Identification of Key Factors Affecting the Trophic State of Four Tropical Small Water Bodies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Sampling and Analysis of Water Parameters
2.3. Trophic State Index (TSI)
2.4. Water Residence Time (WRT)
2.5. Hydrogeochemical Modelling of Divalent Cations Minerals
2.6. Organic Carbon in Sediments (OC)
2.7. Spatial and Temporal Differences in Water Parameters
2.8. Partial Least Squares Regression (PLSR)
2.8.1. Determination Coefficient (R2) and Stone–Geisser Index (Q2)
2.8.2. Root of the Mean Square Error of Prediction (RMSEP)
2.8.3. Significance and Importance of Variables in PLSR Models
3. Results
3.1. Environmental Conditions in the Lakes (2013–2016)
3.2. Spatial and Temporal Differences in Water Parameters
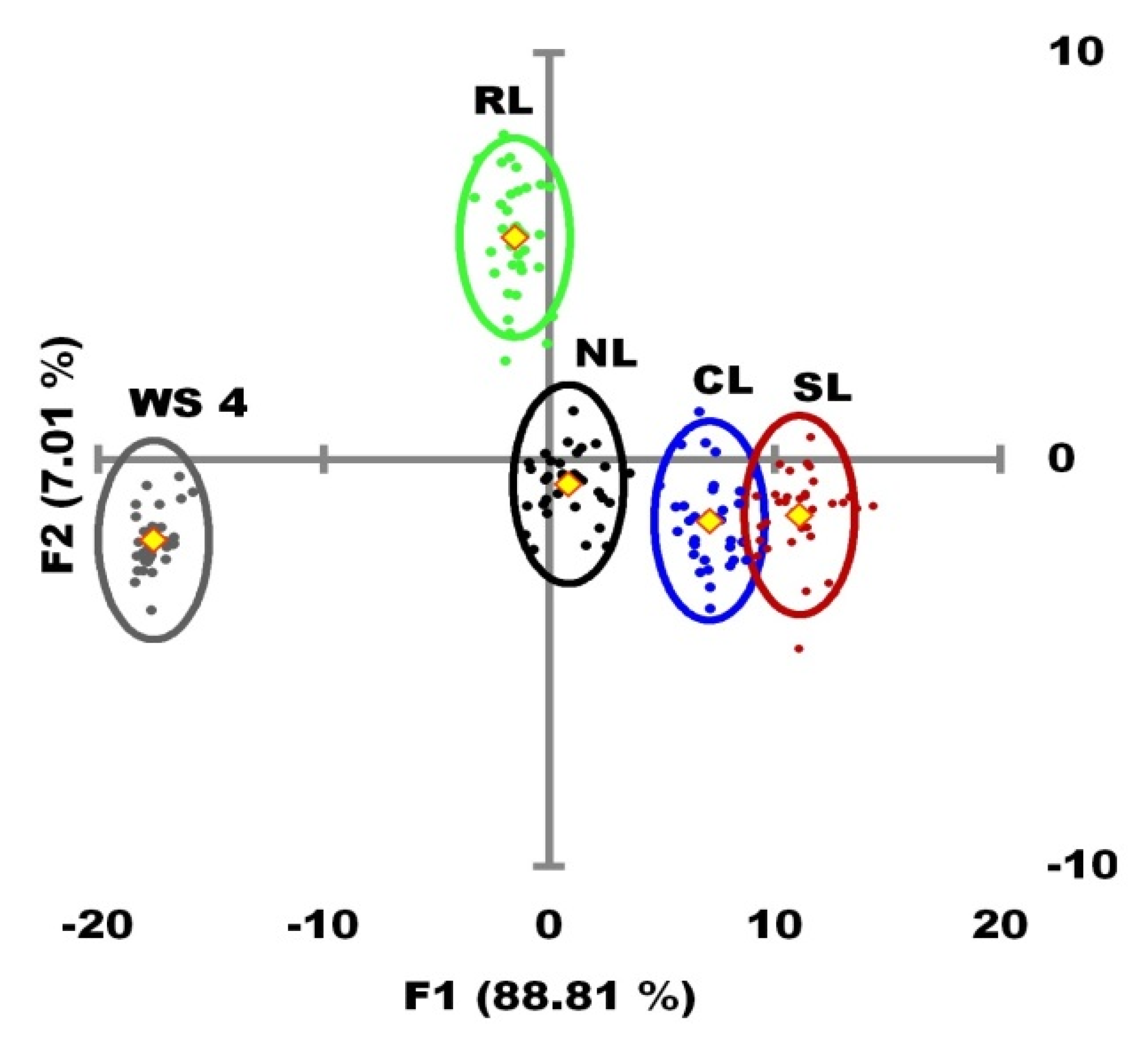
3.3. Identification of Key Factors in the Trophic State by PLSR
3.3.1. North Lake (NL)
3.3.2. Central Lake (CL)
3.3.3. South Lake (SL)
3.3.4. Regulation Lake (RL)
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Downing, J.A. Emerging global role of small lakes and ponds: Little things mean a lot. Limnetica 2010, 29, 9–24. [Google Scholar]
- Beklioğlu, M.; Meerhoff, M.; Davidson, T.A.; Ger, K.A.; Havens, K.; Moss, B. Preface: Shallow lakes in a fast changing world: The 8th International Shallow Lakes Conference. Hydrobiologia 2016, 778, 9–11. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Xu, M.; Li, R.; Zhang, L.; Deng, Q. Estimating the ecological water levels of shallow lakes: A case study in Tangxun Lake, China. Sci. Rep. 2020, 10, 5637. [Google Scholar] [CrossRef] [Green Version]
- Shueler, T.; Simpson, J. Why urban lakes are different. Watershed Prot. Tech. 2003, 3, 747–750. [Google Scholar]
- Schindler, D.W. Recent advances in the understanding and management of eutrophication. Limnol. Oceanogr. 2006, 51, 356–363. [Google Scholar] [CrossRef] [Green Version]
- Downing, J.A. Limnology and Oceanography: Two estranged twins reunited by global change. Inland Waters 2014, 4, 215–232. [Google Scholar] [CrossRef] [Green Version]
- Moss, B. Allied attack: Climate change and eutrophication. Inland Waters 2011, 1, 101–105. [Google Scholar] [CrossRef] [Green Version]
- Osborne, P. Eutrophication of Shallow Tropical Lakes. In The Lakes Handbook: Lake Restoration and Rehabilitation Volume 2; O’Sullivan, P.E., Reynolds, C.S., Eds.; Blackwell Publishing: Malden, MA, USA, 2005; pp. 279–299. [Google Scholar]
- Edmondson, W.T. Phosphorous, nitrogen and algae in Lake Washington after divertion of sewage. Science 1970, 169, 690–691. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.E.; Wu, X.; Hao, H.; He, Z. Mechanisms and assessment of water eutrophication. J. Zhejiang Univ. Sci. B 2008, 9, 1–2. [Google Scholar] [CrossRef]
- Lot, A.; Pérez, M.; Gil, G.; Rodríguez, S.; Camarena, P. La Reserva Ecológica del Pedregal de San Ángel: Atlas de Riesgos, 1st ed.; Reserva Ecológica del Pedregal de San Ángel UNAM, Ed.; UNAM: México, Mexico, 2012; pp. 6–9. [Google Scholar]
- Lot, A. Guía Ilustrada de la Cantera Oriente. Caracterización Ambiental e Inventario Biológico, 1st ed.; Lot, A., Ed.; UNAM: México, Mexico, 2007; pp. 7–29. [Google Scholar]
- Canteiro, M.; Olea, S.; Escolero, O.; Zambrano, L. Relationships between urban aquifers and preserved areas south of Mexico City. Groundw. Sustain. Dev. 2019, 8, 373–380. [Google Scholar] [CrossRef]
- Gutiérrez, S.G.; Sarma, S.S.S.; Nandini, S. Seasonal variations of rotifers from a high altitude urban shallow water body, La Cantera Oriente (Mexico City, Mexico). Chin. J. Oceanol. Limnol. 2017, 35, 1387–1397. [Google Scholar] [CrossRef]
- Google Inc. Google Earth Pro 2018; Google Inc.: Mountain View, CA, USA, 2018. [Google Scholar]
- SMN-CNA Resúmenes Mensuales de Temperaturas y Lluvia. Available online: https://smn.cna.gob.mx/es/climatologia/temperaturas-y-lluvias/resumenes-mensuales-de-temperaturas-y-lluvias (accessed on 4 December 2018).
- Martínez, O.H.; Flores, A.Q.; Ramírez-García, P.; Lot, A. Paisaje lacustre: Ecología de la vegetación acuática. In Guía Ilustrada de la Cantera Oriente. Caracterización Ambiental e Inventario Biológico; Lot, A., Ed.; UNAM: Mexico, Mexico, 2007; pp. 45–59. [Google Scholar]
- Novelo, E.; Ponce, E.; Ramírez, R. Las microalgas de la Cantera Oriente. In Biodiversidad del Ecosistema del Pedregal de San Ángel; Lot, A., Cano, S., Eds.; UNAM: Mexico, Mexico, 2009; pp. 71–80. [Google Scholar]
- Espinosa Pérez, H. Los peces y sus hábitats. In Biodiversidad del Pedregal de San Ángel; Cano-Santana, Z., Lot, A., Eds.; UNAM: México, Mexico, 2009; pp. 357–362. [Google Scholar]
- De la Cruz, F.M.; Vega, J.Z.; de la Vega Perez, A.D.; Resendiz, R.L.; Méndez, N.M. Anfibios y Reptiles. In Guía Ilustrada de la Cantera Oriente. Caracterización Ambiental e Inventario Biológico; Lot, A., Ed.; UNAM: México, Mexico, 2007; pp. 29–40. [Google Scholar]
- Fundación UNAM Crea UNAM Primer Albergue de Axolotes. Available online: http://www.fundacionunam.org.mx/ecopuma/alberguedeaxolotes/ (accessed on 21 October 2019).
- Chávez, N.; Gurrola, M.A. Aves. In Guía Ilustrada de la Cantera Oriente. Caracterización Ambiental e Inventario Biológico; UNAM, Ed.; UNAM: México, Mexico, 2007; pp. 221–253. [Google Scholar]
- APHA/AWWA/WEF. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; Rice, E., Baird, R., Eaton, A., Clesceri, L., Eds.; Port City Press: Baltimore, MD, USA, 2012. [Google Scholar]
- Arar, E.; Collins, G. Method: 445.0 In Vitro Determination of Chlorophyll a and Pheophytin a in Marine and Freshwater Algae by Fluorocence; EPA: Cincinnati, OH, USA, 1997; pp. 1–22. [Google Scholar]
- Carlson, R.E. Trophic state index for lakes. Limnol. Ocean. 1977, 22, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Yu, X.; Liu, L.; Zhang, W.; Guo, P. Algae community and trophic state of subtropical reservoirs in southeast Fujian, China. Environ. Sci. Pollut. Res. 2012, 19, 1432–1442. [Google Scholar] [CrossRef] [Green Version]
- Galizia Tundisi, J.; Matsumura-Tundisi, T. Limnology, 1st ed.; CRC Press: Boca Raton, FL, USA, 2011; p. 402. [Google Scholar]
- Rodier, J.; Lagube, B.; Merlet, N. Análsis del Agua, 9th ed.; Ediciones Omega: Barcelona, Spain, 2011; pp. 23–27. [Google Scholar]
- Inskeep, W.P.; Silvertooth, J.C. Kinetics of hydroxyapatite precipitation at pH 7.4 to 8.4. Geochim. Cosmochim. Acta 1988, 52, 1883–1893. [Google Scholar] [CrossRef]
- Danen-Louwerse, H.J.; Lijklema, L.; Coenraats, M. Coprecipitation of Phosphate with Calcium Carbonate in Lake Veluwe. Water Res. 1995, 29, 1781–1785. [Google Scholar] [CrossRef]
- Ryan, P. Environmental and Low Temperature Geochemistry, 1st ed.; Wiley Blackwell: Hoboken, NJ, USA, 2014; p. 126. [Google Scholar]
- Kalka, H. Aqion Hydrochemistry Software. Available online: www.aqion.de/download/ (accessed on 29 November 2019).
- Loring, D.H.; Rantala, R.R.T. Manual for the Geochemical analyses of Marine Sediments and Suspended Particulate Matter. Earth Sci. Rev. 1992, 32, 235–283. [Google Scholar] [CrossRef]
- Calvo, A.; Rodríguez, M. Análisis discriminante múltiple. In Análisis Multivariante para las Ciencias Sociales; Lévy, J.-P., Varela, J., Eds.; Pearson Educación: Madrid, Spain, 2003; pp. 249–276. [Google Scholar]
- Stern, L. A Visual Approach to SPSS for Windows. A guide to SPSS 17.0, 2nd ed.; Pearson-Allyn & Bacon: Boston, MA, USA, 2010; p. 169. [Google Scholar]
- Addinsoft. XLStat A Data Analysis and Statistical Solution for Microsoft Excel; Addinsoft: Paris, France, 2014. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Wold, S. PLS for multivariate linear modelling. In Chemometric Methods in Molecular Design; van de Waterbeemd, H., Ed.; Verlag Chemie: Weinheim, Germany, 1995; pp. 195–218. [Google Scholar]
- Dixon, W.T. Processing Data for Outliers. Biometrics 1953, 9, 74–89. [Google Scholar] [CrossRef]
- Valdéz, D. Regresión por Mínimos Cuadrados Parciales. Varianza 2010, 7, 18–22. [Google Scholar]
- Barroso, C.; Carrión, G.C.; Roldán, J. Applying Maximum Likelihood and PLS on Different Sample Sizes: Studies on SERVQUAL Model and Employee Behavior Model. In Handbook of Partial Least Squares Concepts, Methods and Applications; Esposito, V., Chin, W., Henseler, J., Wang, H., Eds.; Springer-Science+Business Media, B.V.: Berlin, Germany, 2010; pp. 427–447. [Google Scholar]
- Mevik, B.H.; Cederkvist, H.R. Mean squared error of prediction (MSEP) estimates for principal component regression (PCR) and partial least squares regression (PLSR). J. Chemom. 2004, 18, 422–429. [Google Scholar] [CrossRef]
- Vega-Vilca, C.; Guzmán, J. Regresión PLS y PCA como solución al problema de multicolinealidad en regresion múltiple. Rev. Matemática Teoría Y Apl. 2011, 18, 9–20. [Google Scholar] [CrossRef] [Green Version]
- Tenenhaus, M.; Pagès, J.; Ambroisine, L.; Guinot, C. PLS methodology to study relationships between hedonic judgements and product characteristics. Food Qual. Prefer. 2005, 16, 315–325. [Google Scholar] [CrossRef]
- Farrés, M.; Platikanov, S.; Tsakovski, S.; Tauler, R. Comparison of the variable importance in projection (VIP) and of the selectivity ratio (SR) methods for variable selection and interpretation. J. Chemom. 2015, 29, 528–536. [Google Scholar] [CrossRef]
- Eriksson, L.; Byrne, T.; Johansson, E.; Trygg, J.; Vikström, C. Multi- and Megavariate Data Analysis, 3rd ed.; Umetrics Academy: Malmö, Sweden, 2013; p. 75. [Google Scholar]
- Eggleton, R.A.; Foudoulis, C.; Varkevisser, D. Weathering of Basalt: Changes in Rock Chemistry and Mineralogy. Clays Clay Miner. 1987, 35, 161–169. [Google Scholar] [CrossRef]
- Kessler, T.J.; Harvey, C.F. The global flux of carbon dioxide into groundwater. Geophys. Res. Lett. 2001, 28, 279–282. [Google Scholar] [CrossRef] [Green Version]
- Ramírez, C.A.S. Calidad de Agua. Evaluación y diagnóstico, 1st ed.; Ediciones de la U-Universidad de Medellín: Medellín, Colombia, 2011; pp. 267–268. [Google Scholar]
- Liu, Y.; Li, L.; Jia, R. The optimum resource ratio (N:P) for the growth of Microcystis aeruginosa with abundant nutrients. Procedia Environ. Sci. 2011, 10, 2134–2140. [Google Scholar] [CrossRef] [Green Version]
- Guildford, S.J.; Hecky, R.E. Total nitrogen, total phosphorus, and nutrient limitation in lakes and oceans: Is there a common relationship? Limnol. Oceanogr. 2000, 45, 1213–1223. [Google Scholar] [CrossRef] [Green Version]
- Tung, M. Calcium phosphates: Structure, composition, solubility, and stability. In Calcium Phosphates in Biological and Industrial Systems; Amjad, Z., Ed.; Kluwer Academic Publishers: Amsterdam, The Netherlands, 1998; pp. 1–19. [Google Scholar]
- Wetzel, R.G. Limnology Lake and Rivers Ecosistems, 3rd ed.; Academic Press: San Diego, CA, USA, 2001; pp. 190–191, 409–411. [Google Scholar]
- Contreras, F. Manual de Técnicas Hidrobiológicas, 1st ed.; Trillas: México, Mexico, 1994; p. 103. [Google Scholar]
- Jeff, S.; Hunter, K.; Vandergucht, D.; Hudson, J. Photochemical mineralization of dissolved organic nitrogen to ammonia in prairie lakes. Hydrobiologia 2012, 693, 71–80. [Google Scholar] [CrossRef]
- Weiner, E. Applications of Environmental Aquatic Chemistry A Practical Guide, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2013; p. 442. [Google Scholar]
- Dodds, W.K.; Smith, V.H.; Lohman, K. Nitrogen and phosphorus relationships to benthic algal biomass in temperate streams. Can. J. Fish. Aquat. Sci. 2002, 59, 865–874. [Google Scholar] [CrossRef] [Green Version]
- Rocha, R.; Thomaz, S.; Carvalho, P.; Gomes, L. Modeling chlorophyll-a and dissolved oxygen concentration in tropical floodplain lakes (Paraná River, Brazil). Braz. J. Biol. 2009, 69, 491–500. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Ha, S.-Y.; Park, H.-K.; Han, M.-S.; Shin, K.-H. Identification of key factors influencing primary productivity in two river-type reservoirs by using principal component regression analysis. Environ. Monit. Assess. 2015, 187, 213. [Google Scholar] [CrossRef]
- Çamdevýren, H.; Demýr, N.; Kanik, A.; Keskýn, S. Use of principal component scores in multiple linear regression models for prediction of Chlorophyll-a in reservoirs. Ecol. Model. 2005, 181, 581–589. [Google Scholar] [CrossRef]
- Secretaría de Salud. NOM-127-SSA1-1994 Salud Ambiental. Agua para uso y Consumo Humano. Límites Permisibles de Calidad y Tratamientos a que debe Someterse el Agua para su Potabilización; Diario Oficial de la Federación: México D.F, México, 2000. Available online: http://www.dof.gob.mx/nota_detalle.php?codigo=2063863&fecha=31/12/1969/ (accessed on 5 July 2019).
- Palma, S.M.; Armienta Hernández, M.A.; Rodríguez Castillo, R.; Domínguez Mariani, E. Identificación de zonas de contaminación por nitratos en el agua subterránea de la zona sur de la cuenca de México. Rev. Int. Contam. Ambie. 2014, 30, 137–142. [Google Scholar]
- Negendank, J.F.W. Volcanics of the Valley of Mexico. Description of some mexican volcanic rocks with special consideration of the opaques. Part I: Petrography of the volcanics. Neues Jarhb. Fuer Miner. Abh. 1972, 116, 308–320. [Google Scholar]
- Wedepohl, K.H. The composition of the continental crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Badilla Cruz, R. Estudio Petrológico de la Lava de la Parte Noreste del Pedregal de San Ángel, D.F. Boletín Soc. Geológica Mex. 1977, 38, 40–57. [Google Scholar] [CrossRef]
- Smith, K.L.; Milnes, A.R.; Eggleton, R.A. Weathering of basalt: Formation of iddingsite. Clays Clay Miner 1987, 35, 418–428. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, C.; Ji, L.; Liu, Y.; Xiao, J.; Cao, X.; Zhou, Y. Cause and effect of N/P ratio decline with eutrophication aggravation in shallow lakes. Sci. Total Environ. 2018, 627, 1294–1302. [Google Scholar] [CrossRef]
- Schallenberg, M.; Burns, C.W. Phytoplankton biomass and productivity in two oligotrophic lakes of short hydraulic residence time. N. Z. J. Mar. Freshw. Res. 1997, 31, 119–134. [Google Scholar] [CrossRef] [Green Version]
- Momberg, G.A.; Oellermann, R.A. The removal of phosphate by hydroxyapatite and struvite crystallisation in South Africa. Water Sci. Technol. 1992, 26, 987–996. [Google Scholar] [CrossRef]
- Inskeep, W.P.; Silvertooth, J.C. Inhibition of Hydroxyapatite Precipitation. Soil Sci. Soc. Am. J. 1998, 52, 941–946. [Google Scholar] [CrossRef]
- Kleiner, J. Coprecipitation of phosphate with calcite in lake water: A laboratory experiment modelling phosphorus removal with calcite in Lake Constance. Water Res. 1988, 22, 1259–1265. [Google Scholar] [CrossRef]
- Murphy, T.P.; Hall, K.J.; Yesaki, I. Coprecipitation of phosphate with calcite in a naturally eutrophic lake. Limnol. Oceanogr. 1983, 28, 58–69. [Google Scholar] [CrossRef]
- Jensen, H.S.; Andersen, F.O. Importance of temperature, nitrate, and pH for phosphate release from aerobic sediments of four shallow, eutrophic lakes. Limnol. Oceanogr. 1992, 37, 577–589. [Google Scholar] [CrossRef]
- Kosmulski, M. Compilation of PZC and IEP of sparingly soluble metal oxides and hydroxides from literature. Adv. Colloid Interface Sci. 2009, 152, 14–25. [Google Scholar] [CrossRef]
- Song, K.; Burgin, A.J. Perpetual Phosphorus Cycling: Eutrophication Amplifies Biological Control on Internal Phosphorus Loading in Agricultural Reservoirs. Ecosystems 2017, 20, 1483–1493. [Google Scholar] [CrossRef]
- Hogsett, M.; Li, H.; Goel, R. The Role of Internal Nutrient Cycling in a Freshwater Shallow Alkaline Lake. Environ. Eng. Sci. 2019, 36, 551–563. [Google Scholar] [CrossRef]
- Qin, B.; Zhou, J.; Elser, J.J.; Gardner, W.S.; Deng, J.; Brookes, J.D. Water Depth Underpins the Relative Roles and Fates of Nitrogen and Phosphorus in Lakes. Environ. Sci. Technol. 2020, 54, 3191–3198. [Google Scholar] [CrossRef]
- Lugo-Vázquez, A.; Sánchez-Rodríguez, M.; Morlán-Mejía, J.; Peralta-Soriano, L.; Arellanes-Jiménez, E.A.; Oliva Martínez, M.G. Ciliates and trophic state: A study in five adjacent urban ponds in Mexico City. J. Environ. Biol. 2017, 38, 1161–1169. [Google Scholar] [CrossRef]
- Jeppesen, E.; Meerhoff, M.; Jacobsen, B.A.; Hansen, R.S.; Søndergaard, M.; Jensen, J.P.; Lauridsen, T.L.; Mazzeo, N.; Branco, C.W.C. Restoration of shallow lakes by nutrient control and biomanipulation—The successful strategy varies with lake size and climate. Hydrobiologia 2007, 581, 269–285. [Google Scholar] [CrossRef]
- Hosper, S.H. Restoration of Lake Veluwe, The Netherlands, by reduction of phosphorus loading and flushing. Water Sci. Technol. 1985, 17, 757–768. [Google Scholar] [CrossRef]
- Spears, B.M.; Carvalho, L.; Paterson, D.M. Phosphorus partitioning in a shallow lake: Implications for water quality management. Water Environ. J. 2007, 21, 47–53. [Google Scholar] [CrossRef]
- Yang, S.Q.; Liu, P.W. Strategy of water pollution prevention in Taihu Lake and its effects analysis. J. Great Lakes Res. 2010, 36, 150–158. [Google Scholar] [CrossRef] [Green Version]





| Parameter | NL Mean ± SD (Min-Max) | CL Mean ± SD (Min-Max) | SL Mean ± SD (Min-Max) | RL Mean ± SD (Min-Max) | WS 4 Mean ± SD (Min-Max) |
|---|---|---|---|---|---|
| Depth (cm) | 94 ± 17 (60–120) | 118 ± 13 (100–150) | 109 ± 16 (80–150) | 121 ± 11 (100–140) | 65 ± 14 (20–100) |
| WRT (days) | 5 ± 5 (2–16) | 9 ± 11 (2–31) | 15 ± 14 (3–90) | 3 ± 5 (1–9) | ≈0 |
| T (°C) | 16.5 ± 1.7 (12.1–18.6) | 17.5 ± 1.7 (13.4–19.8) | 18.8 ± 1.8 (14.1–21.1) | 17.8 ± 1 (14.9–19.5) | 17 ± 0.6 (15.2–18.5) |
| K25 (µS/cm) | 411 ± 33 (362–480) | 398 ± 34 (331–472) | 385 ± 23 (340–423) | 400 ± 21 (358–452) | 415 ± 35 (364–491) |
| Ca2+ (mg/L) | 26 ± 4 (19–42) | 26 ± 4 (17–40) | 23 ± 3 (19–34) | 24 ± 3 (21–36) | 26 ± 4 (22–37) |
| Mg2+ (mg/L) | 14 ± 3 (7–22) | 13 ± 2 (6–19) | 13 ± 3 (7–20) | 14 ± 3 (7–19) | 13 ± 3 (5–20) |
| CO32− (mg/L) | 6 ± 32 (0–192) | 37 ± 38 (0–160) | 52 ± 29 (0–144) | 5 ± 22 (0–128) | 0 ± 0 (0–0) |
| HCO3− (mg/L) | 75 ± 31 (32–184) | 39 ± 27 (12–112) | 22 ± 20 (2–72) | 66 ± 26 (36–168) | 67 ± 42 (32–256) |
| N-NO3− (mg/L) | 4.7 ± 1.1 (2.1–6.5) | 4.6 ± 0.9 (2.4–6.3) | 4.4 ± 1.3 (1.9–8.8) | 7.2 ± 1.3 (2.9–9.1) | 7.3 ± 1.4 (3.7–10.4) |
| N-NO2− (mg/L) | 0.03 ± 0.2 (0.01–0.12) | 0.05 ± 0.02 (0.01–0.13) | 0.09 ± 0.04 (0.02–0.18) | 0.03 ± 0.02 (0.01–0.13) | 0.01 ± 0.01 (0.01–0.07) |
| N-NH3 (mg/L) | 0.08 ± 0.05 (0.01–0.23) | 0.07 ± 0.06 (0.01–0.27) | 0.07 ± 0.05 (0.01–0.25) | 0.02 ± 0.02 (0.01–0.09) | 0.03 ± 0.03 (0.01–0.17) |
| N-Org (mg/L) | 1.7 ± 1.3 (0.2–4.9) | 2.7 ± 2.1 (0.3–8.8) | 3.4 ± 1.9 (0.3–9.5) | 2.0 ± 1.7 (0.1–6.3) | 2.5 ± 2.3 (0.1–11.2) |
| TN (mg/L) | 6.6 ± 1.3 (4.5–11.8) | 7.9 ± 3.2 (5.1–22.2) | 8.3 ± 2.7 (5.1–18.6) | 9.9 ± 3.5 (6.6–28.1) | 11.1 ± 7.5 (6.1–52.7) |
| P-PO43− (mg/L) | 0.10 ± 0.4 (0.05–0.25) | 0.08 ± 0.07 (0.01–0.35) | 0.05 ± 0.05 (0.01–0.25) | 0.13 ± 0.07 (0.06–0.38) | 0.13 ± 0.03 (0.09–0.25) |
| P-Org (mg/L) | 0.05 ± 0.03 (0.02–0.11) | 0.12 ± 0.09 (0.02–0.42) | 0.12 ± 0.16 (0.01–0.97) | 0.06 ± 0.06 (0.01–0.29) | 0.03 ± 0.03 (0.01–0.15) |
| TP (mg/L) | 0.21 ± 0.13 (0.10–0.55) | 0.19 ± 0.13 (0.05–0.58) | 0.17 ± 0.16 (0.06–1.03) | 0.18 ± 0.12 (0.09–0.58) | 0.18 ± 0.10 (0.10–0.52) |
| P-HAP (mg/L) | 0 ± 0 (0–0) | 0.033 ± 0.026 (0–0.14) | 0.006 ± 0.007 (0.001–0.035) | 0 ± 0 (0–0) | 0 ± 0 (0–0) |
| TN:TP (by mass) | 39 ± 16 (13–63) | 49 ± 18 (14–84) | 69 ± 37 (10–199) | 67 ± 23 (14–120) | 62 ± 20 (18–133) |
| Chl a (µg/L) | 57 ± 39 (7–147) | 131 ± 80 (16–336) | 162 ± 120 (39–622) | 21 ± 16 (4–63) | 6 ± 8 (1–36) |
| zSD (cm) | 77 ± 19 (45–120) | 54 ± 23 (20–120) | 37 ± 14 (8–80) | 112 ± 16 (65–140) | 61 ± 11 (43–100) |
| DO (mg/L) | 9.4 ± 3.1 (5–17.2) | 14.1 ± 4.9 (6–23.3) | 17 ± 4.7 (5.3–25.3) | 12.9 ± 4.2 (7.9–28.4) | 6.8 ± 1.7 (4.8–13.5) |
| pH | 7.4 ± 0.5 (6.7–9.0) | 8.3 ± 0.5 (7.2–9.7) | 9.0 ± 0.4 (8.1–10) | 7.8 ± 0.6 (6.9–9.9) | 7.3 ± 0.4 (6.6–8.6) |
| TSI-Int | 69 ± 5 (60–77) Medium Eutrophic | 75 ± 5 (63–83) Hyper Eutrophic | 77 ± 5 (66–88) Hyper Eutrophic | 61 ± 4 (52–73) Medium Eutrophic | 57 ± 5 (48–68) Light Eutrophic |
| pH | NL SICalcite | CL SICalcite | SL SICalcite | RL SICalcite | WS 4 SICalcite |
|---|---|---|---|---|---|
| Mean ± SD 1 | −1.30 to 0.49 | 0.22 to 1.13 | 0.99 to 1.59 | −0.42 to 0.88 | −0.77 to 0.10 |
| Parameter | NL | CL | SL | RL | WS 4 |
|---|---|---|---|---|---|
| OC (%) | 11.75 ± 0.12 | 4.37 ± 0.10 | 1.96 ± 0.09 | 3.09 ± 0.10 | Not Measured |
| Parameter | F1 | F2 |
|---|---|---|
| K25 | −0.295 | −0.012 |
| DO | 0.695 | 0.147 |
| pH | 0.755 | −0.104 |
| Chl a | 0.866 | −0.214 |
| zSD | −0.341 | 0.691 |
| Ca2+ | 0.021 | −0.918 |
| Mg2+ | −0.067 | 0.786 |
| CO32− | 0.682 | −0.360 |
| HCO3− | −0.429 | 0.248 |
| N-NO3− | −0.578 | 0.429 |
| N-NO2− | 0.625 | −0.207 |
| N-NH3 | 0.341 | −0.271 |
| P-PO43− | −0.487 | 0.186 |
| P-Org | 0.387 | −0.141 |
| WRT | 0.997 | −0.022 |
| Variance Explained | 88.81% | 7.01% |
| Wilk’s lambda | <0.001 | 0.014 |
| p-value | <0.001 | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuevas Madrid, H.; Lugo Vázquez, A.; Peralta Soriano, L.; Morlán Mejía, J.; Vilaclara Fatjó, G.; Sánchez Rodríguez, M.d.R.; Escobar Oliva, M.A.; Carmona Jiménez, J. Identification of Key Factors Affecting the Trophic State of Four Tropical Small Water Bodies. Water 2020, 12, 1454. https://doi.org/10.3390/w12051454
Cuevas Madrid H, Lugo Vázquez A, Peralta Soriano L, Morlán Mejía J, Vilaclara Fatjó G, Sánchez Rodríguez MdR, Escobar Oliva MA, Carmona Jiménez J. Identification of Key Factors Affecting the Trophic State of Four Tropical Small Water Bodies. Water. 2020; 12(5):1454. https://doi.org/10.3390/w12051454
Chicago/Turabian StyleCuevas Madrid, Homero, Alfonso Lugo Vázquez, Laura Peralta Soriano, Josué Morlán Mejía, Gloria Vilaclara Fatjó, María del Rosario Sánchez Rodríguez, Marco Antonio Escobar Oliva, and Javier Carmona Jiménez. 2020. "Identification of Key Factors Affecting the Trophic State of Four Tropical Small Water Bodies" Water 12, no. 5: 1454. https://doi.org/10.3390/w12051454
APA StyleCuevas Madrid, H., Lugo Vázquez, A., Peralta Soriano, L., Morlán Mejía, J., Vilaclara Fatjó, G., Sánchez Rodríguez, M. d. R., Escobar Oliva, M. A., & Carmona Jiménez, J. (2020). Identification of Key Factors Affecting the Trophic State of Four Tropical Small Water Bodies. Water, 12(5), 1454. https://doi.org/10.3390/w12051454





