Bioremediation of Landoltia punctata to Microcystis aeruginosa Contaminated Waters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Organisms
2.2. Medium Formula and Culture Conditions
2.2.1. BG11 Culture Medium
2.2.2. Hoagland Culture Medium
2.3. Experimental Design
2.4. Experimental Methods
2.4.1. Determination of Growth Status of L. punctata
2.4.2. Screening Co-Culture Density of L. punctata and M. aeruginosa
2.4.3. Determination of Antioxidase Activities of L. punctata
2.4.4. Determination of MC Content
2.4.5. Determination of Removal Rate of TN and TP
2.5. Statistical Analysis
3. Results
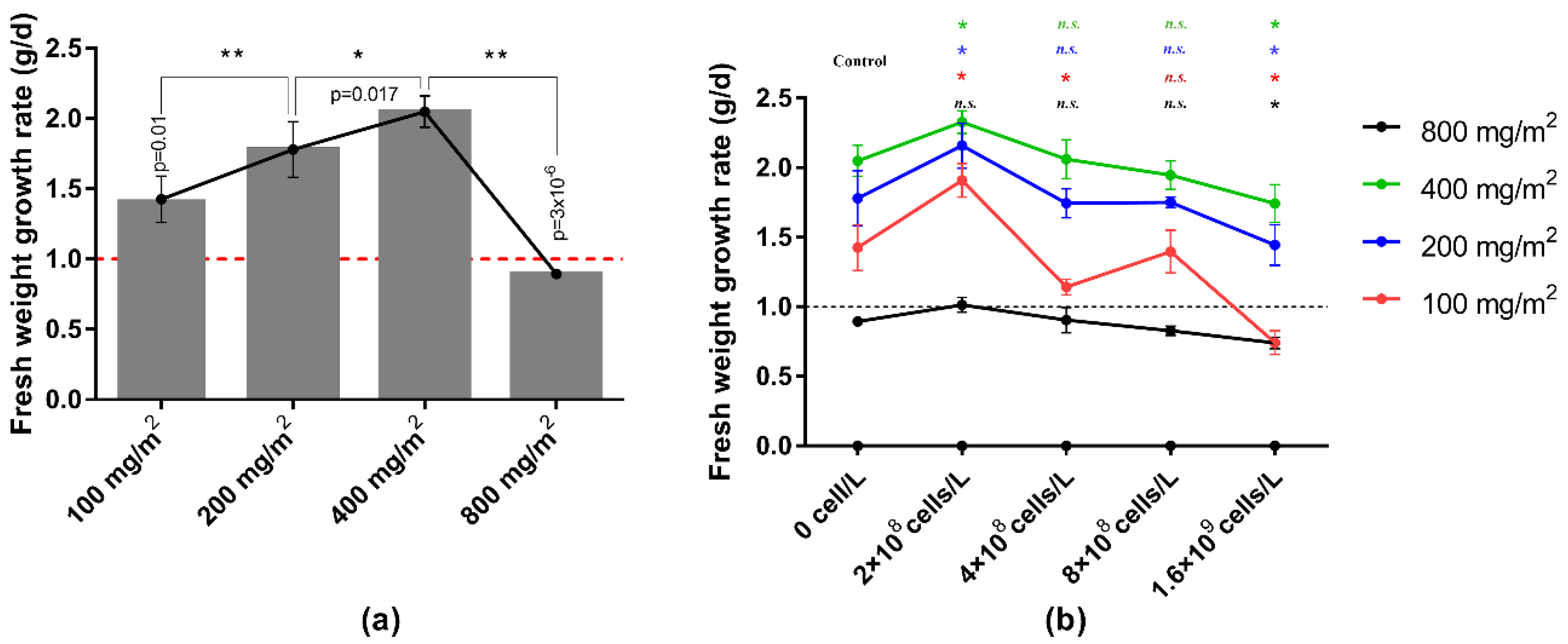
3.1. Screening Co-Culture Density of L. punctata and M. aeruginosa
3.2. Effects of M. aeruginosa on the Growth of L. punctata
3.3. Effects of M. aeruginosa on Amylase and Starch Content of L. punctata
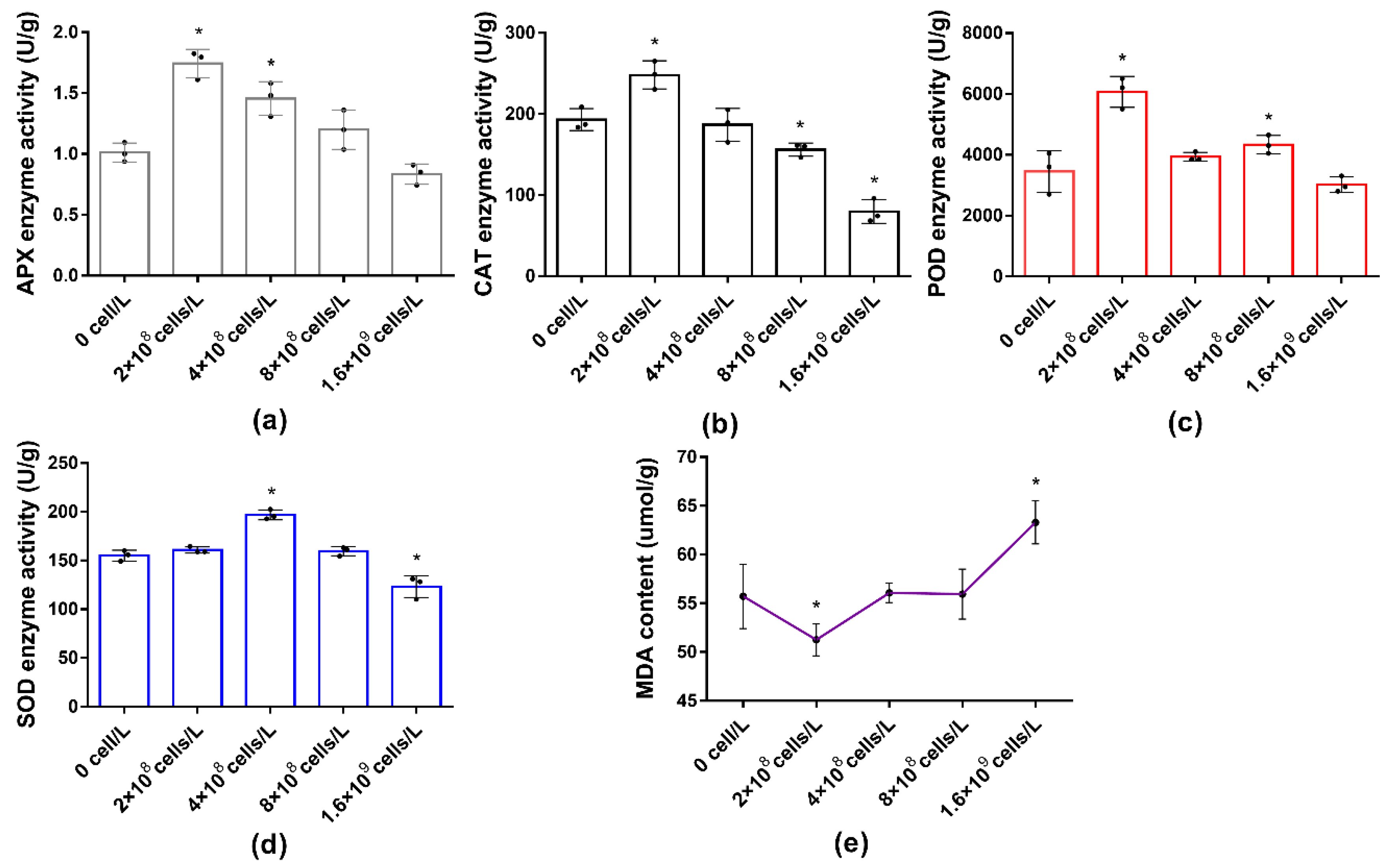
3.4. Effects of M. aeruginosa on Antioxidant System and MDA Accumulation of L. punctata
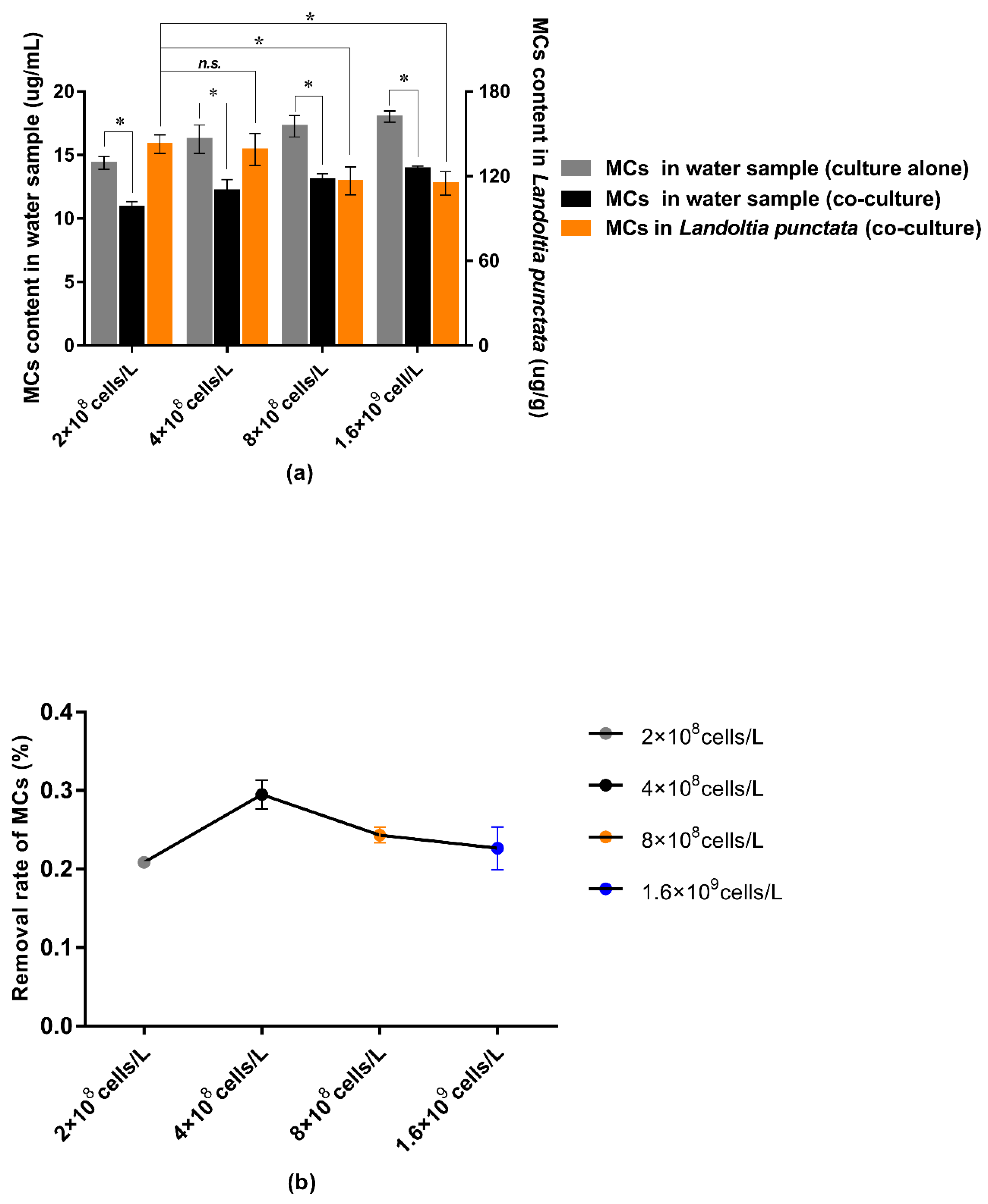
3.5. Effect of Water Purification by L. punctata
4. Discussion
4.1. Effects of M. aeruginosa on the Growth of L. punctata
4.2. Effects of M. aeruginosa on Amylase and Starch Content of L. punctata
4.3. Effects of M. aeruginosa on Antioxidant System and MDA Accumulation of L. punctata
4.4. Effect of Water Purification by L. punctata
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sinha, E.; Michalak, A.M.; Balaji, V. Eutrophication will increase during the 21st century as a result of precipitation changes. Science 2017, 357, 405–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanabe, Y.; Hodoki, Y.; Sano, T.; Tada, K.; Watanabe, M.M. Adaptation of the Freshwater Bloom-Forming Cyanobacterium Microcystis aeruginosa to Brackish Water Is Driven by Recent Horizontal Transfer of Sucrose Genes. Front. Microbiol. 2018, 9, 1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, T.; Kao, C.M.; Chen, W.H. Integrated phytoremediation focused on microbial investigation. RSC Adv. 2018, 8, 4680–4685. [Google Scholar] [CrossRef] [Green Version]
- Bassuney, D.; Tawfik, A. Baffled duck weed pond system for treatment of agricultural drainage water containing pharmaceuticals. Int. J. Phytoremediat. 2017, 19, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ma, S.; Huang, M.; Peng, M.; Bog, M.; Sree, K.S.; Appenroth, K.-J.; Zhang, J. Species distribution, genetic diversity and barcoding in the duckweed family (Lemnaceae). Hydrobiologia 2014, 743, 75–87. [Google Scholar] [CrossRef]
- Gupta, C.; Prakash, D. Duckweed: An effective tool for phyto-remediation. Toxicol. Environ. Chem. 2013, 95, 1256–1266. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Zhang, P.; Zeng, G.; Cai, X.; Liu, S.; Yin, Y.; Hu, X.; Hu, X.; Tan, X. Growth inhibition and oxidative damage of Microcystis aeruginosa induced by crude extract of Sagittaria trifolia tubers. J. Environ. Sci. 2016, 43, 40–47. [Google Scholar] [CrossRef]
- Massey, I.Y.; Yang, F.; Ding, Z.; Yang, S.; Guo, J.; Tezi, C.; Al-Osman, M.; Kamegni, R.B.; Zeng, W. Exposure routes and health effects of microcystins on animals and humans: A mini-review. Toxicon 2018, 151, 156–162. [Google Scholar] [CrossRef]
- Tsuji, K.; Watanuki, T.; Kondo, F.; Watanabe, M.F.; Suzuki, S.; Nakazawa, H.; Suzuki, M.; Uchida, H.; Harada, K.-I. Stability of microcystins from cyanobacteria—II. Effect of UV light on decomposition and isomerization. Toxicon 1995, 33, 1619–1631. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Sinha, R.P.; Incharoensakdi, A. The cyanotoxin-microcystins: Current overview. Rev. Environ. Sci. Bio/Technol. 2014, 13, 215–249. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Y.; Li, H.; Gong, A.; Han, J.; Qian, Z.; Chao, W. Removal of MCs by Bi2O2CO3: Adsorption and the potential of photocatalytic degradation. Environ. Sci. Pollut. Res. 2018, 25, 11867–11874. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Mennerich, A.; Urban, B. Coupled nutrient removal and biomass production with mixed algal culture: Impact of biotic and abiotic factors. Bioresour. Technol. 2012, 118, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Ying, L.I.; Shi, Z.; Zhang, Y.X.; Zhao, Q.L.; Aijun, L.I.; Jin, Y.; Cheng, T. Evaluation Method and Application on Cyanobacteria Bloom Degree Classification With Algal Density. Environ. Sustain. Dev. 2014, 2, 67–68. [Google Scholar]
- Chen, Q.; Jin, Y.; Zhang, G.; Fang, Y.; Xiao, Y.; Zhao, H. Improving Production of Bioethanol from Duckweed (Landoltia punctata) by Pectinase Pretreatment. Energies 2012, 5, 3019–3032. [Google Scholar] [CrossRef] [Green Version]
- Silva, P.D.P.; De Nys, R.; Paul, N. Seasonal growth dynamics and resilience of the green tide alga Cladophora coelothrix in high-nutrient tropical aquaculture. Aquac. Environ. Interact. 2012, 2, 253–266. [Google Scholar] [CrossRef] [Green Version]
- Arnon, D.I. Copper Enzymes in Isolated Chloroplasts. Polyphenoloxidase in Beta Vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Pavlović, I.; Pěnčík, A.; Novak, O.; Vujčić, V.; Brkanac, S.R.; Lepedus, H.; Strnad, M.; Sondi, B.S. Short-term salt stress in Brassica rapa seedlings causes alterations in auxin metabolism. Plant Physiol. Biochem. 2018, 125, 74–84. [Google Scholar] [CrossRef]
- Lin, Y.; Chang, Q.R.; Liu, M.Y.; Wang, S. Estimation of chlorophyll content in rice at different growth stages based on hyperspectral in yellow river irrigation zone. Agric. Res. Arid Areas 2018, 2, 45–52. [Google Scholar]
- Chen, G.; Fang, Y.; Huang, J.; Zhao, Y.; Li, Q.; Lai, F.; Xu, Y.; Tian, X.; He, K.; Jin, Y.; et al. Duckweed systems for eutrophic water purification through converting wastewater nutrients to high-starch biomass: Comparative evaluation of three different genera (Spirodela polyrhiza, Lemna minor and Landoltia punctata) in monoculture or polyculture. RSC Adv. 2018, 8, 17927–17937. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.-Q.; Liu, Y.-D.; Li, D.; Dauta, A. Growth and antioxidant system of the cyanobacterium Synechococcus elongatus in response to microcystin-RR. Hydrobiologia 2005, 534, 23–29. [Google Scholar] [CrossRef]
- Ziková, A.; Palíková, M.; Mareš, J.; Navrátil, S.; Kopp, R. Impacts of dietary cyanobacteria on fish. Acta Univ. Agric. Silvic. Mendel. Brun. 2010, 58, 277–284. [Google Scholar] [CrossRef] [Green Version]
- Monroe, J.D.; Storm, A.R. Review: The Arabidopsis β-amylase (BAM) gene family: Diversity of form and function. Plant Sci. 2018, 276, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Yu, D.J.; Kim, S.J.; Choi, I.; Lee, H.J. Intraspecies differences in cold hardiness, carbohydrate content and -amylase gene expression of Vaccinium corymbosum during cold acclimation and deacclimation. Tree Physiol. 2012, 32, 1533–1540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benina, M.; Obata, T.; Mehterov, N.; Ivanov, I.; Petrov, V.; Toneva, V.; Fernie, A.R.; Gechev, T.S. Comparative metabolic profiling of Haberlea rhodopensis, Thellungiella halophyla, and Arabidopsis thaliana exposed to low temperature. Front. Plant Sci. 2013, 4, 499–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smeekens, S.; Ma, J.; Hanson, J.; Rolland, F.A. Sugar signals and molecular networks controlling plant growth. Curr. Opin. Plant Boil. 2010, 13, 273–278. [Google Scholar] [CrossRef]
- Meng, N.; He, M.; Bai, Y.; Xu, H.; Dandekar, A.M.; Fei, Z.; Cheng, L. Decreased sorbitol synthesis leads to abnormal stamen development and reduced pollen tube growth via an MYB transcription factor, MdMYB39L, in apple (Malus domestica). New Phytol. 2017, 217, 641–656. [Google Scholar] [CrossRef] [Green Version]
- Ha, M.-H.; Pflugmacher, S. Time-dependent alterations in growth, photosynthetic pigments and enzymatic defense systems of submerged Ceratophyllum demersum during exposure to the cyanobacterial neurotoxin anatoxin-a. Aquat. Toxicol. 2013, 138, 26–34. [Google Scholar] [CrossRef] [PubMed]



| L. punctata density (g/m2) | 100 | 200 | 400 | 800 |
| Water Surface Coverage Rate (%) | 25 | 50 | 100 | 100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Le, S.; Li, G.; Luo, M.; Wang, R.; Zhao, Y. Bioremediation of Landoltia punctata to Microcystis aeruginosa Contaminated Waters. Water 2020, 12, 1764. https://doi.org/10.3390/w12061764
Li S, Le S, Li G, Luo M, Wang R, Zhao Y. Bioremediation of Landoltia punctata to Microcystis aeruginosa Contaminated Waters. Water. 2020; 12(6):1764. https://doi.org/10.3390/w12061764
Chicago/Turabian StyleLi, Shi, Sixiu Le, Guolin Li, Mei Luo, Rui Wang, and Yun Zhao. 2020. "Bioremediation of Landoltia punctata to Microcystis aeruginosa Contaminated Waters" Water 12, no. 6: 1764. https://doi.org/10.3390/w12061764





