Nitrogen Fertilization, Container Type, and Irrigation Frequency Affect Mineral Nutrient Uptake of Hydrangea
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Macronutrient Concentrations (mg g−1) and Contents (mg per plant)
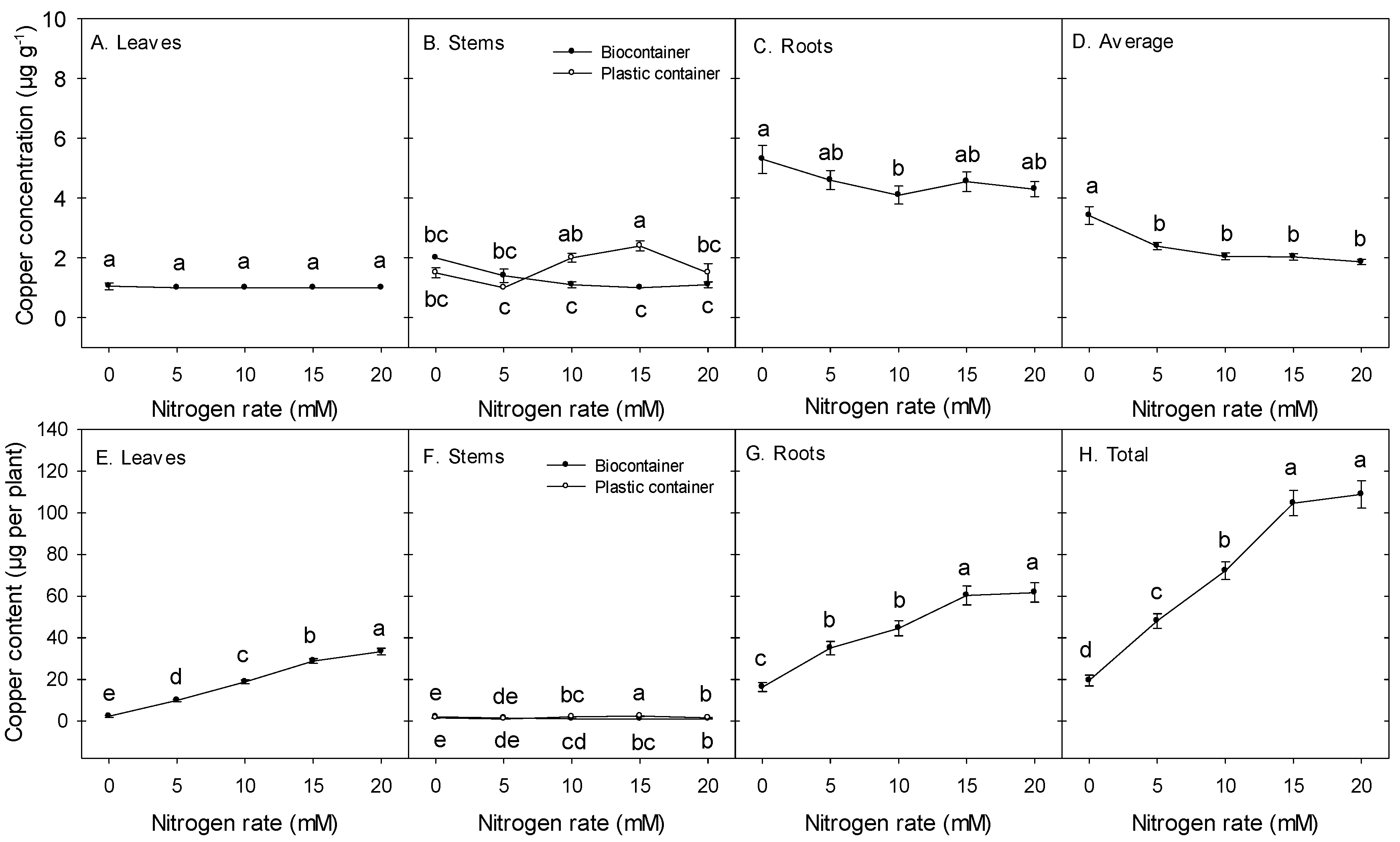
3.2. Micronutrient Concentrations (µg g−1) and Contents (µg per plant)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Orozco-Obando, W.; Hirsch, G.N.; Wetzstein, H.Y. Genotypic variation in flower induction and development in Hydrangea macrophylla. HortScience 2005, 40, 1695–1698. [Google Scholar] [CrossRef]
- Reed, S.M.; Jones, K.D.; Rinehart, T.A. Production and characterization of intergeneric hybrids between Dichroa febrifuga and Hydrangea macrophylla. J. Am. Soc. Hort. Sci. 2008, 133, 84–91. [Google Scholar] [CrossRef]
- Van Gelderen, C.J.; van Gelderen, D.M. Encyclopedia of Hydrangeas; Timber Press: Portland, OR, USA, 2004. [Google Scholar]
- Dirr, M.A. Hydrangeas for American Aardens; Timber Press: Portland, OR, USA, 2004. [Google Scholar]
- U.S. Department of Agriculture. 2012 Census of Agriculture. Census of Horticultural Specialties (2014). Available online: http://www.agcensus.usda.gov/Publications/2012/Online_Resources/Census_of_Horticulture_Specialties/HORTIC.pdf (accessed on 27 May 2020).
- Bi, G.; Scagel, C.F. Nitrogen uptake and mobilization by hydrangea leaves from foliar-sprayed urea in fall depend on plant nitrogen status. HortScience 2008, 43, 2151–2154. [Google Scholar] [CrossRef]
- Bi, G.; Scagel, C.F.; Harkess, R.L. Rate of nitrogen fertigation during vegetative growth and spray application of urea in the fall alters growth and flowering of florists’ hydrangeas. HortScience 2008, 43, 472–477. [Google Scholar] [CrossRef]
- Li, T.; Bi, G.; Harkess, R.L.; Denny, G.C.; Scagel, C. Nitrogen fertilization and irrigation frequency affect hydrangea growth and nutrient uptake in two container types. HortScience 2019, 54, 167–174. [Google Scholar] [CrossRef]
- Millard, P. Internal cycling of nitrogen in trees. Acta Hort. 1995, 383, 3–13. [Google Scholar] [CrossRef]
- Sanchez, E.E.; Righetti, T.L.; Sugar, D.; Lombard, P.B. Recycling of nitrogen in field-grown ‘Comice’ pears. J. Hort. Sci. 1991, 66, 479–486. [Google Scholar] [CrossRef]
- Li, T.; Bi, G.; Harkess, R.L.; Blythe, E.K. Mineral nutrient uptake of Encore Azalea ‘Chiffon’ affected by nitrogen, container, and irrigation frequency. HortScience 2019, 54, 2240–2248. [Google Scholar] [CrossRef]
- Gastal, F.; Lemaire, G. N uptake and distribution in crops: An agronomical and ecophysiological perspective. J. Exp. Bot. 2002, 53, 789–799. [Google Scholar] [CrossRef]
- O’Meara, L.; Chappell, M.R.; van Iersel, M.W. Water use of Hydrangea macrophylla and Gardenia jasminoides in response to a gradually drying substrate. HortScience 2014, 49, 493–498. [Google Scholar] [CrossRef]
- Jahromi, N.B.; Walker, F.; Fulcher, A.; Altland, J.; Wright, W.C. Growth response, mineral nutrition, and water utilization of container-grown woody ornamentals grown in biochar-amended pine bark. HortScience 2018, 53, 347–353. [Google Scholar] [CrossRef]
- Scagel, C.F.; Bi, G.; Fuchigami, L.H.; Regan, R.P. Effects of irrigation frequency and nitrogen fertilizer rate on water stress, nitrogen uptake, and plant growth of container-grown rhododendron. HortScience 2011, 46, 1569–1603. [Google Scholar] [CrossRef]
- Scagel, C.F.; Bi, G.; Fuchigami, L.H.; Regan, R.P. Irrigation frequency alters nutrient uptake in container-grown Rhododendron plants grown with different rates of nitrogen. HortScience 2012, 47, 189–197. [Google Scholar] [CrossRef]
- Beeks, S.A.; Evans, M.R. Growth of cyclamen in biocontainers on an ebb-and-flood subirrigation system. HortTechnology 2013, 23, 173–176. [Google Scholar] [CrossRef]
- Conneway, R.; Verlinder, S.; Koeser, A.K.; Evans, M.; Schnelle, R.; Anderson, V.; Stewart, J.R. Use of alternative containers for long- and short-term greenhouse crop production. HortTechnology 2015, 25, 26–34. [Google Scholar] [CrossRef]
- Koeser, A.; Kling, G.; Miller, C.; Warnock, D. Compatibility of biocontainer in commercial greenhouse crop production. HortTechnology 2013, 23, 149–156. [Google Scholar] [CrossRef]
- Kratsch, H.A.; Schrader, J.A.; McCabe, K.G.; Srinivasan, G.; Grewell, D.; Graves, W.R. Performance and biodegradable in soil of novel horticulture containers made from bioplastics and biocomposites. HortTechnology 2015, 25, 119–131. [Google Scholar] [CrossRef]
- Kuehny, J.S.; Taylor, M.; Evans, M.R. Greenhouse and landscape performance of bedding plants in biocontainers. HortTechnology 2011, 21, 155–161. [Google Scholar] [CrossRef]
- Li, T.; Bi, G.; Niu, G.; Nambuthiri, S.S.; Geneve, R.L.; Wang, X.; Fernandez, T.; Sun, Y.; Zhao, X. Feasibility of using biocontainers in a pot-in-pot system for nursery production of river birch. HortTechnology 2015, 25, 57–62. [Google Scholar] [CrossRef]
- Nambuthiri, B.; Geneve, R.L.; Sun, Y.; Wang, X.; Fernandez, R.T.; Niu, G.; Bi, G.; Fulcher, A. Substrate temperature in plastic and alternative nursery containers. HortTechnology 2015, 25, 50–56. [Google Scholar] [CrossRef]
- Evans, M.R.; Tayler, M.; Kuehny, J. Physical properties of biocontainer for greenhouse crops production. HortTechnology 2010, 20, 549–555. [Google Scholar] [CrossRef]
- Flax, N.J.; Currey, C.J.; Schrader, J.A.; Grewell, D.; Graves, W.R. Commercial greenhouse growers can produce high-quality bedding plants in bioplastic-based biocontainers. HortTechnology 2017, 27, 472–481. [Google Scholar] [CrossRef]
- Flax, N.J.; Currey, C.J.; Schrader, J.A.; Grewell, D.; Graves, W.R. Coconut coir and peat biocontainers influence plant growth retardant drench efficacy. HortTechnology 2018, 28, 370–377. [Google Scholar] [CrossRef]
- Evans, M.R.; Hensley, D.L. Plant growth in plastic, peat, and processed poultry feather fiber growing containers. HortScience 2004, 39, 1012–1014. [Google Scholar] [CrossRef]
- Evans, M.R.; Karcher, D. Properties of plastic, peat, and processed poultry feather fiber growing containers. HortScience 2004, 39, 1008–1011. [Google Scholar] [CrossRef]
- Evans, M.R.; Koeser, A.K.; Bi, G.; Nambuthiri, S.; Geneve, R.; Lovell, S.T.; Stewart, J.R. Impact of biocontainers with and without shuttle trays on water use in the production of a containerized ornamental greenhouse crop. HortTechnology 2015, 25, 35–41. [Google Scholar] [CrossRef]
- Koeser, A.; Lovell, S.T.; Evans, M.; Stewart, J.R. Biocontainer water use in short-term greenhouse crop production. HortTechnology 2013, 23, 215–219. [Google Scholar] [CrossRef]
- Wang, X.; Fernandez, R.T.; Cregg, B.M.; Auras, R.; Fulcher, A.; Cochran, D.R.; Niu, G.; Sun, Y.; Bi, G.; Nambuthiri, S.; et al. Multistate evaluation of plant growth and water use in plastic and alternative nursery containers. HortTechnology 2015, 25, 42–49. [Google Scholar] [CrossRef]
- Li, T.; Bi, G.; Harkess, R.L.; Denny, G.C.; Blythe, E.K.; Zhao, X. Nitrogen rate, irrigation frequency, and container type affect plant growth and nutrient uptake of encore azalea ‘Chiffon’. HortScience 2018, 53, 560–566. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: San Diego, CA, USA, 2012. [Google Scholar]
- Scagel, C.F.; Bi, G.; Fuchigami, L.H.; Regan, R.P. Nitrogen availability alters mineral nutrient uptake and demand in container-grown deciduous and evergreen rhododendron. J. Environ. Hort. 2008, 26, 177–187. [Google Scholar]
- Bryson, G.M.; Mills, H.A.; Sasseville, D.N.; Jones, J.B., Jr.; Barker, A.V. Plant Analysis Handbook III; Micro-Macro Publishing: Athens, GA, USA, 2014; p. 491. [Google Scholar]
- Barker, A.V.; Pilbeam, D.J. Handbook of Plant Nutrition; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Strik, B.C. Seasonal variation in mineral nutrient content of primocane-fruiting blackberry leaves. HortScience 2015, 50, 540–545. [Google Scholar] [CrossRef]



| Container Type | Macronutrient Concentrations (mg g−1) z | Micronutrient Concentrations (µg g−1) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | P | P | Mg | Fe | Fe | Mn | Mn | Mn | Mn | Cu | Cu | Zn | B | |
| Stem | Root | Average | Root | Root | Average | Leaf | Stem | Root | Average | Root | Average | Root | Average | |
| Biocontainer | 1.59 | 2.35 | 2.16 | 1.59 | 36.3 | 42.9 | 49.2 | 32.6 | 47.4 | 46.2 | 3.78 | 2.09 | 36.6 | 25.7 |
| Plastic container | 1.75 | 2.93 | 2.41 | 1.69 | 41.6 | 47.8 | 53.6 | 38.4 | 66.5 | 55.4 | 5.36 | 2.61 | 41.9 | 27.2 |
| p-value | 0.008 | <0.0001 | <0.0001 | 0.039 | 0.0025 | 0.014 | 0.48 | 0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0002 | 0.0033 | 0.049 |
| Macronutrient Content (mg Per Plant) z | Micronutrient Content (µg Per Plant) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Container Type | P | P | K | Mn | Cu | Cu | Zn | Zn | B |
| Root | Total | Root | Total | Root | Total | Root | Total | Root | |
| Biocontainer | 20.2 | 68.14 | 89.8 | 1892 | 34.9 | 60 | 350.7 | 1146 | 91.4 |
| Plastic container | 26.2 | 74.91 | 112.5 | 2252 | 52.4 | 81.3 | 415 | 1308 | 103 |
| p-value | <0.0001 | 0.0072 | <0.0001 | 0.0008 | <0.0001 | <0.0001 | 0.012 | 0.04 | 0.0028 |
| Nutrient Concentrations | ||||||
|---|---|---|---|---|---|---|
| P | P | Ca | Zn | Zn | Zn | |
| Irrigation Frequency z | (mg g−1) | (µg g−1) | ||||
| Leaf | Stem | Leaf | Leaf | Stem | Average | |
| Once | 2.21 | 1.73 | 12.1 | 32.1 | 64.2 | 39.2 |
| Twice | 1.97 | 1.61 | 11.4 | 28.1 | 55.7 | 35.4 |
| p-value | 0.039 | 0.012 | 0.036 | 0.038 | 0.0047 | 0.034 |
| Macronutrient Content (mg Per Plant) z | Micronutrient Content (μg Per Plant) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| P | K | Ca | Mg | Fe | Mn | Cu | Zn | B | |
| Leaf | 38.9 a | 548.7 a | 220.4 a | 108.6 a | 1087 a | 1211 a | 18.6 b | 519.7 a | 740.1 a |
| Stem | 9.4 c | 51.3 c | 20.4 c | 9.6 c | 216.8 c | 240 c | 8.4 c | 324.5 c | 60 c |
| Root | 23.2 b | 101.1 b | 41.2 b | 15.5 b | 422.1 b | 620.2 b | 43.6 a | 382.9 b | 97.2 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Bi, G.; Zhao, X.; Harkess, R.L.; Scagel, C. Nitrogen Fertilization, Container Type, and Irrigation Frequency Affect Mineral Nutrient Uptake of Hydrangea. Water 2020, 12, 1987. https://doi.org/10.3390/w12071987
Li T, Bi G, Zhao X, Harkess RL, Scagel C. Nitrogen Fertilization, Container Type, and Irrigation Frequency Affect Mineral Nutrient Uptake of Hydrangea. Water. 2020; 12(7):1987. https://doi.org/10.3390/w12071987
Chicago/Turabian StyleLi, Tongyin, Guihong Bi, Xiaojie Zhao, Richard L. Harkess, and Carolyn Scagel. 2020. "Nitrogen Fertilization, Container Type, and Irrigation Frequency Affect Mineral Nutrient Uptake of Hydrangea" Water 12, no. 7: 1987. https://doi.org/10.3390/w12071987
APA StyleLi, T., Bi, G., Zhao, X., Harkess, R. L., & Scagel, C. (2020). Nitrogen Fertilization, Container Type, and Irrigation Frequency Affect Mineral Nutrient Uptake of Hydrangea. Water, 12(7), 1987. https://doi.org/10.3390/w12071987





