Impact of Cold Temperatures on Nitrogen Removal in Denitrifying Down-Flow Hanging Sponge (DDHS) Reactors
Abstract
1. Introduction
2. Methods
2.1. Reactor Configuration
2.2. Influent
2.3. Reactor Operation
2.4. Sample Analysis
2.5. Data Analysis and Statistics
3. Results and Discussion
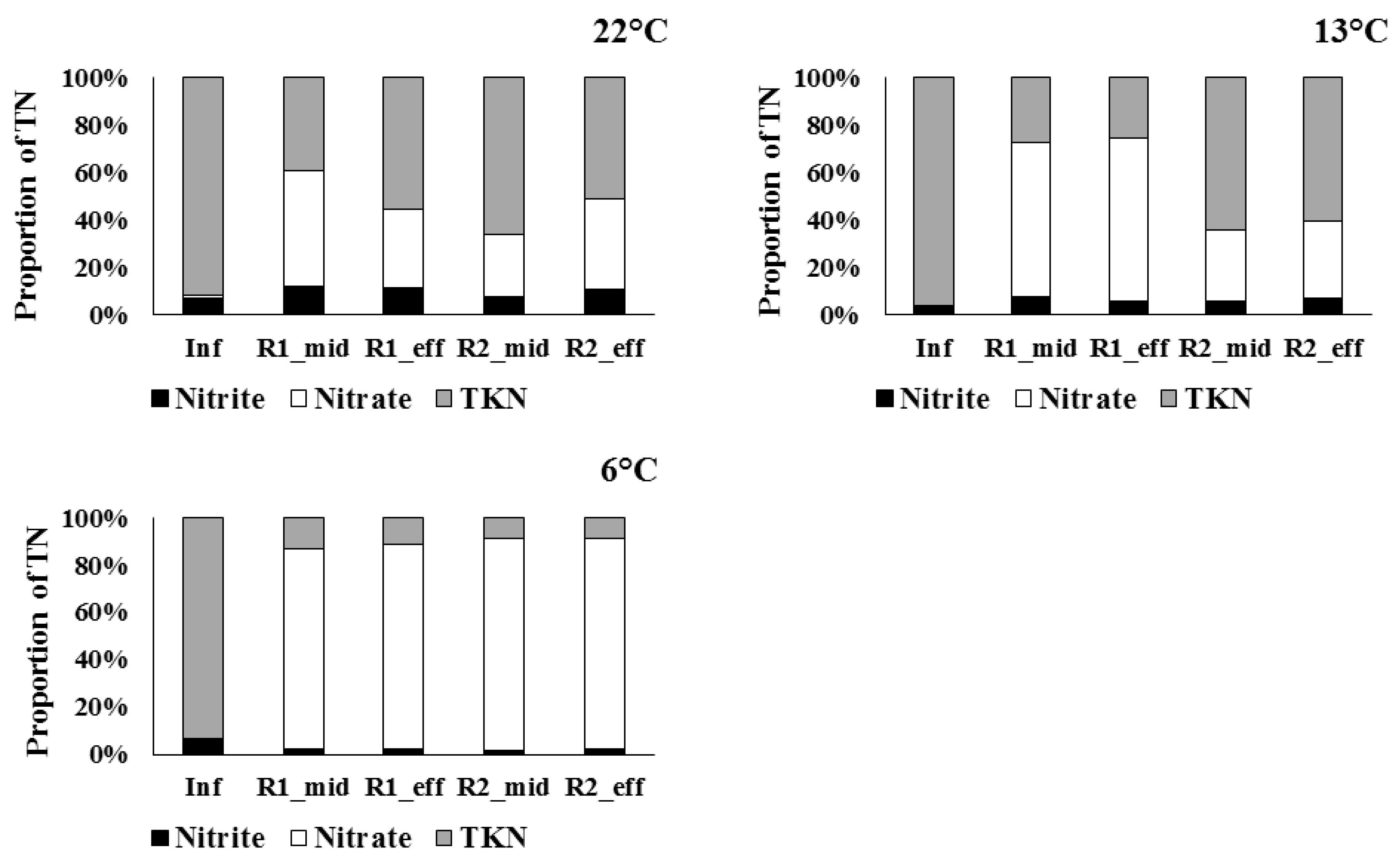
3.1. Trends in TN Removal and Effluent Quality
3.2. Nitrification at Low Temperatures
3.3. Implications for Reactor Design
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- DEFRA. Environmental Permitting (England and Wales) Regulations 2010; Statutory Instrument No. 675 Environmental Permitting (England and Wales) Regulations 2010; DEFRA: London, UK, 2010.
- Pujol, R.; Lienard, A. Qualitative and quantitative characterization of wastewater for small communities. In Proceedings of the International Specialized Conference on Design and Operation of Small Wastewater Treatment Plants, Trondheim, Norway, 26–28 June 1989; Odegaard, H., Ed.; Tapir: Bergen, Norway, 1989; pp. 267–274. [Google Scholar]
- Council of European Communities. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. Off. J. Eur. Parliam. 2000, L327, 1–82. [Google Scholar]
- Graham, D.W.; Bunce, J.T.; Giesen, M.J. Strategic approach for prioritising local and regional sanitation interventions for reducing global antibiotic resistance. Water 2019, 11, 27. [Google Scholar] [CrossRef]
- Bunce, J.T.; Ndam, E.; Ofiteru, I.D.; Moore, A.; Graham, D.W. A review of phosphorus removal technologies and their applicability to small-scale domestic wastewater treatment systems. Front. Environ. Sci. 2018, 6, 8. [Google Scholar] [CrossRef]
- Bundy, C.A.; Wu, D.; Jong, M.C.; Edwards, S.R.; Ahammad, Z.S.; Graham, D.W. Enhanced denitrification in Downflow Hanging Sponge reactors for decentralised domestic wastewater treatment. Bioresour. Technol. 2017, 226, 1–8. [Google Scholar] [CrossRef]
- Jong, M.C.; Su, J.Q.; Bunce, J.T.; Harwood, C.R.; Snape, J.R.; Zhu, Y.G.; Graham, D.W. Co-optimization of sponge-core bioreactors for removing total nitrogen and antibiotic resistance genes from domestic wastewater. Sci. Total Environ. 2018, 634, 1417–1423. [Google Scholar] [CrossRef]
- Kobayashi, N.; Oshiki, M.; Ito, T.; Segawa, T.; Hatamoto, M.; Kato, T.; Yamaguchi, T.; Kubota, K.; Takahashi, M.; Iguchi, A.; et al. Removal of human pathogenic viruses in a down-flow hanging sponge (DHS) reactor treating municipal wastewater and health risks associated with utilization of the effluent for agricultural irrigation. Water Res. 2017, 110, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Beas, Y.R.E.; Kujawa-Roeleveld, K.; van Lier, J.B.; Zeeman, G.A. Downflow Hanging Sponge (DHS) reactor for faecal coliform removal from an Upflow Anaerobic Sludge Bed (UASB) effluent. Water Sci. Technol. 2015, 72, 2034–2044. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Harrison, J.R.; Daigger, G.T. Comparison of Trickling Filter Media. J. Water Pollut. Control Fed. 1987, 59, 679–685. [Google Scholar]
- Tawfik, A.; Ohashi, A.; Harada, H. Effect of sponge volume on the performance of down-flow hanging sponge system treating UASB reactor effluent. Bioprocess Biosyst. Eng. 2010, 33, 779–785. [Google Scholar] [CrossRef]
- Onodera, T.; Tandukar, M.; Sugiyana, D.; Uemura, S.; Ohashi, A.; Harada, H. Development of a sixth-generation down-flow hanging sponge (DHS) reactor using rigid sponge media for post-treatment of UASB treating municipal sewage. Bioresour. Technol. 2014, 152, 93–100. [Google Scholar] [CrossRef]
- Ikuma, K.; Decho, A.W.; Lau, B.L.T. The Extracellular Bastions of Bacteria—A Biofilm Way of Life. Nat. Educ. Knowl. 2013, 4, 2. [Google Scholar]
- Schipper, L.A.; Robertson, W.D.; Gold, A.J.; Jaynes, D.B.; Cameron, S.C. Denitrifying bioreactors—An approach for reducing nitrate loads to receiving waters. Ecol. Eng. 2010, 36, 1532–1543. [Google Scholar] [CrossRef]
- Met Office. Met Office: UK Climate—Historic Station Data 1981–2010. Available online: https://www.metoffice.gov.uk/public/weather/climate-historic/#?tab=climateHistoric (accessed on 26 May 2020).
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2009. [Google Scholar]
- R Foundation for Statistical Computing. R Core R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Paerl, H.W.; Xu, H.; Mccarthy, M.J.; Zhu, G.; Qin, B.; Li, Y.; Gardner, W.S. Controlling harmful cyanobacterial blooms in a hyper-eutrophic lake (Lake Taihu, China): The need for a dual nutrient (N & P) management strategy. Water Res. 2011, 45, 1973–1983. [Google Scholar] [PubMed]
- Lishman, L.A.; Legge, R.L.; Farquhar, G.J. Temperature effects on wastewater treatment under aerobic and anoxic conditions. Water Res. 2000, 34, 2263–2276. [Google Scholar] [CrossRef]
- Ducey, T.F.; Vanotti, M.B.; Shriner, A.D.; Szogi, A.A.; Ellison, A.Q. Characterization of a microbial community capable of nitrification at cold temperature. Bioresour. Technol. 2010, 101, 491–500. [Google Scholar] [CrossRef]
- Council of European Communities. Directive concerning urban waste water treatment. Off. J. Eur. Commun. 1991, 135, 40–52. [Google Scholar]
- Daigger, G.T. Oxygen and carbon requirements for biological nitrogen removal processes accomplishing nitrification, nitritation, and anammox. Water Environ. Res. 2014, 86, 204–209. [Google Scholar] [CrossRef]
- Nedwell, D.B. Effect of low temperature on microbial growth: Lowered affinity for substrates limits growth at low temperature. FEMS Microbiol. Ecol. 1999, 30, 101–111. [Google Scholar] [CrossRef]
- Weiss, R.F. The solubility of nitrogen, oxygen and argon in water and seawater. Deep Sea Res. Oceanogr. Abstr. 1970, 17, 721–735. [Google Scholar] [CrossRef]
- Hwang, J.H.; Oleszkiewicz, J.A. Effect of cold-temperature shock on nitrification. Water Environ. Res. 2007, 79, 964–968. [Google Scholar] [CrossRef]
- Oleszkiewicz, J.A.; Berquist, S.A. Low temperature nitrogen removal in sequencing batch reactors. Water Res. 1988, 22, 1163–1171. [Google Scholar] [CrossRef]
- Painter, H.A.; Loveless, J.E. Effect of temperature and pH value on the growth-rate constants of nitrifying bacteria in the activated sludge process. Water Res. 1983, 17, 237–248. [Google Scholar] [CrossRef]
- Head, M.A.; Oleszkiewicz, J.A. Bioaugmentation for nitrification at cold temperatures. Water Res. 2004, 38, 523–530. [Google Scholar] [CrossRef]
- Onodera, T.; Okubo, T.; Uemura, S.; Yamaguchi, T.; Ohashi, A.; Harada, H. Long-term performance evaluation of down-flow hanging sponge reactor regarding nitrification in a full-scale experiment in India. Bioresour. Technol. 2016, 204, 177–184. [Google Scholar] [CrossRef]


| R1 | 22 °C | 13 °C | 6 °C | |||||||||
| Inf (mg/L) | Eff (mg/L) | R% | Load Removal (g/L/day) | Inf (mg/L) | Eff (mg/L) | R% | Load Removal (g/L/day) | Inf (mg/L) | Eff (mg/L) | R% | Load Removal (g/L/day) | |
| sCOD | 365 (65.5) | 67.4 (9.8) | 81.5 | 1.04 | 195 (75.3) | 50.4 (22.4) | 74.1 | 0.51 | 271 (34.6) | 38.8 (15.9) | 85.6 | 0.81 |
| tCOD | 569 (107) | 85.0 (27.5) | 85.1 | 1.70 | 322 (124.5) | 56.7 (16.3) | 82.4 | 0.93 | 487 (29.7) | 61.4 (48.7) | 87.4 | 1.49 |
| TN | 51.8 (23.2) | 20.0 (12.2) | 61.4 | 0.11 | 33.1 (15.8) | 45.9 (30.8) | −38.7 | −0.05 | 54.7 (5.6) | 129 (46.3) | −136 | −0.26 |
| NH4–N | 47.4 (15.0) | 13.0 (8.9) | 72.6 | 0.12 | 29.8 (10.4) | 5.2 (5.2) | 82.6 | 0.09 | 51.5 (6.0) | 1.6 (0.9) | 91.4 | 0.17 |
| pH | 7.3 (0.4) | 7.4 (0.2) | - | - | 7.1 (0.5) | 6.8 (0.4) | - | - | 7.6 (0.2) | 7.2 (0.5) | - | - |
| DO | 1.4 (0.4) | 1.6 (0.4) | - | - | 2.5 (0.3) | 3.2 (1.6) | - | - | 2.0 (1.5) | 6.0 (3.5) | - | - |
| R2 | 22 °C | 13 °C | 6 °C | |||||||||
| Inf (mg/L) | Eff (mg/L) | R% | Load Removal (g/L/day) | Inf (mg/L) | Eff (mg/L) | R% | Load Removal (g/L/day) | Inf (mg/L) | Eff (mg/L) | R% | Load Removal (g/L/day) | |
| sCOD | 365 (65.5) | 54.8 (26.8) | 85.0 | 1.09 | 195 (75.3) | 42.0 (17.8) | 78.4 | 0.54 | 271 (34.6) | 42.9 (21.9) | 84.2 | 0.80 |
| tCOD | 569 (107) | 54.1 (21.1) | 90.5 | 1.80 | 322 (124.5) | 42.5 (16.1) | 86.8 | 0.98 | 487 (29.7) | 64.9 (44.8) | 86.7 | 1.48 |
| TN | 51.8 (23.2) | 21.6 (14.3) | 58.3 | 0.11 | 33.1 (15.8) | 22.8 (14.8) | 31.1 | 0.04 | 54.7 (5.6) | 196 (58.3) | −258 | −0.49 |
| NH4–N | 47.4 (15.0) | 12.6 (5.7) | 73.4 | 0.12 | 29.8 (10.4) | 6.6 (6.1) | 77.9 | 0.08 | 51.5 (6.0) | 4.0 (7.1) | 92.2 | 0.17 |
| pH | 7.3 (0.4) | 7.4 (0.4) | - | - | 7.1 (0.5) | 7.0 (0.4) | - | - | 7.6 (0.2) | 6.9 (0.2) | - | - |
| DO | 1.4 (0.4) | 1.7 (0.2) | - | - | 2.5 (0.3) | 2.1 (0.4) | - | - | 2.0 (1.5) | 5.5 (3.7) | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Collings, E.J.; Bunce, J.T.; Jong, M.-C.; Graham, D.W. Impact of Cold Temperatures on Nitrogen Removal in Denitrifying Down-Flow Hanging Sponge (DDHS) Reactors. Water 2020, 12, 2029. https://doi.org/10.3390/w12072029
Collings EJ, Bunce JT, Jong M-C, Graham DW. Impact of Cold Temperatures on Nitrogen Removal in Denitrifying Down-Flow Hanging Sponge (DDHS) Reactors. Water. 2020; 12(7):2029. https://doi.org/10.3390/w12072029
Chicago/Turabian StyleCollings, Emily J., Joshua T. Bunce, Mui-Choo Jong, and David W. Graham. 2020. "Impact of Cold Temperatures on Nitrogen Removal in Denitrifying Down-Flow Hanging Sponge (DDHS) Reactors" Water 12, no. 7: 2029. https://doi.org/10.3390/w12072029
APA StyleCollings, E. J., Bunce, J. T., Jong, M.-C., & Graham, D. W. (2020). Impact of Cold Temperatures on Nitrogen Removal in Denitrifying Down-Flow Hanging Sponge (DDHS) Reactors. Water, 12(7), 2029. https://doi.org/10.3390/w12072029








