Metagenomic Profiles of Antibiotic Resistance Genes in Activated Sludge, Dewatered Sludge and Bioaerosols
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling
2.2. DNA Extraction and High-Throughput Sequencing
2.3. Bioinformatics Analysis
3. Results and Discussion
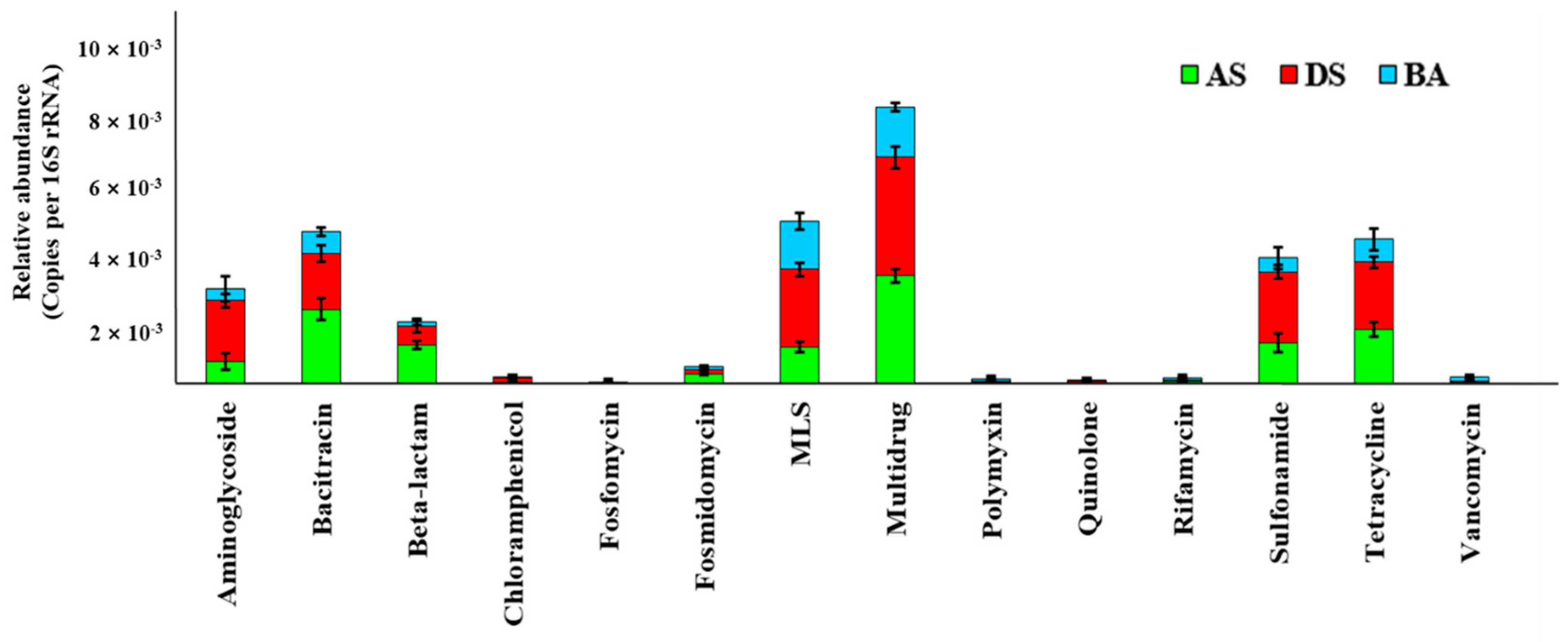
3.1. Prevalence of ARGs in AS, DS, and BA
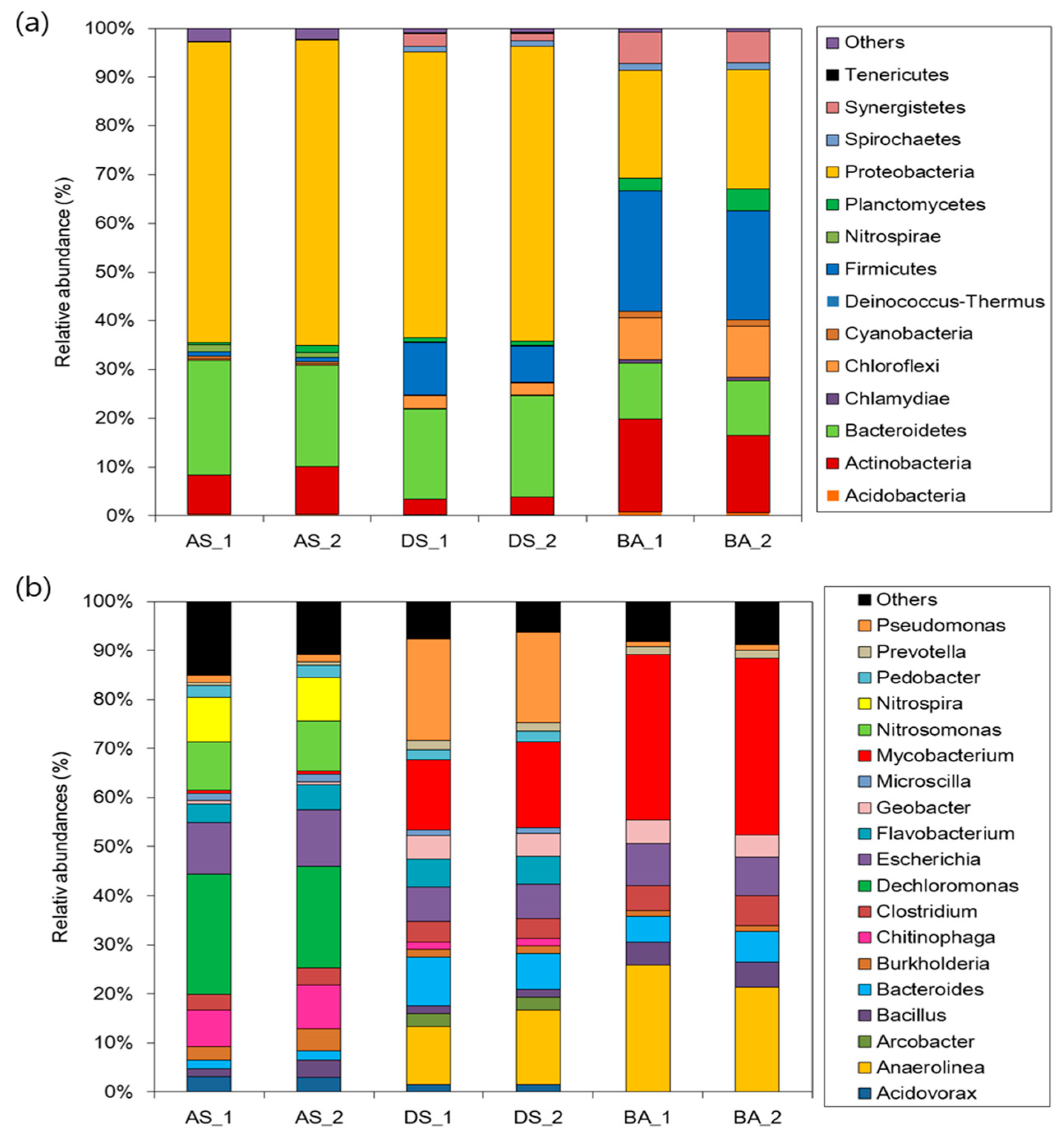
3.2. Phylogenetic Characterization of the Bacterial Community
3.3. Prevalence of Mobile Genetic Elements (MGEs)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Walsh, C. Antibiotics: Actions, Origins, Resistance; ASM Press: Washington, DC, USA, 2003. [Google Scholar]
- O’Neill, J. 2016 Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. Available online: https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf (accessed on 10 December 2018).
- Rizzo, L.; Manaia, C.; Merlin, C.; Schwartz, T.; Dagot, C.; Ploy, M.C.; Michael, I.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: A review. Sci. Total Environ. 2013, 447, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Bouki, C.; Venieri, D.; Diamadopoulos, E. Detection and fate of antibiotic resistant bacteria in wastewater treatment plants: A review. Ecotoxicol. Environ. Saf. 2013, 91, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pruden, A.; Larsson, D.J.; Amézquita, A.; Collignon, P.; Brandt, K.K.; Graham, D.W.; Lazorchak, J.M.; Suzuki, S.; Silley, P.; Snape, J.R.; et al. Management options for reducing the release of antibiotics and antibiotic resistance genes to the environment. Environ. Heal. Persp. 2013, 121, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yang, Y.; Ma, L.; Ju, F.; Guo, F.; Tiedje, J.M.; Zhang, T. Metagenomic and network analysis reveal wide distribution and co-occurrence of environmental antibiotic resistance genes. ISME J. 2015, 9, 2490–2502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, K.; Yoo, H.; Lee, J.; Choi, E.; Park, J. Exploring the antibiotic resistome in activated sludge and anaerobic digestion sludge in an urban wastewater treatment plant via metagenomic analysis. J. Microbiol. 2020, 58, 123–130. [Google Scholar] [CrossRef]
- Quach-Cu, J.; Herrera-Lynch, B.; Marciniak, C.; Adams, S.; Simmerman, A.; Reinke, R.A. The Effect of Primary, Secondary, and Tertiary Wastewater Treatment Processes on Antibiotic Resistance Gene (ARG) Concentrations in Solid and Dissolved Wastewater Fractions. Water 2018, 10, 37. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Li, J.; Chen, H.; Bond, P.L.; Yuan, Z. Metagenomic analysis reveals wastewater treatment plants as hotspots of antibiotic resistance genes and mobile genetic elements. Water Res. 2017, 123, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.L. Environmental pollution by antibiotics and by antibiotic resistance determinants. Environ. Pollut. 2009, 157, 2893–2902. [Google Scholar] [CrossRef]
- Li, J.; Cheng, W.; Xu, L.; Jiao, Y.; Baig, S.A.; Chen, H. Occurrence and removal of antibiotics and the corresponding resistance genes in wastewater treatment plants: Effluents’ influence to downstream water environment. Environ. Sci. Pollut. R. 2015, 23, 6826–6835. [Google Scholar] [CrossRef]
- Karkman, A.; Do, T.T.; Walsh, F.; Virta, M.P. Antibiotic-resistance genes in wastewater. Trends Microbiol. 2018, 26, 220–228. [Google Scholar] [CrossRef] [Green Version]
- Mao, D.; Yu, S.; Rysz, M.; Lou, Y.; Yang, F.; Li, F.; Hou, J.; Mu, Q.; Alvarez, P.J.J. Prevalence and proliferation of antibiotic resistance genes in two municipal wastewater treatment plants. Water Res. 2015, 85, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Diehl, D.L.; LaPara, T.M. Effect of temperature on the fate of genes encoding tetracycline resistance and the integrase of class 1 integrons within anaerobic and aerobic digesters treating municipal wastewater solids. Environ. Sci. Technol. 2010, 44, 9128–9133. [Google Scholar] [CrossRef]
- Chen, B.; Yang, Y.; Liang, X.; Yu, K.; Zhang, T.; Li, X. Metagenomic profiles of antibiotic resistance genes (ARGs) between human impacted estuary and deep ocean sediments. Environ. Sci. Technol. 2013, 47, 12753–12760. [Google Scholar] [CrossRef] [PubMed]
- Burch, T.R.; Sadowsky, M.J.; LaPara, T.M. Fate of Antibiotic Resistance Genes and Class 1 Integrons in Soil Microcosms Following the Application of Treated Residual Municipal Wastewater Solids. Environ. Sci. Technol. 2014, 46, 5620–5627. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, Y.; Wang, H.; Guo, C.; Qiu, H.; He, Y.; Zhang, Y.; Li, X.; Meng, W. Occurrence of antibiotics and antibiotic resistance genes in a sewage treatment plant and its effluent-receiving river. Chemosphere 2015, 119, 1379–1385. [Google Scholar] [CrossRef]
- Ross, J.; Topp, E. Abundance of antibiotic resistance genes in bacteriophage following soil fertilization with dairy manure or municipal biosolids, and evidence for potential transduction. Appl. Environ. Microbiol. 2015, 81, 7905–7913. [Google Scholar] [CrossRef] [Green Version]
- Yoo, K.; Lee, T.K.; Choi, E.J.; Yang, J.; Shukla, S.K.; Hwang, S.I.; Park, J. Molecular approaches for the detection and monitoring of microbial communities in bioaerosols: A review. J. Environ. Sci. 2017, 51, 234–247. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, R.; Chen, B.; Zhang, T.; Hu, L.; Zou, S. Characterization of airborne antibiotic resistance genes from typical bioaerosol emission sources in the urban environment using metagenomic approach. Chemosphere 2018, 213, 463–471. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, X.; Li, Z.; Kuang, Y.; Mao, D.; Lou, Y. Occurrence and Distribution of Urban Dust-Associated Bacterial Antibiotic Resistance in Northern China. Environ. Sci. Technol. Lett. 2018, 5, 50–55. [Google Scholar] [CrossRef]
- Brandi, G.; Sisti, M.; Amagliani, G. Evaluation of the environmental impact of microbial aerosols generated by wastewater treatment plants utilizing different aeration systems. J. Appl. Microbiol. 2000, 88, 845–852. [Google Scholar] [CrossRef]
- Li, J.; Zhou, L.; Zhang, X.; Xu, C.; Dong, L.; Yao, M. Bioaerosol emissions and detection of airborne antibiotic resistance genes from a wastewater treatment plant. Atmos. Environ. 2016, 124, 404–412. [Google Scholar] [CrossRef]
- Makowska, N.; Koczura, R.; Mokracka, J. Class1 integrase, sulfonamide and tetracycline resistance genes in wastewater treatment plant and surface water. Chemosphere 2016, 144, 1665–1673. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jeon, J.H.; Shin, J.; Jang, H.M.; Kim, S.; Song, M.S.; Kim, Y.M. Quantitative and qualitative changes in antibiotic resistance genes after passing through treatment processes in municipal wastewater treatment plants. Sci. Total Environ. 2017, 605–606, 906–914. [Google Scholar] [CrossRef]
- Yoo, K.; Yoo, H.; Lee, J.M.; Shukla, S.K.; Park, J. Classification and Regression Tree Approach for Prediction of Potential Hazards of Urban Airborne Bacteria during Asian Dust Events. Sci. Rep. 2018, 8, 11823. [Google Scholar] [CrossRef]
- Radosevich, J.L.; Wilson, W.J.; Shinn, J.H.; DeSanties, T.Z.; Andersen, G.L. Development of a high-volume aerosol collection system for the identification of air-borne micro-organisms. Lett. Appl. Microbiol. 2002, 34, 162–167. [Google Scholar] [CrossRef] [Green Version]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, X.L.; Jiang, X.T.; Chai, B.L.; Li, L.G.; Yang, Y.; Cole, J.R.; Tiedje, J.M.; Zhang, T. ARGs-OAP v2.0 with an expanded SARG database and Hidden Markov Models for enhancement characterization and quantification of antibiotic resistance genes in environmental metagenomes. Bioinformatics 2018, 34, 2263–2270. [Google Scholar] [CrossRef] [Green Version]
- Kristiansson, E.; Fick, J.; Janzon, A.; Grabic, R.; Rutgersson, C.; Weijdegard, B.; Soderstrom, H.; Larsson, D.G.J. Pyrosequencing of antibiotic-contaminated river sediments reveals high levels of resistance and gene transfer elements. PLoS ONE 2011, 6, e17038. [Google Scholar] [CrossRef]
- Fang, H.; Huang, K.; Yu, J.; Ding, C.; Wang, Z.; Zhao, C.; Yuan, H.; Wang, Z.; Wang, S.; Hu, J.; et al. Metagenomic analysis of bacterial communities and antibiotic resistance genes in the Eriocheir sinensis freshwater aquaculture environment. Chemosphere 2019, 224, 202–211. [Google Scholar] [CrossRef]
- Truong, D.T.; Franzosa, E.A.; Tickle, T.L.; Scholz, M.; Weingart, G.; Pasolli, E.; Tett, A.; Huttenhower, C.; Segata, N. MetaPhlAn2 for enhanced metagenomic taxonomic profiling. Nat. Methods 2015, 12, 902–903. [Google Scholar] [CrossRef]
- Brown, K.D.; Kulis, J.; Thomson, B.; Chapman, T.H.; Mawhinney, D.B. Occurrence of antibiotics in hospital, residential, and dairy effluent, municipal wastewater, and the Rio Grande in New Mexico. Sci. Total Environ. 2006, 366, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Kim, Y.; Park, J.; Park, C.K.; Kim, M.; Kim, H.S.; Kim, P. Seasonal variations of several pharmaceutical residues in surface water and sewage treatment plants of Han River, Korea. Sci. Total Environ. 2008, 405, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Ok, Y.S.; Kim, S.C.; Kim, K.R.; Lee, S.S.; Moon, D.H.; Lim, K.J.; Sung, J.-K.; Hur, S.-O.; Yang, J.E. Monitoring of selected veterinary antibiotics in environmental compartments near a composting facility in Gangwon Province, Korea. Environ. Monit. Assess. 2011, 174, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Han, E.; Lee, S.O.; Kim, D.S. Antibiotic use in South Korea from 2007 to 2014: A health insurance database-generated time series analysis. PLoS ONE 2017, 12, e0177435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yang, Y.; Pruden, A. Effect of temperature on removal of antibiotic resistance genes by anaerobic digestion of activated sludge revealed by metagenomic approach. Appl. Microbiol. Biotechnol. 2015, 99, 7771–7779. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Munir, M.; Xagoraraki, I. Correlation of tetracycline and sulfonamide antibiotics with corresponding resistance genes and resistant bacteria in a conventional municipal wastewater treatment plant. Sci. Total Environ. 2012, 421–422, 173–183. [Google Scholar] [CrossRef]
- Toth, M.; Antunes, N.T.; Stewart, N.K.; Frase, H.; Bhattacharya, M.; Smith, C.A.; Vakulenko, S.B. Class D β-lactamases do exist in Gram-positive bacteria. Nat. Chem. Biol. 2015, 12, 9. [Google Scholar] [CrossRef]
- Bonomo, R.A. β-Lactamases: A Focus on Current Challenges. Cold Spring Harb. Perspect Med. 2017, 7, a025239. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Huang, K.; Miao, Y.; Shi, P.; Liu, B.; Long, C.; Li, A. Metagenomic Profiling of Antibiotic Resistance Genes and Mobile Genetic Elements in a Tannery Wastewater Treatment Plant. PLoS ONE 2013, 8, e76079. [Google Scholar] [CrossRef] [PubMed]
- Munir, M.; Wong, K.; Xagoraraki, I. Release of antibiotic resistant bacteria and genes in the effluent and biosolids of five wastewater utilities in Michigan. Water Res. 2011, 45, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, M.E.; Meyer, T.M.; Thurman, E.M. Analysis of trace levels of sulfonamide and tetracycline antimicrobials in groundwater and surface water using solid-phase extraction and liquid chromatography/mass spectrometry. Anal. Chem. 2001, 73, 4640–4646. [Google Scholar] [CrossRef] [PubMed]
- Batt, A.L.; Bruce, I.B.; Aga, D.S. Evaluating the vulnerability of surface waters to antibiotic contamination from varying wastewater treatment plant discharges. Environ. Pollut. 2006, 142, 295–302. [Google Scholar] [CrossRef]
- Antunes, P.; Machado, J.; Sousa, J.C.; Peixe, L. Dissemination of sulfonamide resistance genes (sul1, sul2, and sul3) in Portuguese Salmonella enterica strains and relation with integrons. Antimicrob. Agents Chemother. 2005, 49, 836–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Wilson, C.A.; Novak, J.T.; Riffat, R.; Aynur, S.; Murthy, S.; Pruden, A. Effect of various sludge digestion conditions on sulfonamide, macrolide, and tetracycline resistance genes and class I integrons. Environ. Sci. Technol. 2011, 45, 7855–7861. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.E.; Jin, L.; Luo, X.; Zhao, Z.; Li, X. Seasonal disparities in airborne bacteria and associated antibiotic resistance Genes in PM2.5 between Urban and Rural Sites. Environ. Sci. Technol. Lett. 2018, 5, 74–79. [Google Scholar] [CrossRef]
- Sapkota, A.R.; Ojo, K.K.; Roberts, M.C.; Schwab, K.J. Antibiotic resistance genes in multidrug-resistant Enterococcus spp. and Streptococcus spp. recovered from the indoor air of a large-scale swine-feeding operation. Lett. Appl. Microbiol. 2006, 43, 534–540. [Google Scholar] [CrossRef]
- McEachran, A.D.; Blackwell, B.R.; Delton Hanson, J.; Wooten, K.J.; Mayer, G.D.; Cox, S.B.; Smith, P.N. Antibiotics, Bacteria, and Antibiotic Resistance Genes: Aerial Transport from Cattle Feed Yards via Particulate Matter. Environ. Heal. Persp. 2015, 123, 337–343. [Google Scholar] [CrossRef] [Green Version]
- Gandolfi, I.; Franzetti, A.; Bertolini, V.; Gaspari, E.; Bestetti, G. Antibiotic resistance in bacteria associated with coarse atmospheric particulate matter in an urban area. J. Appl. Microbiol. 2011, 110, 1612–1620. [Google Scholar] [CrossRef]
- Novo, A.; André, S.; Viana, P.; Nunes, O.C.; Manaia, C.M. Antibiotic resistance, antimicrobial residues and bacterial community composition in urban wastewater. Water Res. 2013, 47, 1875–1887. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Huang, Z.; Yang, K.; Graham, D.; Xie, B. Relationships between antibiotics and antibiotic resistance gene levels in municipal solid waste leachates in Shanghai, China. Environ. Sci. Technol. 2015, 49, 4122–4128. [Google Scholar] [CrossRef]
- Penton, C.R.; Gupta, V.V.S.R.; Yu, J.; Tiedje, J.M. Size matters: Assessing optimum soil sample size for fungal and bacterial community structure analyses using high throughput sequencing of rRNA gene amplicons. Front. Microbiol. 2016, 7, 824. [Google Scholar] [CrossRef] [PubMed]
- Fahrenfeld, N.L.; Reyes, H.D.; Eramo, A.; Akob, D.M.; Mumford, A.C.; Cozzarelli, I.M. Shifts in microbial community structure and function in surface waters impacted by unconventional oil and gas wastewater revealed by metagenomics. Sci. Total Environ. 2017, 580, 1205–1231. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Loy, A. Bacterial community composition and function in sewage treatment systems. Curr. Opin. Biotechnol. 2002, 13, 218–227. [Google Scholar] [CrossRef]
- Zhang, T.; Shao, M.F.; Ye, L. 454-Pyrosequencing reveals bacterial diversity of activated sludge from 14 sewage treatment plants. ISME J. 2012, 6, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, Y.; Li, L.; Xu, G.; Liu, J.; Yang, K. Bacterial population and chemicals in bioaerosols from indoor environment: Sludge dewatering houses in nine municipal wastewater treatment plants. Sci. Total Environ. 2018, 618, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Gillings, M.R.; Gaze, W.H.; Pruden, A.; Smalla, K.; Tiedje, J.M.; Zhu, T. Using the class 1 integron-integrase gene as a proxy for anthropogenic pollution. ISME J. 2015, 9, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, X.X.; Ye, L. Plasmid metagenome reveals high levels of antibiotic resistance genes and mobile genetic elements in activated sludge. PLoS ONE 2011, 6, e26041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Souza, M.L.; Wackett, L.P.; Sadowsky, M.J. The atzABC genes encoding atrazine catabolism are located on a self-transmissible plasmid in Pseudomonas sp. strain ADP. Appl. Environ. Microbiol. 1998, 64, 2323–2326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, K.S.; Danzeisen, J.L.; Xu, W.; Johnson, T.J. Transcriptome mapping of pAR060302, a bla CMY-2-positive broad-host-range IncA/C plasmid. Appl. Environ. Microbiol. 2012, 78, 3379–3386. [Google Scholar] [CrossRef] [Green Version]
- Kieffer, N.; Nordmann, P.; Millemann, Y.; Poirel, L. Functional characterization of a Miniature Inverted Transposable Element at the origin of mcr-5 gene acquisition in Escherichia coli. Antimicrob. Agents Chemother. 2017, 63, e00559-19. [Google Scholar] [CrossRef] [Green Version]
- Ju, F.; Li, B.; Ma, L.; Wang, Y.; Huang, D.; Zhang, T. Antibiotic resistance genes and human bacterial pathogens: Co-occurrence, removal, and enrichment in municipal sewage sludge digesters. Water Res. 2016, 91, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Shi, P.; Hu, Q.; Li, B.; Zhang, T.; Zhang, X.X. Bacterial community shift drives antibiotic resistance promotion during drinking water chlorination. Environ. Sci. Technol. 2015, 49, 12271–12279. [Google Scholar] [CrossRef] [PubMed]
- Su, J.Q.; Wei, B.; Ou-Yang, W.; Huang, F.; Zhao, Y.; Xu, H.; Zhu, Y. Antibiotic resistome and its association with bacterial communities during sewage sludge composting. Environ. Sci. Technol. 2015, 49, 7356–7363. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, I.; Yoo, K. Metagenomic Profiles of Antibiotic Resistance Genes in Activated Sludge, Dewatered Sludge and Bioaerosols. Water 2020, 12, 1516. https://doi.org/10.3390/w12061516
Han I, Yoo K. Metagenomic Profiles of Antibiotic Resistance Genes in Activated Sludge, Dewatered Sludge and Bioaerosols. Water. 2020; 12(6):1516. https://doi.org/10.3390/w12061516
Chicago/Turabian StyleHan, Il, and Keunje Yoo. 2020. "Metagenomic Profiles of Antibiotic Resistance Genes in Activated Sludge, Dewatered Sludge and Bioaerosols" Water 12, no. 6: 1516. https://doi.org/10.3390/w12061516




