Combining Water Quality Indices and Multivariate Modeling to Assess Surface Water Quality in the Northern Nile Delta, Egypt
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Area
2.2. Sampling and Analyses
2.3. Indexing Approach
2.3.1. Drinking Water Quality Index (DWQI)
2.3.2. Pollution Indices (PIs)
Heavy Metal Pollution Index (HPI)
Heavy Metal Evaluation Index (HEI)
Contamination Index (CD)
Pollution Index (PI)
2.4. Data Analysis
3. Results
3.1. Physicochemical Data
3.2. Geochemical Facies and Controlling Mechanisms
3.3. Water Quality Indices
Relationships between the Drinking Water Quality Index and Pollution Indices
3.4. Multivariate Statistical Analysis
3.4.1. Principal Component Analysis
3.4.2. The Performance of Partial Least Square Regression Models and Stepwise Multiple Linear Regressions to Predict the Drinking Water Quality Index and Pollution Indices
4. Discussion
4.1. Physiochemical Parameters
4.2. Assessment of Water Quality Indices
4.3. Multivariate Statistical Analysis
4.3.1. Principal Component Analysis
4.3.2. Partial Least Square Regression Models and Stepwise Multiple Linear Regressions to Predict the Drinking Water Quality Index and Pollution Indices
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gad, M.; El-Hattab, M. Integration of water pollution indices and DRASTIC model for assessment of groundwater quality in El Fayoum Depression, Western Desert, Egypt. J. Afr. Earth Sci. 2019, 158, 103554. [Google Scholar] [CrossRef]
- Goher, M.E.; Farhat, H.I.; Abdo, M.H.; Salem, S.G. Metal pollution assessment in the surface sediment of Lake Nasser, Egypt. Egypt. J. Aquat. Res. 2014, 40, 213–224. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Sharma, A.; Kumar, R.; Bhardwaj, R.; Thukral, A.K.; Rodrigo-Comino, J. Assessment of heavy-metal pollution in three different Indian water bodies by combination of multivariate analysis and water pollution indices. Hum. Ecol. Risk Assess. 2018, 26, 1–16. [Google Scholar] [CrossRef]
- Taheri, M.; Mehrzad, J.; MahmudyGharaie, M.H.; Afshari, R.; Dadsetan, A.; Hami, S. High soil and groundwater arsenic levels induce high body arsenic loads, health risk and potential anemia for inhabitants of northeastern Iran. Environ. Geochem. Health 2016, 38, 469–482. [Google Scholar] [CrossRef]
- Smith, V.H. Effects of eutrophication on maximum algal biomass in lake and river ecosystems. Inland Waters 2016, 6, 147–154. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Wang, X.; Chen, Y.; Cai, Y.; Deng, J. Assessing river water quality using water quality index in Lake Taihu Basin, China. Sci. Total Environ. 2018, 612, 914–922. [Google Scholar] [CrossRef]
- Zhao, Y.; Xia, X.H.; Yang, Z.F.; Wang, F. Assessment of water quality in Baiyangdian Lake using multivariate statistical techniques. Procedia Environ. Sci. 2012, 13, 1213–1226. [Google Scholar] [CrossRef] [Green Version]
- Masoud, A.H.; El-Fakharany, I.I.; Abd El-Razik, M.A.S. Monitoring of some agrochemical pollutants in surface water in Kafr El-Sheikh Governorate. J. Pest. Cont. Environ. Sci. 2007, 15, 21–41. [Google Scholar]
- El-Bouraie, M.M.; El-Barbary, A.A.; Yehia, M.M.; Motawea, E.A. Heavy metal concentrations in surface river water and bed sediments at Nile Delta in Egypt. Suoseura 2010, 61, 1–12. [Google Scholar]
- El-Bana, T.A. Agro-Ecological Assessment of Land and Water Resources Norther Nile Delta: A Case Study in Kafr El-Sheikh Governorate. Master’s Thesis, Alexandria University, Alexandria, Egypt, 2003. [Google Scholar]
- El-Bana, T.A.; Gaber, H.M.; Bahnassy, M.H.; Suliman, A.S. Quality assessment of water resources in Northern Nile Delta: A case study in Kafr El-Sheikh Governorate. Egypt. J. Soil Sci. 2006, 46, 409–423. [Google Scholar]
- Bora, M.; Goswami, D.C. Water quality assessment in terms of water quality index (DWQI): Case study of the Kolong River, Assam, India. Appl. Water Sci. 2017, 7, 3125–3135. [Google Scholar] [CrossRef] [Green Version]
- Jalali, M. Salinization of groundwater in arid and semiarid zones: An example from Tajarak, Western Iran. Environ. Geol. 2007, 52, 1133–1149. [Google Scholar] [CrossRef]
- Mondal, N.C.; Singh, V.P. Hydrochemical analysis of salinization for a tannery belt in Southern India. J. Hydrol. 2011, 405, 235–247. [Google Scholar] [CrossRef]
- Mondal, N.C.; Tiwari, K.K.; Sharma, K.C.; Ahmed, S. A diagnosis of groundwater quality from a semiarid region in Rajasthan, India. Arab. J. Geosci. 2016, 9, 1–22. [Google Scholar] [CrossRef]
- Edet, A.E.; Offiong, O.E. Evaluation of water quality pollution indices for heavy metal contamination monitoring. A study case from Akpabuyo-Odukpani area, Lower Cross River Basin (south Nigeria). GeoJ 2002, 4, 295–304. [Google Scholar] [CrossRef]
- Sheykhi, V.; Moore, F. Geochemical characterization of Kor River water quality, Fars Province, Southwest Iran. Water Qual. Expo Health 2012, 4, 25–38. [Google Scholar] [CrossRef]
- Sobhanardakani, S.; Yari, A.R.; Taghavi, L.; Tayebi, L. Water quality pollution indices to assess the heavy metal contamination, case study: Groundwater resources of Asadabad Plain in 2012. Arch. Hyg. Sci. 2016, 5, 221–228. [Google Scholar]
- AbouZakhem, B.; Hafez, R. Heavy metal pollution index for groundwater quality assessment in Damascus Oasis, Syria. Environ. Earth Sci. 2015, 73, 6591–6600. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Ramu, A. Evaluation of heavy metal pollution index (HPI) of ground water in and around the coastal area of Gulf of Mannar biosphere and Palk strait. J. Adv. Chem. Sci. 2016, 2, 331–333. [Google Scholar]
- El Fehri, I.C.; El Abidi, A.; Fekhaoui, M.; Bellaouchou, A.; Benakan, R.; El Jaoudi, R. Level of contamination by trace metals in groundwater in the agricultural area of Sidi abdelrrazak (Province of Khémisset, Morocco). J. Mater. Environ. Sci. 2014, 5, 705–710. [Google Scholar]
- Sobhanardakani, S.; Taghavi, L.; Shahmoradi, B.; Jahangard, A. Groundwater quality assessment using the water quality pollution indices in Toyserkan Plain. Environ. Health Eng. Manag. 2017, 4, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Prasad, B.; Kumari, S. Heavy metal pollution index of ground water of an abandoned open cast mine filled with fly ash: A case study. Mine Water Environ. 2008, 27, 265–267. [Google Scholar] [CrossRef]
- Reza, R.; Singh, G. Assessment of heavy metal contamination and its indexing approach for river water. Int. J. Environ. Sci. Tech. 2010, 7, 785–792. [Google Scholar] [CrossRef] [Green Version]
- Reza, R.; Singh, G.; Manish, J. Application of heavy metal pollution index for ground water quality assessment in Angul District of Orissa, India. Int. J. Res. Chem. Environ. 2011, 1, 118–122. [Google Scholar]
- Caerio, S.; Costa, M.H.; Ramos, T.B.; Fernandes, F.; Silveira, N.; Coimbra, A.; Painho, M. Assessing heavy metal contamination in Sado Estuary sediment: An index analysis approach. Ecol. Indic. 2005, 5, 155–169. [Google Scholar] [CrossRef]
- Mustafa, O.M. Evaluating water quality of Waraz mountainous area, using contamination index, Sulaimaniya Governorate, Northeast Iraq. Iraqi Bull. Geol. Min. 2008, 4, 59–66. [Google Scholar]
- Kamtchueng, B.T.; Fantong, W.Y.; Wirmvem, M.J. Hydrogeochemistry and quality of surface water and groundwater in the vicinity of Lake Monoun, West Cameroon: Approach from multivariate statistical analysis and stable isotopic characterization. Environ. Monit. Assess. 2016, 188, 524. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, A.; Chawla, A.; Bhardwaj, R.; Thukral, A.K. Water quality assessment of River Beas, India, using multivariate and remote sensing techniques. Environ. Monit. Assess. 2016, 188, 137. [Google Scholar] [CrossRef]
- Matiatos, I.; Alexopoulos, A.; Godelitsas, A. Multivariate statistical analysis of the hydrogeochemical and isotopic composition of the groundwater resources in northeastern Peloponnesus (Greece). Sci. Total Environ. 2014, 476, 577–590. [Google Scholar] [CrossRef]
- Rakotondrabe, F.; Ngoupayou, J.R.; Mfonka, Z. Water quality assessment in the Betare-Oya gold mining area (East-Cameroon): Multivariate statistical analysis approach. Sci. Total Environ. 2018, 610, 831–844. [Google Scholar] [CrossRef]
- Li, P.; Tian, R.; Liu, R. Solute geochemistry and multivariate analysis of water quality in the Guohua Phosphorite Mine, Guizhou Province, China. Expo Health 2019, 11, 81–94. [Google Scholar] [CrossRef]
- Ahmed, U.; Mumtaz, R.; Anwar, H.; Shah, A.A.; Irfan, R.; García-Nieto, J. Efficient water quality prediction using supervised machine learning. Water 2019, 11, 2210. [Google Scholar] [CrossRef] [Green Version]
- Banda, T.D.; Kumarasamy, M. Application of multivariate statistical analysis in the development of a surrogate water quality index. Water 2020, 12, 1584. [Google Scholar] [CrossRef]
- Chowdhury, R.M.; Muntasir, S.Y.; Hossain, M.M. Water quality index of water bodies along Faridpur-Barisal road in Bangladesh. Glob. Eng. Technol. Rev. 2012, 2, 1–8. [Google Scholar]
- Tamasi, G.; Cini, R. Heavy metals in drinking waters from Mount Amiata (Tuscany, Italy) possible risks from arsenic for public health in the province of Siena. Sci. Total Environ. 2004, 327, 41–51. [Google Scholar] [CrossRef]
- Ahmad, S.; Ghani, M.M.I. Stepwise multiple regression method to forecast fish landing. Procedia Soc. Behav. Sci. 2010, 8, 549–554. [Google Scholar]
- Mustapha, A.; Aris, Z.A. Multivariate statistical analysis and environmental modeling of heavy metals pollution by industries. Pol. J. Environ. Stud. 2012, 21, 1359–1367. [Google Scholar]
- Chen, J.; Lu, J. Effects of land use, topography and socio-economic factors on river water quality in a mountainous watershed with intensive agricultural production in East China. PLoS ONE 2014, 9, e102714. [Google Scholar] [CrossRef] [PubMed]
- Garriga, M.; Romero-Bravo, S.; Estrada, F.; Escobar, A.; Matus, I.A.; del Pozo, A.; Astudillo, C.A.; Lobos, G.A. Assessing wheat traits by spectral reflectance: Do we really need to focus on predicted trait-values or directly identify the elite genotypes group? Front. Plant Sci. 2017, 8, 280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, H.J.; Lee, H.J. Multiple linear regression models for predicting nonpoint-source pollutant discharge from a highland agricultural region. Water 2018, 10, 1156. [Google Scholar] [CrossRef] [Green Version]
- Wei, L.; Huang, C.; Zhong, Y.; Wang, Z.; Hu, X.; Lin, L. Inland waters suspended solids concentration retrieval based on PSO-LSSVM for UAV-borne hyperspectral remote sensing imagery. Remote Sens. 2019, 11, 1455. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.; Ren, X.; Yang, Z.; Long, H.; Xiao, J. Influence of landscape structures on water quality at multiple temporal and spatial scales: A case study of Wujiang River Watershed in Guizhou. Water 2019, 11, 159. [Google Scholar] [CrossRef] [Green Version]
- CAPMAS. Egypt in Figures; Central Agency for Public Mobilization and Statistics (CAPMAS): Cairo, Egypt, 2012. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Nasrabadi, T. An index approach to metallic pollution in river waters. Int. J. Environ. Res. 2015, 9, 385–394. [Google Scholar]
- Piper, A.M. A graphic procedure in the geochemical interpretation of water-analyses. Trans. Am. Geophys. Union 1944, 25, 914–928. [Google Scholar] [CrossRef]
- Gibbs, R.J. Mechanisms controlling world water chemistry. Science 1970, 170, 10881. [Google Scholar] [CrossRef]
- Madrid, C.H.; Vázquez, L.A.; Soriano, P.L.; Mejía, J.M.; Fatjó, V.G.; Rodríguez, S.D.M.; Oliva, E.A.M.; Jiménez, C.J. Identification of key factors affecting the trophic state of four tropical small water bodies. Water 2020, 12, 1454. [Google Scholar] [CrossRef]
- Twisa, S.; Kazumba, S.; Kurian, M.; Buchroithner, F.M. Evaluating and predicting the effects of land use changes on hydrology in Wami River Basin, Tanzania. Hydrology 2020, 7, 17. [Google Scholar] [CrossRef] [Green Version]
- Vasques, G.M.; Grunwald, S.; Sickman, J.O. Comparison of multivariate methods for inferential modeling of soil carbon using visible/near-infrared spectra. Geoderma 2008, 146, 14–25. [Google Scholar] [CrossRef]
- Aktar, M.W.; Paramasivam, M.; Ganguly, M.; Purkait, S.; Sengupta, D. Assessment and occurrence of various heavy metals in surface water of Ganga River around Kolkata: A study for toxicity and ecological impact. Environ. Monit. Assess. 2010, 160, 207–213. [Google Scholar] [CrossRef]
- Begum, A.; Ramaiah, M.; Khan, H.I.; Veena, K. Heavy metal pollution and chemical profile of Cauvery River Water. J. Chem. 2009, 6, 47–52. [Google Scholar] [CrossRef]
- Saxena, V.K.; Singh, V.S.; Mondal, N.C.; Jain, S.C. Use of chemical parameters to delineation fresh ground water resources in Potharlanka Island, India. Environ. Geol. 2003, 44, 516–521. [Google Scholar] [CrossRef]
- Sarwade, D.V.; Nandakumar, M.V.; Kesari, M.P.; Mondal, N.C.; Singh, V.S.; Singh, B. Evaluation of sea water ingress into an Indian Attoll. Environ. Geol. 2007, 52, 1475–1483. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, L.; Abuduwaili, J.; Ge, Y.; Issanova, G.; Saparov, G. Hydrochemical characteristics and irrigation suitability of surface water in the Syr Darya River, Kazakhstan. Environ. Monit. Assess. 2019, 191, 572. [Google Scholar] [CrossRef] [Green Version]
- Bhat, A.N.; Bhat, A.A.; Nath, S.; Singh, P.B.; Guha, B.D. Assessment of drinking and irrigation water quality of surface water resources of South-West Kashmir, India. J. Civ. Environ. Eng. 2016, 6, 222. [Google Scholar]
- Kachroud, M.; Trolard, F.; Kefi, M.; Jebari, S.; Bourrié, G. Water quality indices: Challenges and application limits in the literature. Water 2019, 11, 361. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.F.; Liu, C.Q.; Tu, C.L. Atmospheric deposition of metals in TSP of Guiyang, PR China. Bull. Environ. Contam. Toxicol. 2008, 80, 465–468. [Google Scholar] [CrossRef]
- Abdel-Satar, A.M. Environmental studies on the impact of the drains effluent upon the southern sector of Lake Manzalah. Egypt. J. Aquat. Biol. Fish. 2001, 5, 17–30. [Google Scholar] [CrossRef] [Green Version]
- Pulles, T.; van der Gon, H.D.; Appelman, W.; Verheul, M. Emission factors for heavy metals from diesel and petrol used in European vehicles. Atmos. Environ. 2012, 61, 641–651. [Google Scholar] [CrossRef]
- Saleem, M.; Iqbal, J.; Shah, M.H. Geochemical speciation, anthropogenic contamination, risk assessment and source identification of selected metals in freshwater sediments—A case study from Mangla Lake, Pakistan. Environ. Nanotechnol. Monit. Manag. 2015, 4, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Liu, G.; Liu, H.; Lam, P.K. Multivariate statistical evaluation of dissolved trace elements and a water quality assessment in the middle reaches of Huaihe River, Anhui, China. Sci. Total Environ. 2017, 583, 421–431. [Google Scholar] [CrossRef]
- Xiao, M.; Bao, F.; Wang, S.; Cui, F. Water quality assessment of the Huaihe River segment of Bengbu (China) using multivariate statistical techniques. Water Res. 2016, 43, 166–176. [Google Scholar]
- Elsayed, S.; Elhewity, M.; Ibrahim, H.H.; Dewir, Y.H.; Migdadi, H.M.; Schmidhalter, U. Thermal imaging and passive reflectance sensing to estimate the water status and grain yield of wheat under different irrigation regimes. Agric. Water Manag. 2017, 189, 98–110. [Google Scholar] [CrossRef]


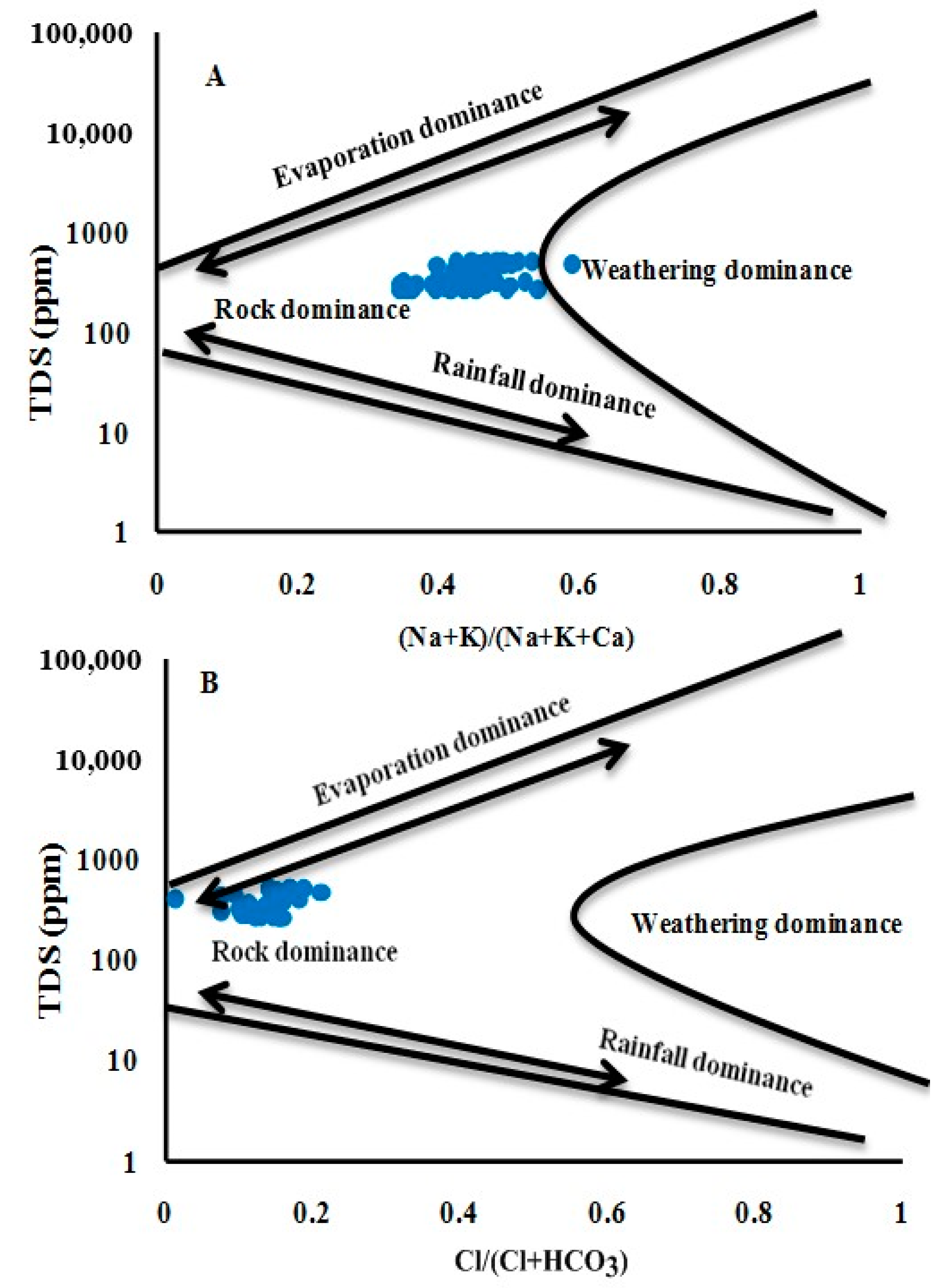
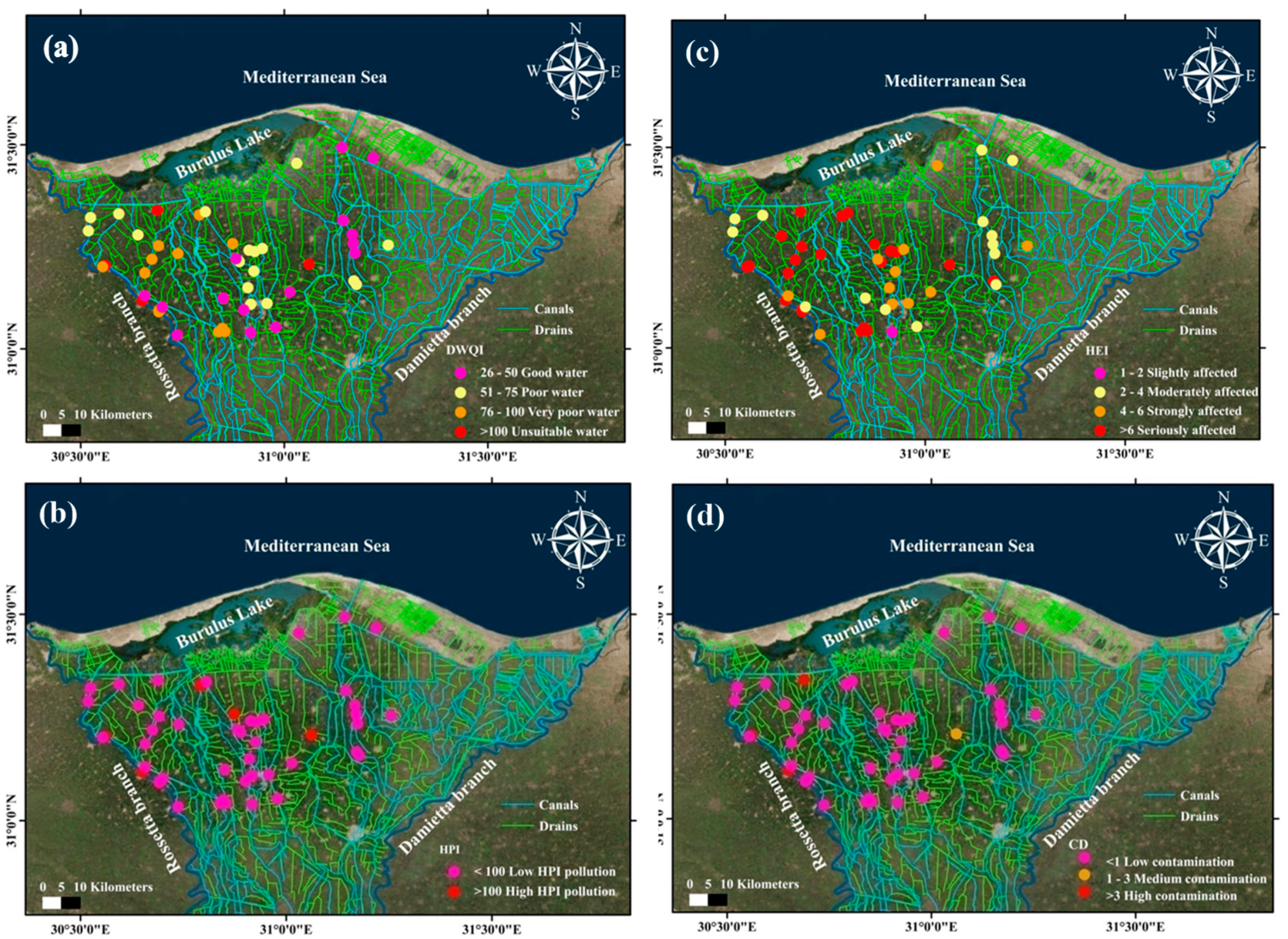



| Parameter | Temperature | pH | EC | TDS | TH | K+ | Na+ | Mg2+ | Ca2+ | Cl− | SO42− | |
| Minimum | 27.0 | 7.2 | 406.3 | 260.0 | 2.80 | 4.5 | 15.0 | 8.2 | 22.6 | 4.0 | 26.2 | |
| Maximum | 33.7 | 8.7 | 790.6 | 506.0 | 236 | 11.2 | 55.0 | 25.0 | 64.6 | 55.0 | 104.1 | |
| Mean | 28.3 | 8.0 | 562.0 | 359.7 | 183.25 | 7.4 | 32.5 | 17.0 | 39.6 | 28.8 | 52.1 | |
| SD | 1.9 | 0.3 | 138.9 | 88.9 | 36.23 | 2.0 | 11.6 | 4.3 | 11.0 | 12.2 | 18.1 | |
| Parameter | Alkalinity | NO3− | B | Cd | Cr | Cu | F | Fe | Mn | Ni | Pb | Zn |
| Minimum | 118.0 | 0.800 | 0.02 | 0.0002 | 0.005 | 0.001 | 0.100 | 0.050 | 0.010 | 0.001 | 0.001 | 0.001 |
| Maximum | 278.3 | 4.420 | 0.49 | 0.0330 | 0.340 | 0.030 | 0.700 | 1.200 | 0.450 | 0.050 | 31.00 | 0.220 |
| Mean | 184.3 | 1.506 | 0.12 | 0.0060 | 0.061 | 0.009 | 0.265 | 0.225 | 0.105 | 0.017 | 0.605 | 0.025 |
| SD | 44.6 | 0.582 | 0.11 | 0.0090 | 0.060 | 0.005 | 0.168 | 0.206 | 0.087 | 0.017 | 4.175 | 0.060 |
| Parameter | Weight (wi) | WHO 2011 [46] | Relative Weight (Wi) |
|---|---|---|---|
| pH | 4 | 7.50 | 0.0548 |
| EC | 4 | 1000 | 0.0548 |
| TDS | 5 | 500 | 0.0685 |
| TH | 2 | 300 | 0.0274 |
| K+ | 2 | 10.0 | 0.0274 |
| Na+ | 3 | 200 | 0.0411 |
| Ca2+ | 2 | 75.0 | 0.0274 |
| Mg2+ | 2 | 30.0 | 0.0274 |
| Cl− | 1 | 250 | 0.0137 |
| SO42− | 3 | 250 | 0.0411 |
| Alkalinity | 3 | 120 | 0.0411 |
| NO3− | 5 | 50.0 | 0.0685 |
| B | 3 | 0.50 | 0.0411 |
| Cd | 3 | 0.003 | 0.0411 |
| Cr | 4 | 0.05 | 0.0548 |
| Cu | 2 | 2.00 | 0.0274 |
| F | 4 | 1.50 | 0.0548 |
| Fe | 4 | 0.30 | 0.0548 |
| Mn | 4 | 0.05 | 0.0548 |
| Ni | 3 | 0.07 | 0.0411 |
| Pb | 5 | 0.01 | 0.0685 |
| Zn | 2 | 3.00 | 0.0274 |
| ∑wi = 73 | ∑Wi = 1 |
| Heavy Metal | WHO 2011 [46] Si (mg/L) | MACi | Unit Weight Wi | Sub index Qi | Wi × Qi |
|---|---|---|---|---|---|
| B | 0.50 | 500 | 0.00404 | 4.00 | 0.016179516 |
| Cd | 0.003 | 3.00 | 0.67415 | 6.66 | 4.494309883 |
| Cr | 0.05 | 50.0 | 0.04045 | 10.00 | 0.404487889 |
| Cu | 2.00 | 2000 | 0.00101 | 0.50 | 0.00050561 |
| F | 1.50 | 1500 | 0.00135 | 20.00 | 0.026965859 |
| Fe | 0.30 | 300 | 0.00674 | 100.0 | 0.674146482 |
| Mn | 0.05 | 50.0 | 0.04045 | 20.00 | 0.808975779 |
| Ni | 0.07 | 70.0 | 0.02889 | 2.85 | 0.082548549 |
| Pb | 0.01 | 10.0 | 0.20224 | 100.0 | 20.22439447 |
| Zn | 3.00 | 3000 | 0.00067 | 0.166 | 0.000112358 |
| ∑ (Wi) = 1 | ∑ (Wi × Qi) |
| Class | PI Value | Effect |
|---|---|---|
| 1 | <1 | No effect |
| 2 | 1–2 | Slightly affected |
| 3 | 2–3 | Moderately affected |
| 4 | 3–5 | Strongly affected |
| 5 | >5 | Seriously affected |
| Water Quality Indices (WQIs) | Sample Range | Range | Water Class | Samples (%) | |||
|---|---|---|---|---|---|---|---|
| Minimum | Maximum | Mean | SD | ||||
| Drinking water quality index (DWQI) | 36.72 | 136.73 | 66.99 | 21.25 | 0–25 | Excellent water | Nil |
| 26–50 | Good water | 33% (18 samples) | |||||
| 51–75 | Poor water | 58% (32 samples) | |||||
| 76–100 | Very poor water | 5% (3 samples) | |||||
| >100 | Unsuitable | 4% (2 samples) | |||||
| Heavy metal pollution index (HPI) | 26.28 | 222.51 | 68.24 | 42.42 | <100 | Low polluted | 87% (48 samples) |
| >100 | High polluted | 13% (7 samples) | |||||
| Heavy metal evaluation index (HEI) | 1.98 | 17.23 | 6.59 | 3.33 | <0.3 | Very pure | Nil |
| 0.3–1.0 | Pure | Nil | |||||
| 1.0–2.0 | Slightly affected | Nil | |||||
| 2.0–3.0 | Moderately affected | 4% (2 samples) | |||||
| 3.0–6.0 | Strongly affected | 49% (27 samples) | |||||
| >6.0 | Seriously affected | 47% (26 samples) | |||||
| Contamination index (CD) | −8.02 | 7.23 | −3.41 | 3.33 | >1 | Low | 91% (50 samples) |
| 1–3 | Medium | 2% (1 sample) | |||||
| <3 | High | 7% (4 samples) | |||||
| Heavy Metal | PI | Class | Effect |
|---|---|---|---|
| B | 0.20 | I | No effect |
| Cd | 0.15 | I | No effect |
| Cr | 1.00 | II | Slightly affected |
| Cu | 0.01 | I | No effect |
| F | 0.24 | I | No effect |
| Fe | 1.17 | II | Slightly affected |
| Mn | 4.50 | IV | Strongly affected |
| Ni | 0.36 | I | No effect |
| Pb | 4.50 | IV | Strongly affected |
| Zn | 0.00 | I | No effect |
| Model No. | Influential Heavy Metals for DWQI | R2 | SE |
| 1 | Pb | 0.774 | 7.62614 |
| 2 | Pb, TDS | 0.921 | 4.54227 |
| 3 | Pb, TDS, Fe | 0.961 | 3.22618 |
| 4 | Pb, TDS, Fe, Cr | 0.985 | 2.02108 |
| 5 | Pb, TDS, Fe, Cr, Ni | 0.994 | 1.24464 |
| 6 | Pb, TDS, Fe, Cr, Ni, Mn | 0.998 | 0.68741 |
| 7 | Pb, TDS, Fe, Cr, Ni, Mn, Cd | 0.999 | 0.63303 |
| 8 | Pb, TDS, Fe, Cr, Ni, Mn, Cd, K | 0.999 | 0.59664 |
| 9 | Pb, TDS, Fe, Cr, Ni, Mn, Cd, K, Mg | 0.999 | 0.56404 |
| 10 | Pb, TDS, Fe, Cr, Ni, Mn, Cd, K, Mg, Alkalinity | 0.999 | 0.51314 |
| 11 | Pb, TDS, Fe, Cr, Ni, Mn, Cd, K, Mg, Alkalinity, F | 0.999 | 0.41374 |
| 12 | Pb, TDS, Fe, Cr, Ni, Mn, Cd, K, Mg, Alkalinity, F, TH | 1.000 | 0.2414 |
| 13 | Pb, TDS, Fe, Cr, Ni, Mn, Cd, K, Mg, Alkalinity, F, TH, SO4 | 1.000 | 0.17705 |
| 14 | Pb, TDS, Fe, Cr, Ni, Mn, Cd, K, Mg, Alkalinity, F, TH, SO4, PH | 1.000 | 0.08731 |
| 15 | Pb, TDS, Fe, Cr, Ni, Mn, Cd, K, Mg, Alkalinity, F, TH, SO4, PH, B | 1.000 | 0.06297 |
| 16 | Pb, TDS, Fe, Cr, Ni, Mn, Cd, K, Mg, Alkalinity, F, TH, SO4, PH, B, NO3 | 1.000 | 0.05622 |
| 17 | Pb, TDS, Fe, Cr, Ni, Mn, Cd, K, Mg, Alkalinity, F, TH, SO4, PH, B, NO3, Ca | 1.000 | 0.04346 |
| 18 | Pb, TDS, Fe, Cr, Ni, Mn, Cd, K, Mg, Alkalinity, F, TH, SO4, PH, B, NO3, Ca, Na | 1.000 | 0.01118 |
| 19 | Pb, TDS, Fe, Cr, Ni, Mn, Cd, K, Mg, Alkalinity, F, TH, SO4, PH, B, NO3, Ca, Na, Cl | 1.000 | 0.00487 |
| 20 | Pb, TDS, Fe, Cr, Ni, Mn, Cd, K, Mg, Alkalinity, F, TH, SO4, PH, B, NO3, Ca, Na, Cl, Cu | 1.000 | 0.00074 |
| 21 | Pb, TDS, Fe, Cr, Ni, Mn, Cd, K, Mg, Alkalinity, F, TH, SO4, PH, B, NO3, Ca, Na, Cl, Cu, Zn | 1.000 | 0.00000 |
| Model No. | Influential Heavy Metals for HPI | R2 | SE |
| 1 | Pb | 0.98 | 5.88732 |
| 2 | Pb, Cd | 0.998 | 1.90212 |
| 3 | Pb, Cd, Cr | 1.000 | 0.72319 |
| 4 | Pb, Cd, Cr, Ni | 1.000 | 0.38778 |
| 5 | Pb, Cd, Cr, Ni, Fe | 1.000 | 0.10818 |
| 6 | Pb, Cd, Cr, Ni, Fe, Mn | 1.000 | 0.01196 |
| 7 | Pb, Cd, Cr, Ni, Fe, Mn, F | 1.000 | 0.00125 |
| 8 | Pb, Cd, Cr, Ni, Fe, Mn, F, B | 1.000 | 0.00024 |
| 9 | Pb, Cd, Cr, Ni, Fe, Mn, F, B, Cu | 1.000 | 0.00001 |
| 10 | Pb, Cd, Cr, Ni, Fe, Mn, F, B, Cu, Zn | 1.000 | 0.00000 |
| Model No. | Influential Heavy Metals for HEI | R2 | SE |
| 1 | Pb | 0.861 | 0.88209 |
| 2 | Pb, Fe | 0.929 | 0.63571 |
| 3 | Pb, Fe, Cr | 0.968 | 0.43119 |
| 4 | Pb, Fe, Cr, Ni | 0.989 | 0.25051 |
| 5 | Pb, Fe, Cr, Ni, Mn | 0.998 | 0.11135 |
| 6 | Pb, Fe, Cr, Ni, Mn, F | 0.999 | 0.08108 |
| 7 | Pb, Fe, Cr, Ni, Mn, F, Cd | 1.000 | 0.01419 |
| 8 | Pb, Fe, Cr, Ni, Mn, F, Cd, B | 1.000 | 0.00221 |
| 9 | Pb, Fe, Cr, Ni, Mn, F, Cd, B, Cu | 1.000 | 0.00027 |
| 10 | Pb, Fe, Cr, Ni, Mn, F, Cd, B, Cu, Zn | 1.000 | 0.00000 |
| Model No. | Influential Heavy Metals For CD | R2 | SE |
| 1 | Pb | 0.861 | 0.88209 |
| 2 | Pb, Fe | 0.929 | 0.63571 |
| 3 | Pb, Fe, Cr | 0.968 | 0.43119 |
| 4 | Pb, Fe, Cr, Ni | 0.989 | 0.25051 |
| 5 | Pb, Fe, Cr, Ni, Mn | 0.998 | 0.11135 |
| 6 | Pb, Fe, Cr, Ni, Mn, F | 0.999 | 0.08108 |
| 7 | Pb, Fe, Cr, Ni, Mn, F, Cd | 1.000 | 0.01419 |
| 8 | Pb, Fe, Cr, Ni, Mn, F, Cd, B | 1.000 | 0.00221 |
| 9 | Pb, Fe, Cr, Ni, Mn, F, Cd, B, Cu | 1.000 | 0.00027 |
| 10 | Pb, Fe, Cr, Ni, Mn, F, Cd, B, Cu, Zn | 1.000 | 0.00000 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gad, M.; Elsayed, S.; Moghanm, F.S.; Almarshadi, M.H.; Alshammari, A.S.; Khedher, K.M.; Eid, E.M.; Hussein, H. Combining Water Quality Indices and Multivariate Modeling to Assess Surface Water Quality in the Northern Nile Delta, Egypt. Water 2020, 12, 2142. https://doi.org/10.3390/w12082142
Gad M, Elsayed S, Moghanm FS, Almarshadi MH, Alshammari AS, Khedher KM, Eid EM, Hussein H. Combining Water Quality Indices and Multivariate Modeling to Assess Surface Water Quality in the Northern Nile Delta, Egypt. Water. 2020; 12(8):2142. https://doi.org/10.3390/w12082142
Chicago/Turabian StyleGad, Mohamed, Salah Elsayed, Farahat S. Moghanm, Mohammed H. Almarshadi, Abdullah S. Alshammari, Khaled M. Khedher, Ebrahem M. Eid, and Hend Hussein. 2020. "Combining Water Quality Indices and Multivariate Modeling to Assess Surface Water Quality in the Northern Nile Delta, Egypt" Water 12, no. 8: 2142. https://doi.org/10.3390/w12082142
APA StyleGad, M., Elsayed, S., Moghanm, F. S., Almarshadi, M. H., Alshammari, A. S., Khedher, K. M., Eid, E. M., & Hussein, H. (2020). Combining Water Quality Indices and Multivariate Modeling to Assess Surface Water Quality in the Northern Nile Delta, Egypt. Water, 12(8), 2142. https://doi.org/10.3390/w12082142










