes, J.A.; Warheit, K.I. Human influences on trophic cascades along rocky shores. Ecol. Appl. 1998, 8, 880–890. [Google Scholar] [CrossRef]
- Underwood, A.J. The ecology of intertidal gastropods. Adv. Mar. Biol. 1979, 16, 111–210. [Google Scholar]
- Nakin, M.D.V.; McQuaid, C.D. Marine reserve effects on population density and size structure of commonly and rarely exploited limpets in South Africa. Afr. J. Mar. Sci. 2014, 3, 1–9. [Google Scholar] [CrossRef]
- Halpern, B.S.; Walbridge, S.; Selkoe, K.A.; Kappel, C.V.; Micheli, F.; D’Agrosa, C.; Bruno, J.F.; Casey, K.S.; Ebert, C.; Fox, H.E.; et al. A global map of human impact on marine ecosystems. Science 2008, 319, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.S. Marine biodiversity: Patterns, threats and conservation needs. Biodivers. Conserv. 1997, 6, 153–175. [Google Scholar] [CrossRef]
- Jackson, J.B.C.; Kirby, M.X.; Berger, W.H.; Bjorndal, K.A.; Botsford, L.W.; Bourque, B.J.; Bradbury, R.H.; Cooke, R.; Erlandson, J.; Estes, J.A.; et al. Historical overfishing and the recent collapse of coastal ecosystems. Science 2001, 293, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Crowe, T.P.; Hawkins, S.J. Rocky intertidal communities: Past environmental changes, present status and predictions for the next 25 years. Environ. Conserv. 2002, 29, 168–191. [Google Scholar] [CrossRef]
- Henriques, P.; Delgado, J.; Sousa, R. Patellid Limpets: An Overview of the Biology and Conservation of Keystone Species of the Rocky Shores; Ray, S., Ed.; Intech, Organismal and Molecular Malacology: Rijeka, Croatia, 2017; pp. 71–95. [Google Scholar]
- Diaz, R.J.; Rosenberg, R. Spreading dead zones and consequences for marine ecosystems. Science 2008, 321, 926–929. [Google Scholar] [CrossRef] [PubMed]
- Molnar, J.L.; Gamboa, R.L.; Revenga, C.; Spalding, M.D. Assessing the global threat of invasive species to marine biodiversity. Front. Ecol. Environ. 2008, 6, 485–492. [Google Scholar] [CrossRef]
- Poloczanska, E.S.; Babcock, R.C.; Butler, A.; Hobday, A.; Hoegh-Guldberg, O.; Kunz, T.J.; Matear, R.; Milton, D.A.; Okey, T.A.; Richardson, A.J. Climate Change and Australian Marine Life, Oceanography and Marine Biology; Crc Press-Taylor & Francis Group: Boca Raton, FL, USA, 2007; Volume 45, pp. 407–478. [Google Scholar]
- Crain, C.M.; Kroeker, K.; Halpern, B.S. Interactive and cumulative effects of multiple human stressors in marine systems. Ecol. Lett. 2008, 11, 1304–1315. [Google Scholar] [CrossRef]
- Little, C.; Trowbridge, C.D.; Pilling, G.M.; Stirling, P.; Morritt, D.; Williams, G.A. Long-term fluctuations in intertidal communities in an Irish sea-lough: Limpet-fucoid cycles. Estuar. Coast. Shelf Sci. 2017, 196, 70–82. [Google Scholar] [CrossRef]
- Jenkins, S.R.; Hartnoll, R.G. Food supply, grazing activity and growth rate in the limpet Patella vulgata L.: A comparison between exposed and sheltered shores. J. Exp. Mar. Biol. Ecol. 2001, 258, 123–139. [Google Scholar] [CrossRef]
- Fernández, N.; Alborés, I.; Aceña-Matarranz, S. Characterization of the reproductive cycle and physiological condition of Patella vulgata in the NW of the Iberian Peninsula: Relevant in-formation for a sustainable exploitation. Fish. Res. 2015, 164, 293–301. [Google Scholar] [CrossRef]
- Fernández, N.; Alborés, I.; Aceña-Matarranz, S. Spatial variability of the reproductive cycle and physiological condition of Patella spp. (Mollusca Gastropoda) in the NW of the Iberian Peninsula: Implications for exploitation. Fish. Res. 2016, 179, 76–85. [Google Scholar] [CrossRef]
- Crothers, J.H. Variation in dog-whelk shells in relation to wave action and crab predation. Biol. J. Linn. Soc. 1983, 20, 85–102. [Google Scholar] [CrossRef]
- Cabral, J.P.; da Silva, A.C.F. Morphometric analysis of limpets from an Iron-Age shell midden found in northwest Portugal. J. Archaeol. Sci. 2003, 30, 817–829. [Google Scholar] [CrossRef]
- Powell, W.B. The Patellid limpets of the world (Patellidae). Indo Pac. Mollusca 1973, 3, 75–206. [Google Scholar]
- Bosman, A.L.; Hockey, P.A.R. Life-history patterns of populations of the limpet Patella granularis: The dominant roles of food supply and mortality rate. Oecologia 1988, 75, 412–419. [Google Scholar] [CrossRef]
- Thompson, G.B. Distribution and population dynamics of the limpet Patella vulgata L in Bantry Bay. J. Exp. Mar. Biol. Ecol. 1980, 45, 173–217. [Google Scholar] [CrossRef]
- Menge, B.A. Predation intensity in a rocky intertidal community: Effect of an algal canopy, wave action and desiccation on predator feeding rates. Oecologia 1978, 34, 17–36. [Google Scholar] [CrossRef]
- Menge, B.A. Predation intensity in a rocky intertidal community: Relationship between predator foraging activity and environmental harshness. Oecologia 1978, 34, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Milton, P. Biology of littoral blennid fishes on the coast of south-west England. J. Mar. Biol. Assoc. UK 1983, 63, 223–237. [Google Scholar] [CrossRef]
- Ambrose, R.F. Effects of octopus predation on motile invertebrates in a rocky subtidal community. Mar. Ecol. Prog. Ser. 1986, 30, 261–273. [Google Scholar] [CrossRef]
- Silva, A.C.F.; Boaventura, D.M.; Flores, A.; Ré, P.; Hawkins, S.J. Rare predation by the intertidal crab Pachygrapsus marmoratus on the limpet Patella depressa. J. Mar. Biol. Assoc. UK 2004, 84, 367–370. [Google Scholar] [CrossRef]
- Black, R. Tactics of whelks preying on limpets. Mar. Biol. 1978, 46, 157–162. [Google Scholar] [CrossRef]
- Markowska, M.; Kidawa, A. Encounters between Antarctic limpets, Nacella concinna, and predatory sea stars, Lysasterias sp., in laboratory and field experiments. Mar. Biol. 2007, 151, 1959–1966. [Google Scholar] [CrossRef]
- Bosman, A.; Hockey, P.A.R. Oystercatcher predation and limpet mortality: The importance of refuges in enhancing the reproductive output of prey populations. Veliger 1989, 32, 120–129. [Google Scholar]
- Weber, L.; Hawkins, S. Evolution of the limpet Patella candei d’Órbigny (Mollusca, Patellidae) in Atlantic archipelagos: Human intervention and natural processes. Biol. J. Linn. Soc. 2002, 77, 341–353. [Google Scholar] [CrossRef]
- Silva, A.C.F.; Hawkins, S.J.; Boaventura, D.M.; Thompson, R.C. Predation by small mobile aquatic predators regulates populations of the intertidal limpet Patella vulgata (L.). J. Exp. Mar. Biol. Ecol. 2008, 367, 259–265. [Google Scholar] [CrossRef]
- Silva, A.C.F. Predation by Crabs on Rocky Shores in North-East Atlantic. Biological Sciences. Ph.D. Thesis, University of Plymouth, Plymouth, UK, 2008; p. 220. [Google Scholar]
- Lewis, J.R.; Bowman, R. Local habitat-induced variations in the population dynamics of Patella vulgata L. J. Exp. Mar. Biol. Ecol. 1975, 17, 165–203. [Google Scholar] [CrossRef]
- Diaz-Agras, G.; Moreira, J.; Tato, R.; Garcia-Regueira, X.; Urgorri, V. Distribution and population structure of Patella vulgata Linnaeus, 1758 (Gastropoda: Patellidae) on intertidal seawalls and rocky shores in the Ria De Ferrol (Galicia, NW Iberian Peninsula). Thalassas 2010, 26, 79–91. [Google Scholar]
- Holmes, S.P.; Walker, G.; Van der Meer, J. Barnacles, limpets and periwinkles: The effects of direct and indirect interactions on cyprid settlement and success. J. Sea Res. 2005, 53, 181–204. [Google Scholar] [CrossRef]
- Bowman, R.S.; Lewis, J.R. Geographical variation in the breeding cycles and recruitment of Patella spp. Hydrobiologia 1986, 142, 41–56. [Google Scholar] [CrossRef]
- McGuinness, K.A. Effects of some natural and artificial substrata on sessile marine organisms at Galeta Reef, Panama. Mar. Ecol. Prog. Ser. 1989, 52, 21–28. [Google Scholar] [CrossRef]
- Underwood, A.J.; Chapman, M.G. Experiments on Topographic Influences on Density and Dispersion of Littorina unifasciata in New South Wales. In Proceedings of the Third International Symposium on Littorinid Biology, The Malacological Society of London, London, UK, 30 August–5 September 1992; pp. 181–195. [Google Scholar]
- Hayes, J.J.; Castillo, O. A new approach for interpreting the Morisita index of aggregation through quadrat size. ISPRS Int. J. Geo Inf. 2017, 6, 296. [Google Scholar] [CrossRef]
- Perkol-Finkel, S.; Benayahu, Y. Differential recruitment of benthic communities on neighboring artificial and natural reefs. J. Exp. Mar. Biol. Ecol. 2007, 340, 25–39. [Google Scholar] [CrossRef]
- Coll, R.C.; Ortuño, P.M.; Aldeguer, M.D.P.; Berenguer, M.L.P.; Carratalá, E.S.; Sánchez-Jerez, P. Aportación al conocimiento de las poblaciones de Patella caerulea y P. aspera en la reserva marina de Tabarca (Alicante): Densidad poblacional y frecuencia de tallas según el tipo de sustrato y grado de presión antrópica. In Trabajos de Campo en la Reserva Marina de Tabarca (Alicante); Universitat d’ Alacant/Universidad de Alicante, Secretariado de Publicaciones de la Universidad de Alicante: Murcia, Spain, 1994; pp. 45–52. [Google Scholar]
- Fretter, V.; Graham, A. The prosobranch molluscs of Britain and Denmark Part I—Pleurotomariacea, Fissurellacea and Patellacea. J. Molluscan Stud. Suppl. 1976, 1, 1–37. [Google Scholar]
- Bacci, G. L’inversione del sesso ed il ciclo stagionale della gonade in Patella caerulea L. Pubbl. Staz. Zool. Napoli 1947, 21, 183–217. (In Italian) [Google Scholar]
- Belkhodja, H.; Jaafoura, M.H.; Missaoui, H.; Romdhane, M.S. Histological investigation of the reproductive cycle of the limpet Patella caerulea Linnaeus, 1758. Cah. Biol. Mar. 2011, 52, 279–290. [Google Scholar]
- Parry, G.D. Reproductive effort in four species in intertidal limpets. Mar. Biol. 1982, 67, 267–282. [Google Scholar] [CrossRef]
- Sella, G. Biometrical relationships between mesolitoral and infralitoral Patella population in the Mediterranean. Pubbl. Staz. Zool. Napoli. 1976, 40, 123–132. [Google Scholar]
- Munoz, M.A.; Acuna, J.D. On the taxonomic discrimination between P. aspera Roding and P. caerulea Linnaeus (Gasteropoda: Patellidae) using conchological traits. J. Conchol. 1994, 35, 37–43. [Google Scholar]
- Amer, L.A.M.; Benali, I.; Dermeche, S.; Bouderbala, M. Seasonal variations of the biometric indices of Patella rustica Linnaeus, 1758 (Gastropoda Patellidae) from contrasted sites of the western Algerian coast. Biodivers. J. 2018, 9, 205–212. [Google Scholar] [CrossRef]
- Littler, M.M.; Murray, S.N. Impact of sewage on the distribution, abundance and community structure of rocky intertidal macro-organisms. Mar. Biol. 1975, 30, 277–291. [Google Scholar] [CrossRef]
- Duran, L.R.; Castilla, J.C. Variation and persistence of the middle rocky intertidal community of central Chile, with and without human harvesting. Mar. Biol. 1989, 103, 555–562. [Google Scholar] [CrossRef]
- Underwood, A.J.; Kennelly, S.J. Pilot studies for designs of surveys of human disturbance of intertidal habitats in New South Wales. Aust. J. Mar. Freshw. Res. 1990, 41, 165–173. [Google Scholar] [CrossRef]
- Povey, A.; Keough, M.J. Effects of trampling on plant and animal populations on rocky shores. Oikos 1991, 61, 355–368. [Google Scholar] [CrossRef]
- Kingsford, M.J.; Underwood, A.J.; Kennelly, S.J. Humans as predators on rocky reefs in New South Wales, Australia. Mar. Ecol. Prog. Ser. 1991, 72, 1–14. [Google Scholar] [CrossRef]
- Keough, M.J.; Quinn, G.P.; King, A. Correlations between human collecting and intertidal mollusc populations on rocky shores. Conserv. Biol. 1993, 7, 378–391. [Google Scholar] [CrossRef]
- Lasiak, T. Multivariate comparisons of rocky infratidal macrofauna assemblages from replicate exploited and non-exploited localities on the Transkei coast of South Africa. Mar. Ecol. Prog. Ser. 1998, 167, 15–23. [Google Scholar] [CrossRef]
- Sala, O.E.; Chapin, F.S.; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Biodiversity—Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Paine, R.T. Ecological determinism in the competition for space: The Robert H. MacArthur Award Lecture. Ecology 1984, 65, 1339–1357. [Google Scholar] [CrossRef]
- Atalah, J.; Crowe, T.P. Combined effects of nutrient enrichment, sedimentation and grazer loss on rock pool assemblages. J. Exp. Mar. Biol. Ecol. 2010, 388, 51–57. [Google Scholar] [CrossRef]




| Sampling Period | Site 1 | Site 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| T (°C) | S (psu) | pH | O2 (mg/L) | T (°C) | S (psu) | pH | O2 (mg/L) | |
| April | 16.06 | 38.54 | 8.32 | 6.79 | 16.95 | 38.16 | 8.32 | 6.86 |
| May | 21.21 | 37.54 | 8.29 | 5.88 | 19.92 | 37.54 | 8.26 | 2.48 |
| June | 25.04 | 37.47 | 8.26 | 6.61 | 26.45 | 36.64 | 8.24 | 5.71 |
| July | 27.08 | 36.46 | 8.26 | 5.44 | 27.12 | 36.18 | 8.23 | 2.45 |
| August | 26.67 | 36.12 | 8.26 | 2.06 | 26.78 | 36.76 | 8.31 | 5.02 |
| September | 24.28 | 36.84 | 8.29 | 4.92 | 25.03 | 36.49 | 8.29 | 5.92 |
| October | 21.79 | 36.42 | 8.31 | 5.22 | 22.04 | 36.84 | 8.35 | 5.54 |
| November | 18.31 | 36.71 | 8.34 | 2.48 | 17.95 | 36.99 | 8.39 | 3.67 |
| December | 16.01 | 36.87 | 8.31 | 2.99 | 15.64 | 36.21 | 8.24 | 4.97 |
| January | 13.96 | 36.99 | 8.27 | 5.21 | 13.17 | 38.21 | 8.33 | 4.01 |
| February | 13.28 | 37.67 | 8.28 | 3.04 | 13.46 | 38.03 | 8.27 | 5.11 |
| March | 13.31 | 37.78 | 8.23 | 4.76 | 13.53 | 38.46 | 8.29 | 6.49 |
| April | 14.62 | 38.12 | 8.24 | 6.45 | 14.93 | 37.78 | 8.24 | 6.12 |
| Sampling Period | No. of Individuals | L ± SE | W ± SE | H ± SE | PL ± SE | Wt ± SE | Wf ± SE |
|---|---|---|---|---|---|---|---|
| Site | (n) | ||||||
| Site 1 | 520 | 23.47 ± 0.23 | 19.09 ± 0.21 | 6.22 ± 0.07 | 11.21 ± 0.13 | 1.43 ± 0.05 | 0.51 ± 0.06 |
| Site 2 | 520 | 23.24 ± 0.15 | 18.83 ± 0.13 | 5.97 ± 0.05 | 10.89 ± 0.09 | 1.24 ± 0.02 | 0.37 ± 0.01 |
| F = 0.66, p = 0.42 | F = 1.13, p = 0.29 | F = 8.51, p < 0.05 | F = 4.12, p < 0.05 | F = 12.23, p < 0.001 | F = 20.58, p < 0.001 | ||
| Season | (n) | ||||||
| Winter | 242 | 22.780 ± 0.27 | 18.716 ± 0.23 | 5.973 ± 0.08 | 10.576 ± 0.14 | 1.150 ± 0.04 | 0.320 ± 0.01 |
| Spring | 318 | 25.140 ± 0.25 | 20.462 ± 0.24 | 6.568 ± 0.08 | 12.506 ± 0.15 | 1.663 ± 0.06 | 0.526 ± 0.02 |
| Summer | 240 | 23.601 ± 0.28 | 19.136 ± 0.25 | 6.398 ± 0.09 | 10.912 ± 0.16 | 1.484 ± 0.06 | 0.467 ± 0.02 |
| Autumn | 240 | 21.322 ± 0.25 | 17.044 ±0.23 | 5.288 ± 0.07 | 9.733 ± 0.13 | 0.943 ± 0.03 | 0.274 ± 0.01 |
| F = 39.02, p < 0.001 | F = 37.07, p < 0.001 | F = 50.82, p < 0.001 | F = 68.59, p < 0.001 | F = 39.80, p < 0.001 | F = 51.18, p < 0.001 | ||
| Total | 1040 | 23.36 ± 0.14 | 18.96 ± 0.12 | 6.09 ± 0.04 | 11.05 ± 0.08 | 1.34 ± 0.03 | 0.41 ± 0.01 |
| Source of Variation | DF | SS | MS | F | p |
|---|---|---|---|---|---|
| Site | 1 | 7884.38 | 7884.38 | 4.45 | 0.077 |
| Season | 6 | 10,397.92 | 1732.99 | 1.25 | 0.334 |
| Error | 16 | 22,195.83 | 1387.24 | ||
| Total | 23 | 40,478.13 |
| Area | Density (ind/m2) | Shell Length (mm) | Shell Width (mm) | Shell Height (mm) | Total Weight (gr) | References |
|---|---|---|---|---|---|---|
| Strait of Gibraltar | 125 | [37] | ||||
| Strait of Gibraltar | 100 | 28.87 ± 3.66 | 2.87 ± 1.09 | [39] | ||
| French Mediterranean coast | 16.5 ± 9.9 | [38] | ||||
| Northern Adriatic | 3–38 | 13.5–56.1 | 19.91–25.52 | 3.5–17.5 | [25] | |
| Tunisia | 21.03–35.57 | 17.93–30.15 | 4.99–9.4 | [40] | ||
| Turkey (Izmir Bay) | 24.36–30.75 | 10.5–46.9 | 6.26–8.9 | 2.59–4.21 | [41] | |
| Pagasitikos Gulf (Central Greece) | 135.63 ± 41.95 | 23.36 ± 4.46 | 18.96 ± 4.01 | 6.09 ± 1.38 | 1.34 ± 0.89 | Present study |
| Sampling Period | Sites | Population Density (ind/m2 ± SD) | Iδ | X2 | Dispersion Pattern | Significance Level | DF |
|---|---|---|---|---|---|---|---|
| April | Site 1 | 55 ± 30.73 | 1.29 | 6.18 | clustered | ns | 9 |
| Site 2 | 177.5 ± 2.56 | 1.13 | 8.29 | random | ns | 9 | |
| May | Site 1 | 122.5 ± 91.6 | 1.51 | 24.74 | clustered | <0.05 | 9 |
| Site 2 | 225 ± 55.3 | 1.05 | 4.89 | random | ns | 9 | |
| June | Site 1 | 132.5 ± 85.8 | 1.38 | 19.98 | clustered | <0.05 | 9 |
| Site 2 | 202.5 ± 113.3 | 1.29 | 22.8 | clustered | <0.05 | 9 | |
| July | Site 1 | 115 ± 69.9 | 1.34 | 15.31 | clustered | ns | 9 |
| Site 2 | 152.5 ± 27.5 | 1.03 | 1.79 | random | ns | 9 | |
| August | Site 1 | 175 ± 76.3 | 1.11 | 7.71 | random | ns | 9 |
| Site 2 | 165 ± 71.9 | 1.17 | 11.27 | random | ns | 9 | |
| September | Site 1 | 70 ± 30.7 | 1.18 | 4.86 | random | ns | 9 |
| Site 2 | 180 ± 85.6 | 1.21 | 14.71 | random | ns | 9 | |
| October | Site 1 | 105 ± 28.4 | 1.07 | 2.76 | random | ns | 9 |
| Site 2 | 105 ± 61.0 | 1.31 | 12.76 | clustered | ns | 9 | |
| November | Site 1 | 85 ± 44.4 | 1.25 | 8.35 | clustered | ns | 9 |
| Site 2 | 145 ± 35.0 | 1.05 | 3.03 | random | ns | 9 | |
| December | Site 1 | 157.5 ± 80.0 | 1.24 | 14.63 | clustered | ns | 9 |
| Site 2 | 127.5 ± 71.2 | 1.29 | 14.59 | clustered | ns | 9 | |
| January | Site 1 | 102.5 ± 60.6 | 1.32 | 12.89 | clustered | ns | 9 |
| Site 2 | 150 ± 60.1 | 1.15 | 8.67 | random | ns | 9 | |
| February | Site 1 | 190 ±62.6 | 1.1 | 7.42 | random | ns | 9 |
| Site 2 | 95 ± 38.7 | 1.15 | 5.68 | random | ns | 9 | |
| March | Site 1 | 87.5 ± 51.7 | 1.32 | 10.99 | clustered | ns | 9 |
| Site 2 | 140 ± 96.9 | 1.44 | 23.95 | clustered | <0.05 | 9 | |
| April | Site 1 | 77.5 ± 36.2 | 1.20 | 6.09 | random | ns | 9 |
| Site 2 | 137.5 ± 71.9 | 1.25 | 13.54 | random | ns | 9 |
| Source of Variation | DF | MS | F | p |
|---|---|---|---|---|
| Abundance | 3 | 773.98 | 34.35 | <0.001 |
| Error | 1036 | 22.53 | ||
| Total | 1039 |
| Age Group | Mean Length (mm) | Standard Deviation | Population Size | Separation Index (SI) | Population % |
|---|---|---|---|---|---|
| 1 | 11.83 | 2.220 | 8 | 3.370 | 0.82 |
| 2 | 18.78 | 1.900 | 228 | 2.620 | 23.41 |
| 3 | 23.13 | 1.420 | 408 | 2.400 | 41.89 |
| 4 | 26.17 | 1.110 | 213 | 3.930 | 21.87 |
| 5 | 30.96 | 1.330 | 78 | 3.010 | 8.01 |
| 6 | 34.62 | 1.100 | 28 | 3.890 | 2.87 |
| 7 | 40.53 | 1.940 | 11 | 3.370 | 1.13 |
| Morphometric Relationships | Equation Comparison | ||||||
|---|---|---|---|---|---|---|---|
| Sampling Site | Equation | N | R2 | t-test | Allometry | Slopes | Intercepts |
| Site 1 | Wt = 0.000083234 × L 3.03929 | 520 | 96.2 | ns | Isometry | * | ns |
| Site 2 | Wt = 0.000133015 × L 2.88733 | 520 | 90.8 | ns | Isometry | ||
| Site 1 | Wt = 0.000496301 × W 2.65566 | 520 | 94.6 | ** | -ve Allometry | * | ns |
| Site 2 | Wt = 0.000750993 × W 2.50773 | 520 | 89.8 | ** | -ve Allometry | ||
| Site 1 | Wt = 0.0135219 × H 2.48436 | 520 | 91.5 | ** | -ve Allometry | ** | ** |
| Site 2 | Wt = 0.0291897 × H 2.07913 | 520 | 82.7 | ** | -ve Allometry | ||
| Site 1 | L = 5.72765 × H 0.774654 | 520 | 88.4 | ** | -ve Allometry | ** | ** |
| Site 2 | L = 8.04943 × H 0.595556 | 520 | 76.2 | ** | -ve Allometry | ||
| Site 1 | L = 1.88689 × W 0.855741 | 520 | 93.2 | ** | -ve Allometry | ns | ns |
| Site 2 | L = 2.14392 × W 0.812553 | 520 | 90.3 | ** | -ve Allometry | ||
| Site 1 | W = 4.12051 × H 0.841331 | 520 | 87.4 | ** | -ve Allometry | ** | ** |
| Site 2 | W = 6.32293 × H 0.612731 | 520 | 70.6 | ** | -ve Allometry | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vafidis, D.; Drosou, I.; Dimitriou, K.; Klaoudatos, D. Population Characteristics of the Limpet Patella caerulea (Linnaeus, 1758) in Eastern Mediterranean (Central Greece). Water 2020, 12, 1186. https://doi.org/10.3390/w12041186
Vafidis D, Drosou I, Dimitriou K, Klaoudatos D. Population Characteristics of the Limpet Patella caerulea (Linnaeus, 1758) in Eastern Mediterranean (Central Greece). Water. 2020; 12(4):1186. https://doi.org/10.3390/w12041186
Chicago/Turabian StyleVafidis, Dimitris, Irini Drosou, Kostantina Dimitriou, and Dimitris Klaoudatos. 2020. "Population Characteristics of the Limpet Patella caerulea (Linnaeus, 1758) in Eastern Mediterranean (Central Greece)" Water 12, no. 4: 1186. https://doi.org/10.3390/w12041186
APA StyleVafidis, D., Drosou, I., Dimitriou, K., & Klaoudatos, D. (2020). Population Characteristics of the Limpet Patella caerulea (Linnaeus, 1758) in Eastern Mediterranean (Central Greece). Water, 12(4), 1186. https://doi.org/10.3390/w12041186






