Phycoremediation of Landfill Leachate with Desmodesmus subspicatus: A Pre-Treatment for Reverse Osmosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Regions Selected for Sampling
2.2. Landfill Leachates
2.3. Microalgae and Growth Medium
2.4. Experimental Set-up
2.5. Analytical Measurements
- Optical density measurement. During cultivation, the microalgae concentration was monitored through OD using the SHIMADZU UV-1900 spectrophotometer (Shimadzu Scientific Instrument, Inc., Columbia, MD, USA) at λ = 560 nm under sterile conditions.
- TOC analysis. An Elementar Vario TOC analyzer (Elementar Analysensysteme, GmbH, Langenselbold, Germany) was used to measure TOC content. For the analysis of each sample, 1 mL of the supernatant was taken out and diluted to 10% with distilled water. The TOC was determined by measuring TC and TIC separately, and calculating their difference.
- Fe analysis. Fe content was measured through atomic absorption spectrometry at a specific wavelength. To determine Fe content decrease, a SensAA atomic absorption spectrometer (GBC Scientific Equipment, Melbourne, Australia) was used.
- TAN analysis. TAN concentration was determined using the phenate method [40]. The absorbance was measured with the SHIMADZU UV-1900 spectrophotometer (Shimadzu Scientific Instruments, Inc., Columbia, MD, USA).
2.6. Statistical Analysis
3. Results and Discussion
3.1. The Initial Ecotoxicity Test
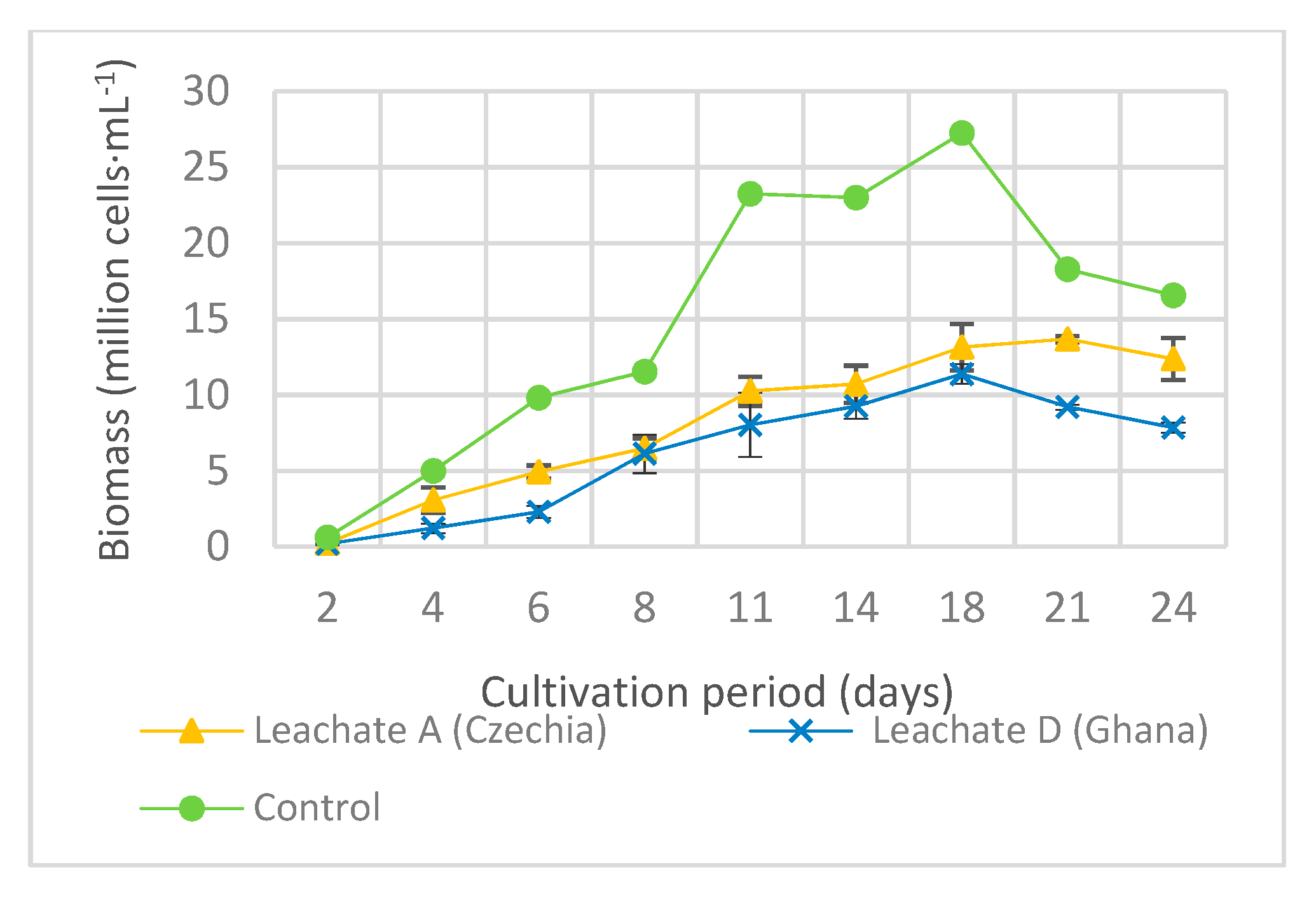
3.2. Effect of Landfill Leachates on Algae Growth.
3.3. Pollutant Removal
3.3.1. TAN and Fe Removal Efficiency of D. subspicatus
3.3.2. Possible Optimizations of the Phycoremediation Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kuraš, M. Odpady a jejich zpracování; Vodní zdroje Ekomonitor, spol. s.r.o.: Prague, Czech Republic, 2014; p. 343. [Google Scholar]
- Kaza, S.; Yao, L.C.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050. Urban Development; World Bank: Washington, DC, USA, 2018. [Google Scholar] [CrossRef]
- Cossu, R. Technical evolution of landfilling. Waste Manag. 2010, 30, 947–948. [Google Scholar] [CrossRef]
- Cingolani, D.; Fatone, F.; Frison, N.; Spinelli, M.; Eusebi, A.L. Pilot-scale multi-stage reverse osmosis (DT-RO) for water recovery from landfill leachate. Waste Manag. 2018, 76, 566–574. [Google Scholar] [CrossRef]
- Berge, N.D.; Reinhart, D.R.; Townsend, T.G. The fate of nitrogen in bioreactor landfills. Crit. Rev. Env. Sci. Technol. 2005, 35, 365–399. [Google Scholar] [CrossRef]
- Bao, X.; Wu, Q.L.; Shi, W.X.; Wang, W.; Zhu, Z.G.; Zhang, Z.Q.; Zhang, R.J.; Zhang, X.Y.; Zhang, B.; Guo, Y.; et al. Insights into simultaneous ammonia-selective and anti-fouling mechanism over forward osmosis membrane for resource recovery from domestic wastewater. J. Membr. Sci. 2019, 573, 135–144. [Google Scholar] [CrossRef]
- Sir, M.; Podhola, M.; Patocka, T.; Honzajkova, Z.; Kocurek, P.; Kubal, M.; Kuras, M. The effect of humic acids on the reverse osmosis treatment of hazardous landfill leachate. J. Hazard. Mater. 2012, 207, 86–90. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Bligh, M.W.; Yuan, X.; Waite, T.D. Ligand-promoted reductive cleaning of iron-fouled membranes from submerged membrane bioreactors. J. Membr. Sci. 2018, 545, 126–132. [Google Scholar] [CrossRef]
- Melliti, E.; Touati, K.; Abidi, H.; Elfil, H. Iron fouling prevention and membrane cleaning during reverse osmosis process. Int. J. Environ. Sci. Technol. 2019, 16, 3809–3818. [Google Scholar] [CrossRef]
- Zakar, M.; Farkas, D.I.; Hanczne-Lakatos, E.; Keszthelyi-Szabo, G.; Laszlo, Z. Purification of Model Dairy Wastewaters by Ozone, Fenton Pre-treatment and Membrane Filtration. Period. Polytech. Chem. Eng. 2020, 64, 357–363. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.; Lim, J.; Lee, S.; Lee, C.; Hong, S. Cold-cathode X-ray irradiation pre-treatment for fouling control of reverse osmosis (RO) in shale gas produced water (SGPW) treatment. Chem. Eng. J. 2019, 374, 49–58. [Google Scholar] [CrossRef]
- Wang, J.H.; Zhou, X.L.; Chen, Y.C.; Hu, Y.Y. Pretreatment in membrane processes for ultra-pure demineralized water production from secondary effluent. Desalin. Water Treat. 2018, 109, 36–44. [Google Scholar] [CrossRef]
- Chaudhary, R.; Tong, Y.W.; Dikshit, A.K. CO2-assisted removal of nutrients from municipal wastewater by microalgae Chlorella vulgaris and Scenedesmus obliquus. Int. J. Environ. Sci. Technol. 2018, 15, 2183–2192. [Google Scholar] [CrossRef]
- Bohutskyi, P.; Chow, S.; Ketter, B.; Shek, C.F.; Yacar, D.; Tang, Y.T.; Zivojnovich, M.; Betenbaugh, M.J.; Bouwer, E.J. Phytoremediation of agriculture runoff by filamentous algae poly-culture for biomethane production, and nutrient recovery for secondary cultivation of lipid generating microalgae. Bioresour. Technol. 2016, 222, 294–308. [Google Scholar] [CrossRef] [PubMed]
- Ajayan, K.V.; Selvaraju, M.; Unnikannan, P.; Sruthi, P. Phycoremediation of Tannery Wastewater Using Microalgae Scenedesmus Species. Int. J. Phytoremediation 2015, 17, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Labbe, J.I.; Ramos-Suarez, J.L.; Hernandez-Perez, A.; Baeza, A.; Hansen, F. Microalgae growth in polluted effluents from the dairy industry for biomass production and phytoremediation. J. Environ. Chem. Eng. 2017, 5, 635–643. [Google Scholar] [CrossRef]
- Sankaran, K.; Premalatha, M.; Vijayasekaran, M.; Somasundaram, V.T. DEPHY project: Distillery wastewater treatment through anaerobic digestion and phycoremediation-A green industrial approach. Renew. Sustain. Energy Rev. 2014, 37, 634–643. [Google Scholar] [CrossRef]
- de Alva, M.S.; Luna-Pabello, V.M.; Cadena, E.; Ortiz, E. Green microalga Scenedesmus acutus grown on municipal wastewater to couple nutrient removal with lipid accumulation for biodiesel production. Bioresour. Technol. 2013, 146, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Sirin, S.; Sillanpaa, M. Cultivating and harvesting of marine alga Nannochloropsis oculata in local municipal wastewater for biodiesel. Bioresour. Technol. 2015, 191, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.; Srivastava, S.; Mishra, S.; Kumar, A.; Tripathi, R.D.; Rai, U.N.; Dave, R.; Tripathi, P.; Charkrabarty, D.; Trivedi, P.K. Characterization of native microalgal strains for their chromium bioaccumulation potential: Phytoplankton response in polluted habitats. J. Hazard. Mater. 2010, 173, 95–101. [Google Scholar] [CrossRef]
- Priatni, S.; Ratnaningrum, D.; Warya, S.; Audina, E. Phycobiliproteins production and heavy metals reduction ability of Porphyridium sp. In Proceedings of the 2nd International Symposium on Green Technology for Value Chains, Jakarta, Indonesia, 23–24 October 2017; Volume 160. [Google Scholar]
- Gao, F.; Li, C.; Yang, Z.H.; Zeng, G.M.; Mu, J.; Liu, M.; Cui, W. Removal of nutrients, organic matter, and metal from domestic secondary effluent through microalgae cultivation in a membrane photobioreactor. J. Chem. Technol. Biotechnol. 2016, 91, 2713–2719. [Google Scholar] [CrossRef]
- Mirghaffari, N.; Moeini, E.; Farhadian, O. Biosorption of Cd and Pb ions from aqueous solutions by biomass of the green microalga, Scenedesmus quadricauda. J. Appl. Phycol. 2015, 27, 311–320. [Google Scholar] [CrossRef]
- Leong, Y.K.; Chang, J.S. Bioremediation of heavy metals using microalgae: Recent advances and mechanisms. Bioresour. Technol. 2020, 303. [Google Scholar] [CrossRef] [PubMed]
- Miazek, K.; Iwanek, W.; Remacle, C.; Richel, A.; Goffin, D. Effect of Metals, Metalloids and Metallic Nanoparticles on Microalgae Growth and Industrial Product Biosynthesis: A Review. Int. J. Mol. Sci. 2015, 16, 23929–23969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, K.; Lee, C.H.; Ko, K.; Lee, Y.J.; Kim, K.N.; Kim, M.K.; Chung, Y.H.; Kim, D.; Yeo, I.K.; Oda, T. Use of phenol-induced oxidative stress acclimation to stimulate cell growth and biodiesel production by the oceanic microalga Dunaliella salina. Algal Res. Biomass Biofuels Bioprod. 2016, 17, 61–66. [Google Scholar] [CrossRef]
- Christensen, T.H.; Kjeldsen, P.; Bjerg, P.L.; Jensen, D.L.; Christensen, J.B.; Baun, A.; Albrechtsen, H.J.; Heron, C. Biogeochemistry of landfill leachate plumes. Appl. Geochem. 2001, 16, 659–718. [Google Scholar] [CrossRef]
- Ministry of the Environment of the Czech Republic. Available online: https://www.mzp.cz/C125750E003B698B/en/waste_management/$FILE/Waste%20Act_1852001.pdf (accessed on 19 June 2020).
- Kusi, E.; Nyarko, A.K.; Boamah, L.A.; Nyamekye, C. Landfills: Investigating Its Operational Practices in Ghana. Int. J. Energy Environ. Sci. 2016, 1, 19–28. [Google Scholar] [CrossRef]
- Miezah, K.; Obiri-Danso, K.; Kadar, Z.; Fei-Baffoe, B.; Mensah, M.Y. Municipal solid waste characterization and quantification as a measure towards effective waste management in Ghana. Waste Manag. 2015, 46, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Owusu, G.; Nketiah-Amponsah, E.; Codjoe, S.N.A.; Afutu-Kotey, R.L. How do Ghana’s landfills affect residential property values? A case study of two sites in Accra. Urban Geogr. 2014, 35, 1140–1155. [Google Scholar] [CrossRef]
- The World Bank. Available online: http://documents.worldbank.org/curated/en/775731551156835790/Environmental-and-Social-Audit-of-Kpone-Landfill (accessed on 19 June 2020).
- Standard Methods for the Examination of Water and Wastewater. Available online: https://www.standardmethods.org/ (accessed on 15 June 2020).
- Culture Collection of Autotrophic Organisms. Available online: https://ccala.butbn.cas.cz/en/bbm-medium (accessed on 17 June 2020).
- Lavens, S. (Ed.) Manual on the Production and Use of Live Food for Aquaculture; Laboratory of Aquaculture and Artemia Reference Center, University of Ghent: Ghent, Belgium, 1996; p. 295. [Google Scholar]
- Lin, L.; Chan, G.Y.S.; Jiang, B.L.; Lan, C.Y. Use of ammoniacal nitrogen tolerant microalgae in landfill leachate treatment. Waste Manag. 2007, 27, 1376–1382. [Google Scholar] [CrossRef]
- El Ouaer, M.; Kallel, A.; Kasmi, M.; Hassen, A.; Trabelsi, I. Tunisian landfill leachate treatment using Chlorella sp.: Effective factors and microalgae strain performance. Arab. J. Geosci. 2017, 10. [Google Scholar] [CrossRef]
- Paskuliakova, A.; McGowan, T.; Tonry, S.; Touzet, N. Microalgal bioremediation of nitrogenous compounds in landfill leachate—The importance of micronutrient balance in the treatment of leachates of variable composition. Algal Res. Biomass Biofuels Bioprod. 2018, 32, 162–171. [Google Scholar] [CrossRef]
- Standard Methods for the Examination of Water and Wastewater/4500-NH3 NITROGEN (AMMONIA). Available online: https://www.standardmethods.org/doi/abs/10.2105/SMWW.2882.087 (accessed on 25 May 2020).
- Rhee, G.Y. Effects of N-P atomic ratios and nitrate limitation on algal growth, cell composition, and nitrate uptake. Limnol. Oceanogr. 1978, 23, 10–25. [Google Scholar] [CrossRef] [Green Version]
- Arbib, Z.; Ruiz, J.; Alvarez-Diaz, P.; Garrido-Perez, C.; Barragan, J.; Perales, J.A. Photobiotreatment: Influence of nitrogen and phosphorus ratio in wastewater on growth kinetics of Scenedesmus obliquus. Int. J. Phytoremediation 2013, 15, 774–788. [Google Scholar] [CrossRef]
- Wang, L.A.; Min, M.; Li, Y.C.; Chen, P.; Chen, Y.F.; Liu, Y.H.; Wang, Y.K.; Ruan, R. Cultivation of Green Algae Chlorella sp in Different Wastewaters from Municipal Wastewater Treatment Plant. Appl. Biochem. Biotechnol. 2010, 162, 1174–1186. [Google Scholar] [CrossRef] [PubMed]
- Shalini, S.S.; Joseph, K. Nitrogen management in landfill leachate: Application of SHARON, ANAMMOX and combined SHARON-ANAMMOX process. Waste Manag. 2012, 32, 2385–2400. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Garcia, A.; Velasquez-Orta, S.B.; Novelo, E.; Yanez-Noguez, I.; Monje-Ramirez, I.; Ledesma, M.T.O. Wastewater-leachate treatment by microalgae: Biomass, carbohydrate and lipid production. Ecotoxicol. Environ. Saf. 2019, 174, 435–444. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Liu, J.G.; Li, R.; Zeng, X.W.; Xue, Y.W.; Liu, J.; Nie, Y.F. Determining the biodegradability of leachate through XAD-8 adsorption. In Proceedings of the Seventh International Conference on Waste Management and Technology, Istanbul, Turkey, 30–31 July 2020; Volume 16, pp. 3–8. [Google Scholar]
- Pereira, S.E.L.; Goncalves, A.L.; Moreira, F.C.; Silva, T.; Vilar, V.J.P.; Pires, J.C.M. Nitrogen Removal from Landfill Leachate by Microalgae. Int. J. Mol. Sci. 2016, 17, 1926. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Ahn, Y.; Park, Y.T.; Ji, M.K.; Choi, J. The Effect of Mixed Wastewaters on the Biomass Production and Biochemical Content of Microalgae. Energies 2019, 12, 3431. [Google Scholar] [CrossRef] [Green Version]
- Rui, H.; Zhen, Y.; Li, J.C.; Min, G.; Ma, W.L.; Yi, Z. Comparative study on cultivation of microalgae for nutrient removal and lipid production in different artificial wastewaters. Int. J. Agric. Biol. Eng. 2017, 10, 107–114. [Google Scholar] [CrossRef]
- Chiellini, C.; Guglielminetti, L.; Pistelli, L.; Ciurli, A. Screening of trace metal elements for pollution tolerance of freshwater and marine microalgal strains: Overview and perspectives. Algal Res. Biomass Biofuels Bioprod. 2020, 45. [Google Scholar] [CrossRef]
- Abla, A.M.F.; Hamedy, H.R.G.; Eman, A.H. Uptake of Iron and Lead from Aqueous Solution by Some Green Microalgae. Fresenius Environ. Bull. 2016, 25, 2613–2619. [Google Scholar]
- Sutkowy, M.; Klosowski, G. Use of the Coenobial Green Algae Pseudopediastrum boryanum (Chlorophyceae) to Remove Hexavalent Chromium from Contaminated Aquatic Ecosystems and Industrial Wastewaters. Water 2018, 10, 712. [Google Scholar] [CrossRef] [Green Version]
- Sibi, G. Biosorption of chromium from electroplating and galvanizing industrial effluents under extreme conditions using Chlorella vulgaris. Green Energy Environ. 2016, 1, 172–177. [Google Scholar] [CrossRef] [Green Version]
- Quan, X.J.; Hu, R.; Chang, H.X.; Tang, X.Y.; Huang, X.X.; Cheng, C.; Zhong, N.B.; Yang, L. Enhancing microalgae growth and landfill leachate treatment through ozonization. J. Clean Prod. 2020, 248. [Google Scholar] [CrossRef]
- Chang, H.X.; Hu, R.; Zou, Y.J.; Quan, X.J.; Zhong, N.B.; Zhao, S.; Sun, Y.H. Highly efficient reverse osmosis concentrate remediation by microalgae for biolipid production assisted with electrooxidation. Water Res. 2020, 174. [Google Scholar] [CrossRef]
- Nawaz, T.; Rahman, A.; Pan, S.; Dixon, K.; Petri, B.; Selvaratnam, T.A. Review of Landfill Leachate Treatment by Microalgae: Current Status and Future Directions. Processes 2020, 8, 384. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Zhou, Y.; Huang, S.; Qiu, D.Y.; Schideman, L.; Chai, X.L.; Zhao, Y.C. Characterization of microalgae-bacteria consortium cultured in landfill leachate for carbon fixation and lipid production. Bioresour. Technol. 2014, 156, 322–328. [Google Scholar] [CrossRef]
- Santaeufemia, S.; Torres, E.; Abalde, J. Biosorption of ibuprofen from aqueous solution using living and dead biomass of the microalga Phaeodactylum tricornutum. J. Appl. Phycol. 2018, 30, 471–482. [Google Scholar] [CrossRef]
- Coimbra, R.N.; Escapa, C.; Vazquez, N.C.; Noriega-Hevia, G.; Otero, M. Utilization of Non-Living Microalgae Biomass from Two Different Strains for the Adsorptive Removal of Diclofenac from Water. Water 2018, 10, 1401. [Google Scholar] [CrossRef] [Green Version]








| Landfill | Location | Operation Period (Year) | Reported Annual Waste Disposal (Metric Tons) |
|---|---|---|---|
| A | Liberec Region, Czechia | 2007–present | 29,000 |
| B | Central Bohemian Region, Czechia | 1993–present | 80,000 |
| C | Ústí nad Labem Region, Czechia | 2004–present | 100,000–140,000 |
| D | Ga South Municipal Assembly, Accra, Ghana | 2002–present | 396,000 |
| E | Ga South Municipal Assembly, Accra, Ghana | first phase: 1991–2001; second phase: 2008–2010 | 438,000 |
| F | Kpone-Katamanso District Assembly, Kpone, Ghana | 2012–present | 295,000 |
| Parameters | A | B | C | D | E | F |
|---|---|---|---|---|---|---|
| pH | 8.0 | 8.0 | 8.2 | 7.8 | 8.3 | 9.5 |
| Electrical conductivity, μS·cm−1 | 12,500 | 18,800 | 10,700 | 18,700 | 19,700 | 19,500 |
| Ammonia nitrogen, mg·L−1 | 380 | 3584 | 387 | 611 | 3156 | 34 |
| Nitrate, mg·L−1 | 224 | 2447 | 133 | 64.8 | 38.9 | <0.05 |
| Phosphorus, mg·L−1 | 3.8 | 7.4 | 4.1 | 5.5 | 12.3 | 14.6 |
| N:P | 95.7 | 473.5 | 85.1 | 94.1 | 212.0 | 1.9 |
| Total organic carbon, mg·L−1 | 248 | 633 | 239 | 348 | 985 | 6919 |
| Total inorganic carbon, mg·L−1 | 614 | 1480 | 796 | 449 | 2326 | 2931 |
| Total carbon, mg·L−1 | 862 | 2114 | 1035 | 796 | 3312 | 9850 |
| Absorbance at 680 nm | 0.054 | 0.142 | 0.051 | 0.141 | 0.398 | 2.884 |
| Absorbance at 455 nm | 0.434 | 0.950 | 0.379 | 0.817 | 2.502 | 4.000 |
| Fe, mg·L−1 | 0.67 | 5.10 | 0.72 | 6.60 | 12.40 | 42.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kholomyeva, M.; Vurm, R.; Tajnaiová, L.; Šír, M.; Maslova, M.; Kočí, V. Phycoremediation of Landfill Leachate with Desmodesmus subspicatus: A Pre-Treatment for Reverse Osmosis. Water 2020, 12, 1755. https://doi.org/10.3390/w12061755
Kholomyeva M, Vurm R, Tajnaiová L, Šír M, Maslova M, Kočí V. Phycoremediation of Landfill Leachate with Desmodesmus subspicatus: A Pre-Treatment for Reverse Osmosis. Water. 2020; 12(6):1755. https://doi.org/10.3390/w12061755
Chicago/Turabian StyleKholomyeva, Marina, Radek Vurm, Lucia Tajnaiová, Marek Šír, Mariya Maslova, and Vladimír Kočí. 2020. "Phycoremediation of Landfill Leachate with Desmodesmus subspicatus: A Pre-Treatment for Reverse Osmosis" Water 12, no. 6: 1755. https://doi.org/10.3390/w12061755







