Abstract
Phosphorus is one of the non-renewable natural resources. High concentration of phosphorus in surface water leads to undesirable eutrophication of the water ecosystem. It is therefore necessary to develop new technologies not only for capturing phosphorus from wastewater but also for phosphorus recovery. The aim of the study was to propose three different integration scenarios for a microalgal biofilm system for phosphorus removal in medium and small wastewater treatment plants, including a comparison of area requirements, a crucial factor in practical application of microalgal biofilm systems. The area requirements of a microalgal biofilm system range from 2.3 to 3.2 m2 per person equivalent. The total phosphorus uptake seems to be feasible for construction and integration of microalgal biofilm systems into small wastewater treatment plants. Application of a microalgal biofilm for phosphorus recovery can be considered one of the more promising technologies related to capturing CO2 and releasing of O2 into the atmosphere.
1. Introduction
Global cycles, being the largest movements of biogeochemical substances on Earth, are important for long-term functioning of the planet’s biosphere. In comparison with cycles of other major biogeochemical elements, “e.g.,” carbon, water or nitrogen, the global cycle of phosphorus (P), an essential nutrient, is quite unique. The P cycle is without any significant gaseous component except for very limited production (pg dm−2 h−1) of phosphine gas (PH3) under highly anaerobic soil conditions [1,2]. Due to its high reactivity, P is never found as a free non-metal element. It is distributed mainly in the form of phosphates, which are included in geological deposits of phosphate rock or phosphorite [3] and in deposits of biological origin such as guano, accumulated seabird excrement [4]. The original P cycle was broken and opened due to modern human activities associated with industrialization. The main flux of the global open P cycle is the transport of P by rivers to the deep sea. The source of the transported P is the P from land, lost as a result of erosion, pollution, and fertilizer runoff [5,6]. Long-term release of P from land starts the process of eutrophication of surface waters [7]. In the long-term, this loss of P from land is unsustainable. Within decades, mining will exhaust naturally occurring phosphate rock deposits of the most accessible high quality P [3,8]. The quality of other P rocks is lower due to higher concentrations of impurities and heavy metals [8]. It is therefore necessary to initiate activities that would gradually close the open global P cycle. Hence, efficient recovery of P contained in wastewater is a necessity [9].
Both problems (surface water eutrophication and sustainability of the P cycle) can be solved by the development of new biologically based technologies for capturing P from eutrophic waters. Biological methods of P removal are considered sustainable and environmentally favorable since they do not require any chemical precipitants [10]. In biological terms, P removal can be carried out by a variety of organisms; polyphosphate accumulating microorganisms, for instance, are able to store phosphates as intracellular polyphosphate in the specific conditions of wastewater treatment [11]. Moreover, biologically based technologies have a high potential for P recovery from the produced biomass [10,11]. The ability of microalgae and cyanobacteria to reduce P and nitrogen (N) concentration in wastewater has been convincingly demonstrated in numerous studies [12,13]. Liu et al. (2017) [14] reviewed a series of studies describing the efficiency of microalgae in nutrient removal. Different algae-based systems for wastewater bioremediation have been developed. Microalgae can be cultivated in liquid suspensions and they can also grow on solid surfaces [15]. However, harvesting of microalgal cells from liquid suspensions is still considered one of the main obstacles to wastewater treatment employing microalgae [16]. Harvesting of small microalgal cells (5–20 μm) accounts for 20–30% of the production costs [17]. Usage of the attached algae-based systems offers several advantages compared to cultivation in liquid suspension [18]. As the algal biomass is concentrated in the cultivation area, the harvest of the microalgal biofilm may be carried out easily by scraping off the attached algae [19].
Microalgal biofilm technologies are considered a promising approach to nutrient removal and P recovery, using the produced biomass as a source of P, captured in the biomass by physiological processes [18,20,21]. A phototrophic microalgal biofilm usually consists of several microalgal and cyanobacterial species and bacteria forming a multilayered, diverse community of photoautotrophs and heterotrophs [22,23]. Algal biofilms can be cultivated on different surfaces such as polystyrene foam, PVC sheeting, nylon membranes, and concrete [24,25,26,27]. Microalgal biofilm-based systems can be designed both in a vertical and a horizontal setup. A typical example of the vertical systems is a “Twin-Layer” photobioreactor, which uses filter paper attached to a glass plate as a support for microalgal growth [26]. In a different type of a “Twin-Layer” photobioreactor, nylon membrane was proposed as the cultivation area. Shi et al. (2014) [28] reported P reduction of about 79% in municipal wastewater after a two-day cycle employing a nylon membrane “Twin-Layer” photobioreactor. The horizontal systems have mostly been studied in a laboratory setting [29,30]. Guzzon et al. (2008) [31] demonstrated that a microalgal biofilm can efficiently remove P from wastewater. Boelee et al. (2011) [25] investigated the feasibility of microalgal biofilms as a post-treatment stage for the effluent of municipal wastewater and their study reported that a microalgal biofilm grown in laboratory conditions decreased P concentration in wastewater to the target value of 0.15 mg L−1.
Studies researching microalgal biofilms have been reviewed by Mantzorou and Ververidis (2019) [30]. Their survey concludes that most of the studies were carried out on a laboratory scale and only a few studies investigated nutrient removal using a microalgal biofilm on a pilot scale. Boelee et al. (2014) [32] tested the practicability of an outdoor pilot-scale biofilm reactor for nutrient removal from municipal wastewater; the obtained results, however, indicated low efficacy, the average P removal efficiency being only 14%. In our previous studies, we presented a pilot-scale Flat Panel bioreactor (4–8 m2) placed in a greenhouse and designed for biofilm growth and P removal [27,33]. We observed an approximately 98% reduction of total P (TP) in wastewater after 24 hours. Our previous studies described promising results suggesting a considerable potential of microalgal-based biotechnology for P removal. Nonetheless, the application of microalgal biofilms in tertiary wastewater treatment remains a rarity. Future advancement of microalgal biofilm-based technologies requires that a scenario be developed for P removal with prospects for recovery in conventional wastewater treatment technologies. At present, there is a notable lack of large-scale practical applications of microalgal biofilm systems. The study presented here therefore focuses on designing scenarios for integrating microalgal biofilm-based technologies into existing wastewater treatment technologies. The design of a specific integration approach to microalgal biofilm technology is closely linked to the question of the biofilm area that is required for sufficient P uptake. The area requirement is generally considered to be one of the main obstacles to introduction of algal technologies into wastewater treatment. This challenge does not exclusively concern the applicability of microalgal biofilms but also the installation of high-rate algal ponds [16,34,35]. It is therefore essential to propose diversified microalgal-biofilm integration scenarios, each paying appropriate attention to area requirements. To the authors’ best knowledge, only one study has tried to theoretically evaluate microalgal biofilm area requirements within the context of biofilm placement in wastewater treatment plants [34]. Boelee et al. [34] reported that the area requirements range from 0.32 to 2.1 m2 per person equivalent (PE) for three hypothetical scenarios of microalgal biofilm integration, to remove P residues after conventional treatment, the tertiary wastewater treatment stage, and a separate wastewater treatment system based on a biofilm. Their values of area requirement, however, were based on theoretical calculations whereas experimental results were not taken into account. Our study presents area requirements based on experimental data. The gist of our study is to propose theoretical scenarios for microalgal biofilm integration into a wastewater treatment plant and to assess the feasibility of each scenario in terms of its area requirement. The microalgal biofilm is proposed as: (1) a post-treatment system for the removal of P residues after conventional wastewater treatment involving a tertiary stage; (2) a tertiary treatment stage for P removal in conventional wastewater treatment (3500 PE); (3) a tertiary treatment stage connected to a small-scale wastewater treatment plant (50 PE). Moreover, our study includes two alternatives for the cultivation area architecture, a horizontal and a vertical setup. To the authors’ best knowledge, no study has yet addressed this issue.
To sum up, our study has three aims: (1) to propose three designs for the integration of a microalgal biofilm system into medium (3500 PE) and small (50 PE) wastewater treatment plants including a comparison of the area requirement for each design; (2) to compare the area requirement for a vertically-oriented cultivation setup based on geotextile (geotextile-based biofilm system for P removal) and a horizontally-oriented cultivation setup based on concrete (concrete-based biofilm system for P removal); and (3) to perform laboratory tests of the microalgal biofilm growth on a geotextile surface to demonstrate the feasibility of the proposed geotextile-based design.
2. Materials and Methods
2.1. Laboratory-Scale Microalgal Biofilm Cultivation on a Geotextile Surface
The microalgal biofilm was cultivated on a geotextile surface on a laboratory scale in order to demonstrate capability of microalgal biofilm growth on non-woven geotextile fabric made from recycled polyester (specific weight 200 g m−2). A microalgal biofilm assemblage was sampled from a concrete-surface cultivation area. The biofilm samples (50 ml in volume) were evenly spread on a geotextile surface that served as a support for biofilm growth. The geotextile patches (3 × 3 cm) with the microalgal biofilm (Figure 1) were placed in three Erlenmeyer flasks containing simulated wastewater. The simulated wastewater medium was prepared according to Sukačová et al. (2017) [27]. The microalgal biofilm on the geotextile patches was cultivated under 70 μmol photons m−2 s−1 and 25 °C for one week. The experiments were performed in three repetitions under identical cultivation conditions. The gradual microalgal growth of the biofilm was evaluated using non-invasive chlorophyll fluorescence measurements. The Fv/Fm (maximum quantum efficiency) ratio was measured each day using an Open FluorCam FC 800-0/2020 (Photon Systems Instruments, Brno, Czech Republic) and analyzed using the FluorCam7 software (Photon Systems Instruments, Brno, Czech Republic). The microalgal growth was expressed as increasing values of the Fv/Fm parameter. The microalgal and cyanobacterial species were identified according to Komárek and Anagnostidis (2005) [36] and Ettl and Gärtner (1995) [37] using an Olympus CX 31 microscope.
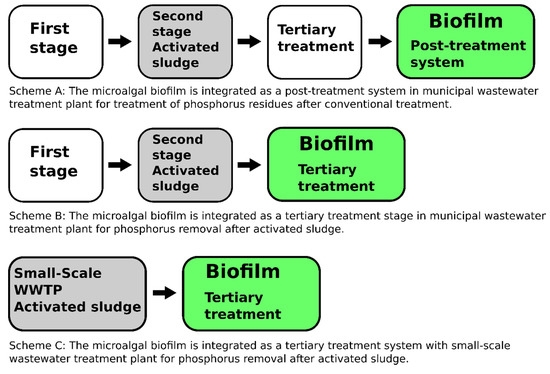
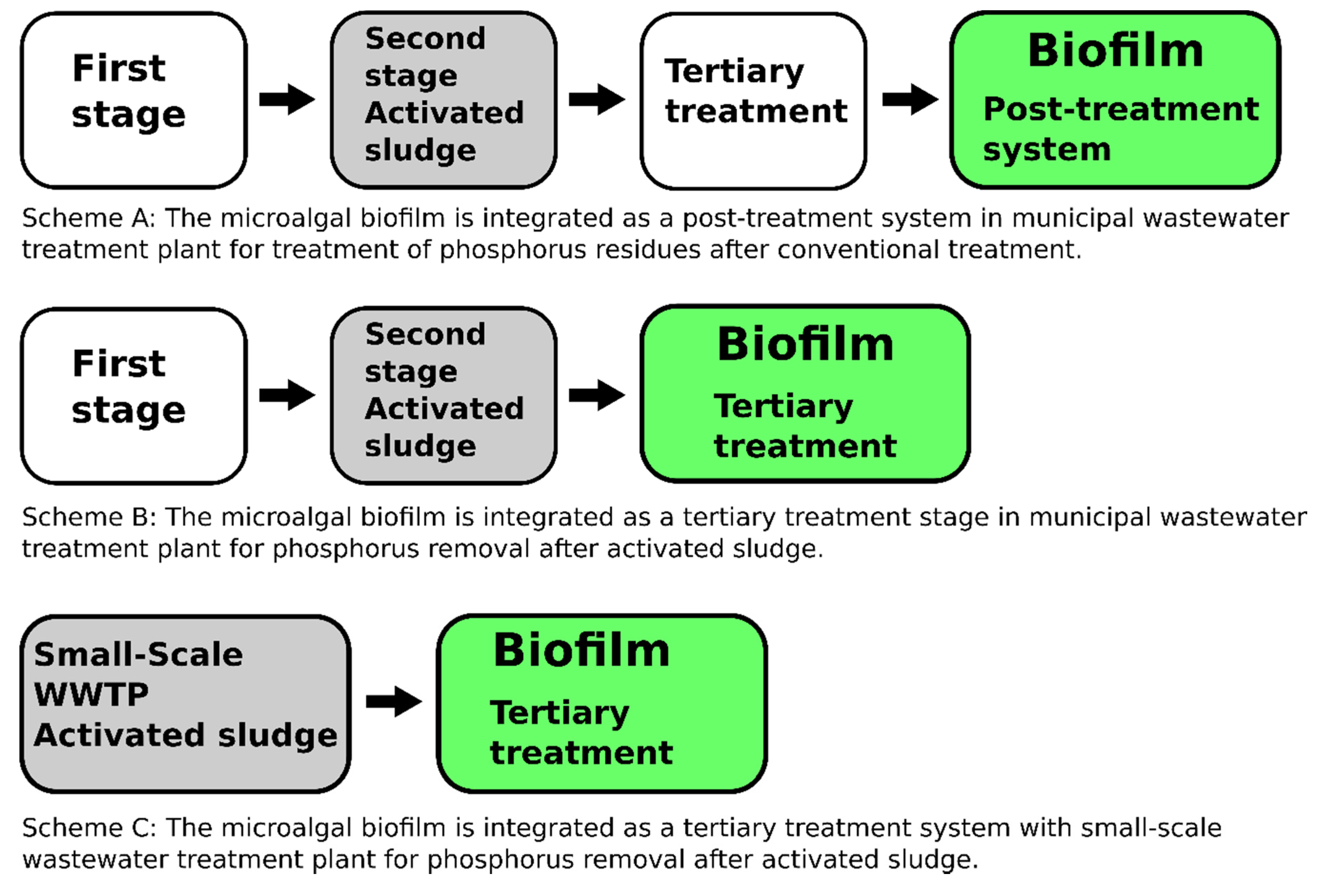
Figure 1.
Designs for microalgal biofilm integration into conventional wastewater treatment systems. Scheme A: The microalgal biofilm is integrated as a post-treatment system in a municipal wastewater treatment plant for the treatment of phosphorus residues after a conventional treatment. Scheme B: The microalgal biofilm is integrated as a tertiary treatment stage in a municipal wastewater treatment plant for phosphorus removal after the activated sludge process. Scheme C: The microalgal biofilm is integrated as a tertiary treatment system in a small-scale wastewater treatment plant for phosphorus removal after the activated sludge process.
2.2. Calculation of the Area Required for the Biofilm
The proposals have been produced according to the fact that the theoretical scenario envisages three schemes of microalgal biofilm integration into a conventional wastewater treatment plant (WWTP) (Figure 1). The proposals are predicated on calculating the required area of biofilm for each proposed design. The required area is defined as the area of cultivation surface for biofilm growth that is necessary for sufficient P removal. The footprint area is based on the particular geometry of the microalgal biofilm module, i.e. its vertical or horizontal architecture.
The TP uptake rate values used for calculations of the required area had been determined for a prototype concrete-based biofilm system in our previous studies [27,33]. We assume an identical TP uptake rate for both systems (the concrete-based and the geotextile-based one). Our assumption is based on a comparable growth of microalgal biofilm on the concrete surface and on the geo-textile surface observed during preliminary experiments (data not shown). Table 1 shows parameters used for area requirement calculations. The studies obtained TP uptake rate for input TP concentrations of 1 mg L−1 for scheme A and 3 mg L−1 for scheme B and C. The selected TP concentrations are considered representative for wastewater effluent from a conventional wastewater treatment plant without a tertiary treatment stage (schemes B and C) and from a conventional wastewater treatment plant involving tertiary treatment (scheme A) [38,39]. The wastewater volume used for the calculations is based on operating conditions for wastewater treatment plants (WWTP) situated in the Czech Republic (Central Europe, temperate zone). The required area (RA) was calculated as a multiplication of the daily wastewater volume (DWW volume) and the difference between the inflow TP concentration (ITP) and the outflow TP concentration (OTP), divided by the P uptake rate of the biofilm (TP uptake). The outflow TP concentration of 0.09 mg L−1 was assumed as the representative outflow TP concentration for each scheme. The footprint area is identical to the required area for a horizontal concrete-based biofilm system. The footprint area of a geotextile-based biofilm system is calculated for a horizontal arrangement of modules (Table 2) where the total cultivation area of all the modules corresponds to the required area.

Table 1.
Summary of parameters used for calculating area requirements: the initial total P concentrations (Inflow TP) in the influent to the microalgal biofilm system, total P concentration (Outflow TP) in the effluent subsequent to treatment using a microalgal biofilm, daily wastewater volume (DWW volume) per person equivalent (PE) and TP uptake rates by the biofilm obtained for the quoted inflow TP concentrations (schemes A, B, and C; Figure 2).

Table 2.
Design parameters of microalgal biofilm modules for a large-scale WWT P removal system.
2.3. Estimated P Recovery Potential
The calculation of daily biomass production was based on the average value for the biomass DW production rate of 23 g m−2 D−1 established in our previous study [27]. Similarly, the calculation of daily P recovery potential was based on the biomass P content of 12 mg g−1 derived from Sukačová et al. [27]. Daily and annual biomass production and daily and annual P recovery potential were estimated for the required area for each of the proposed scenarios.
3. Results
3.1. Laboratory-Scale Microalgal Biofilm Cultivation on the Geotextile Surface
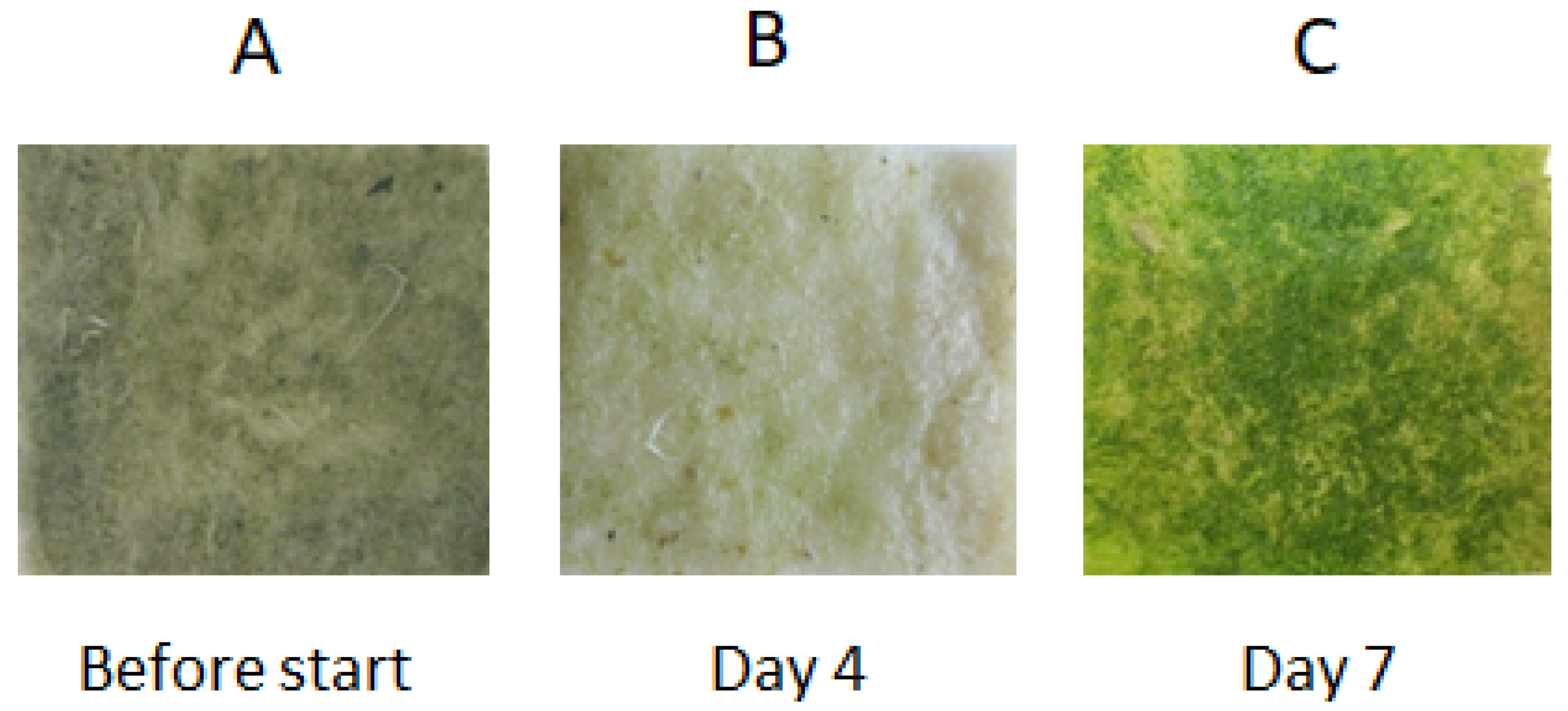
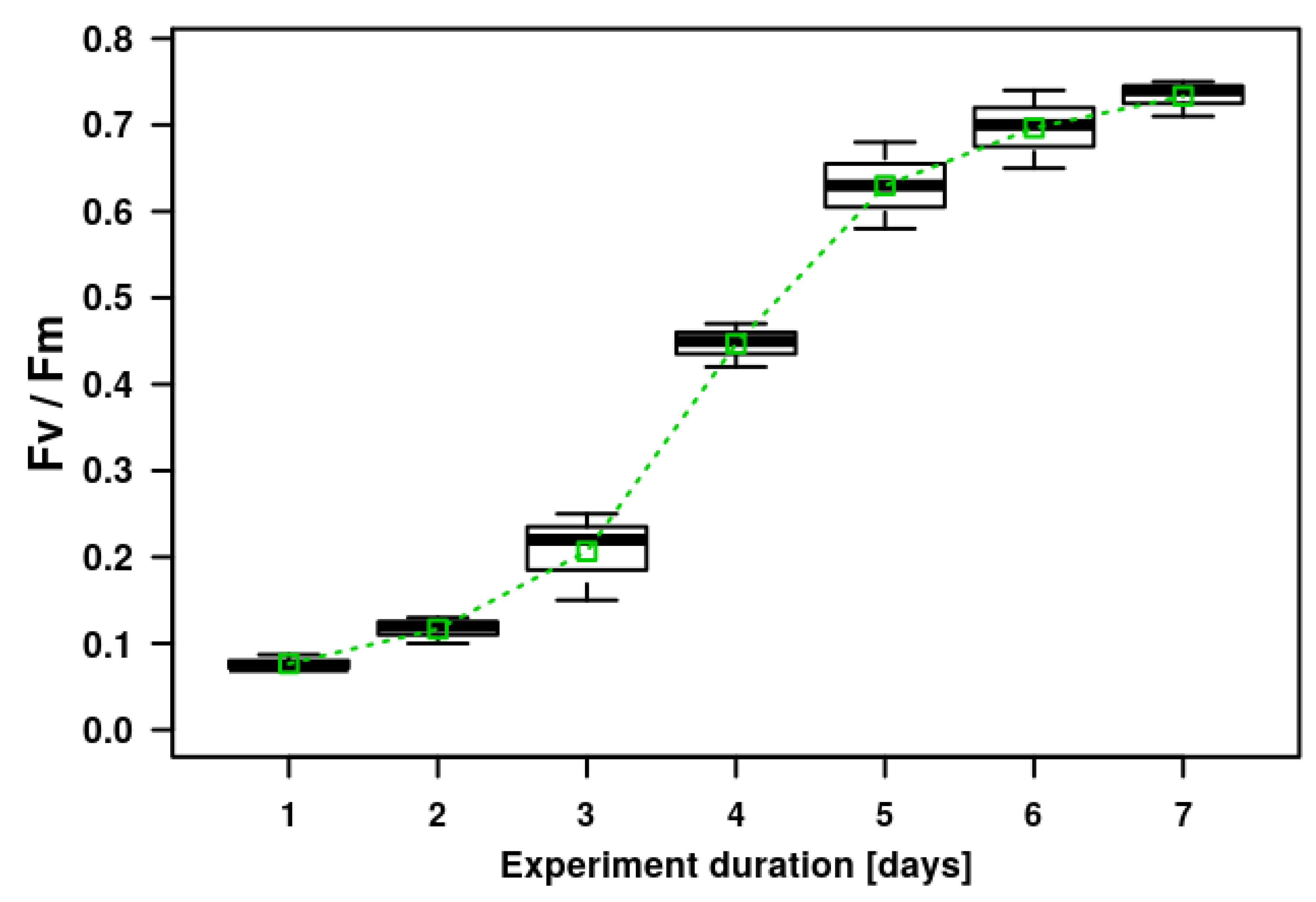
The microalgal biofilm was experimentally cultivated on the geotextile surface over the course of the laboratory experiment in order to demonstrate the possibility of using a geotextile surface for microalgal cultivation (Figure 2). The experimental microalgal biofilm developed on the geotextile patches consisted mostly of the filamentous cyanobacteria Phormidium autumnale and Pseudanabaena sp., and also the coccal green alga Scenedesmus acutus. The composition of the algal assemblages on the geotextile surfaces coincided with dominant species growing on the concrete-based surfaces. The Fv/Fm parameter doubled between day 3 and day 6 of the experiments. The increasing Fv/Fm values indicated a fast growth of the biofilm assemblage on the geotextile surface (Figure 3). The highest variability of Fv/Fm parameter was determined on the third, fifth and sixth day of the experiment duration. These days were at the beginning and end of the fastest biomass growth (Figure 3).
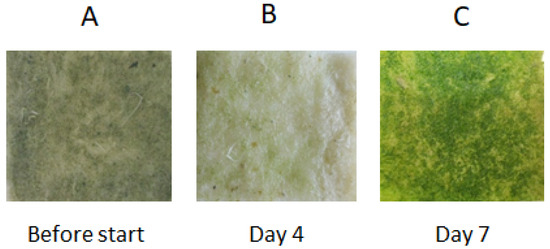
Figure 2.
Growth of the microalgal biofilm assemblage on the geotextile cultivation surface before and during the experiment (after 4 and 7 days). The size of the geotextile patches was 3 × 3 cm. (A) Geotextile cultivation surface before the experiment. (B) Geotextile cultivation surface after 4 days of the experiment. (C) Geotextile cultivation surface after 7 days of the experiment.
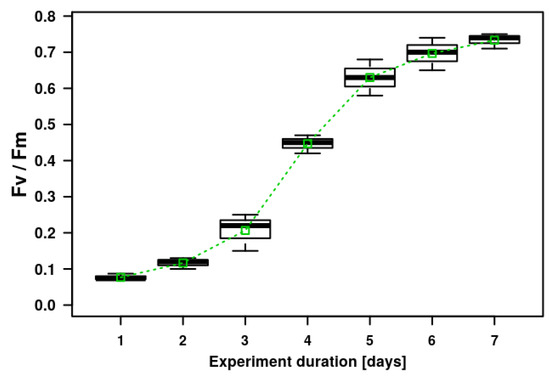
Figure 3.
Microalgal biofilm growth on geotextile patches placed in artificially prepared wastewater during laboratory cultivation in Erlenmeyer flasks. The growth is expressed as the chlorophyll fluorescence parameter Fv/Fm. The graph shows the means (small open green boxes), median values of the second quartile (thick black lines), upper and lower quartile (boxes with 50% of measured data) and error bars with the upper and lower extreme values indicated.
3.2. Design of a Large-Scale Biofilm System for P Removal
The theoretical scenario of the microalgal biofilm system is based on a series of independent microalgal biofilm modules supplied with wastewater (Figure 4). The microalgal biofilm system (individual modules) can be implemented in a wastewater treatment plant (WWTP) in different ways, maintaining an efficient operation of the whole wastewater treatment (WWT) process.
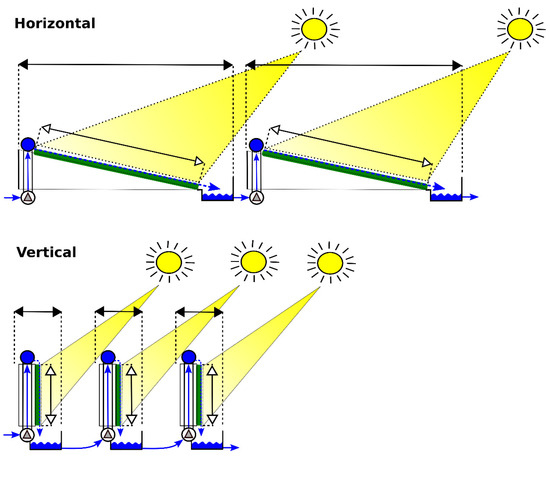
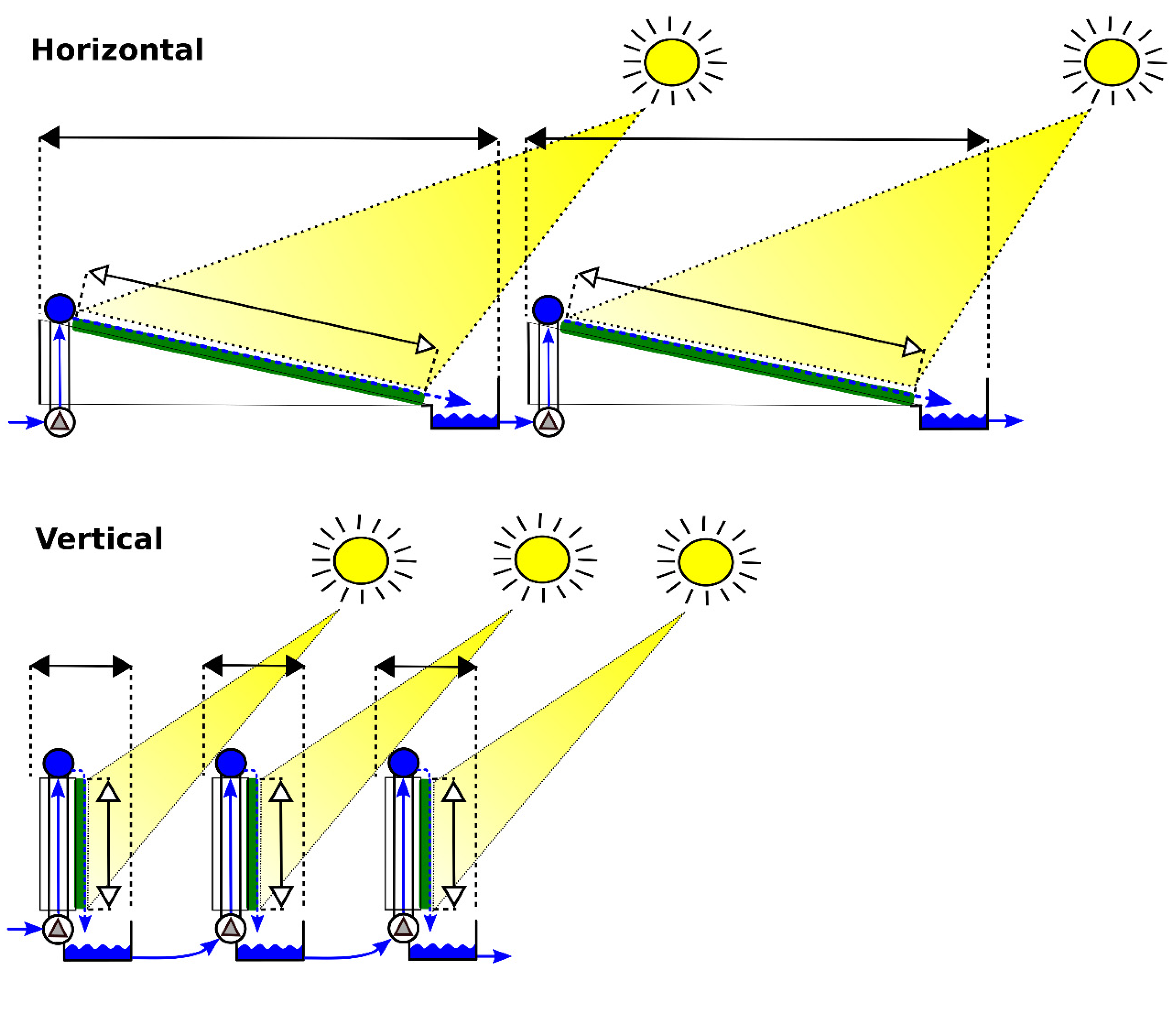
Figure 4.
Design of microalgal biofilm modules supplied with wastewater depicting a concrete-based biofilm system for P removal (Horizontal) with a rectangular concrete cultivation area, and a geotextile-based biofilm system for P removal (Vertical) with a geotextile cultivation area mounted on a rectangular frame.
3.2.1. Geotextile-Based Biofilm System for P Removal
The proposed design for a microalgal biofilm module is based on a geotextile cultivation area mounted on a rectangular frame (Figure 4—Vertical). The projected dimensions of the module are 1 m in height and 10 m in length, the module being oriented in a vertical position (Table 2). The wastewater is to be circulated from the reservoir through the cultivation area. The prospected distance between the modules is 0.8 m. Such distance has been set according a study by Zhang et al. (2018) [13], who experimentally determined the optimal distance of 8 cm between vertical microalgal biofilm modules of 11 cm in height, i.e. in a 5/4 height/distance ratio.
3.2.2. Concrete-Based Biofilm System for P Removal
The theoretical scenario for the microalgal biofilm module proposes a rectangular cultivation area with the dimensions of 2 × 10 m (Table 2). The assumed minimum distance between the modules is 0.2 m. The module of a concrete-based biofilm system consists of a slightly inclined concrete cultivation area with the wastewater circulating through the cultivation area from a reservoir (Figure 4—Horizontal). This theoretical scenario is derived from a functional prototype microalgal biofilm system with an 8 m2 concrete cultivation area presented in our previous studies [27,33].
3.3. Evaluation of the Required Area
The required area determined for schemes A and B was 10,500 m2 and 11,200 m2, respectively. Scheme C projects integration of the microalgal biofilm into a small-scale WWTP, and its required area was only 145 m2. The required area is identical with the footprint identified for a concrete-based biofilm system.
3.4. Comparison of the Vertical and Horizontal Module Geometry of the Biofilm Stage in WWT Systems
The area of the microalgal biofilm is a decisive parameter for an optimally functioning biofilm WWT system (Table 3 and Table 4). The footprint area determined for a geotextile-based biofilm system ranges between 2.3 and 2.6 m2 per person equivalent (PE), as shown in Table 3. The areas for the proposed biofilm WWT system based on concrete slabs are shown in Table 4. The footprint area (area requirement) ranges from 2.9 to 3.2 m2 per PE in all of the proposed schemes. The footprint area is over 20% higher for the system based on a concrete cultivation area.

Table 3.
Calculated design parameters of a biofilm system for P removal based on a geotextile cultivation area. The design of the system assumes several modules with a vertical geotextile arrangement (90° inclination angle). PE = person equivalent, FP Area = footprint area.

Table 4.
Calculated design parameters of a biofilm system for P removal based on concrete slabs. PE = person equivalent. FP Area = footprint area.
3.5. The Footprint Area Within the Context of the Proposed Integration Schemes
The comparison of the biofilm integration scenarios shows that the highest footprint area of 11,200 m2 was determined for the concrete-based system within the integration scheme B. Similarly, the highest footprint area of 9100 m2 was calculated for the integration scheme B in the case of the geotextile-based system. The smallest area was established for the implementation scheme C. The calculated area for integration of the biofilm into a small-scale WWTP (scheme C) ranged from 115 m2 for the geotextile to 145 m2 for the concrete cultivation area.
3.6. Estimated P Recovery Potential
Table 5 shows estimations of P recovery potential determined for the required areas based on DW biomass production. The daily P recovery potential was estimated at 2.9 and 3.1 kg for schemes A respectively B and 0.04 kg for scheme C. The estimated annual P recovery potential was 1059 and 1132 kg P for schemes A respectively B and 14.6 kg P for scheme C.

Table 5.
Estimated P recovery potential of a microalgal biofilm system for the three proposed designs. The values were calculated based on the required areas. Daily DW—biomass production per day expressed as dry weight biomass for the required area; Daily P recovery—the amount of P captured in the microalgal biomass per day; Estimated annual DW—biomass production per year expressed as dry weight biomass for the required area; Estimated P recovery—the amount of P captured in the microalgal biomass per year.
4. Discussion
Our study deals with novel concepts in application of microalgal biofilms for P removal to demonstrate the possibility of bridging the gap between basic research and practical microalgal biofilm applications. The recycled polyester geotextile fabric used in the experiments has been shown as a suitable supporting and surface material for microalgal biofilm growth, being, moreover, a wholly adequate alternative to biofilm growth on a concrete surface. Similarly, research on microalgal assemblages indicates that a biofilm can grow on a variety of supporting materials [30]. Shi et al. (2014) [28] studied successful growth of the coccal microalga Halochlorella rubescens on a nylon membrane, serving as a supporting material. Three types of textiles, i.e. cotton, polyester, and nylon, were successfully tested by De Assis et al. (2019) [40]. Johnson and Wen (2010) [41] discuss polystyrene foam as very suitable material for cultivation of a microalgal biofilm supplied with wastewater. The cited studies clearly indicate the versatility of microalgal biofilm assemblages with regards to growth on a variety of diverse surface types. Our findings confirm this general observation.
Algae-based technologies are considered highly promising for application in WWT, especially due to their potential for nutrient removal [42,43,44] and recycling [45]. There are several ways of implementing microalgal biofilm systems in WWT systems [14,25,46]. Our project proposes three options for integrating microalgal biofilm systems based on a concrete and a geotextile cultivation area in WWT systems (Figure 2). One of the current integration schemes recommends using microalgae for nutrient removal after the conventional stage with activated sludge. Similarly, Zamaloa et al. (2013) [47] carried out laboratory-scale tests of a two-stage sewage treatment process combining a conventional stirring tank reactor with a microalgal biofilm for nutrient removal. Such a system managed to remove approximately 67% of the total N and 96% of the TP from the wastewater. Our previous work demonstrated P removal efficiency averaging 98% [27]. Silveira et al. (2017) [48] presented an integrated system of microalgal cultivation in a suspension culture connected to vertical flow constructed wetlands for treatment of raw municipal wastewater. However, their system was rather inefficient with respect to P removal.
In terms of area requirement, the microalgal biofilm WWT system based on the geotextile cultivation area seems more advantageous. This observation is in line with the results reported by Gross et al. (2013) [49], who demonstrated the operation of rotating algal biofilm growth system for attached microalgae growth. Gross et al. (2013) [49] demonstrated the possibility of planting the total surface area of 3.5 m2 for biofilm growth on the footprint area of 1 m2. The footprint area for the vertical geotextile-based microalgal biofilm system depends on the distance between the modules covered with the geotextile material. The distance is an important factor with respect to the availability of light on the surface of the algal cultivation area (Figure 4). Zhang et al. (2018) [13] studied the distances between vertically arranged modules for microalgal biofilm growth in terms of their impact on biomass production. The study established the optimum distance of 8 cm between vertically oriented modules that were 11 cm high, i.e. the optimum height/distance ratio was 5/4. Following Zhang’s conclusions, we propose a design using one-meter tall vertical geotextile-based modules, evenly spaced at the distance of 0.8 m. A lower height/distance ratio was previously used for flat panel photobioreactors of the “Green Wall Panel” type (panel height 0.7 m). The distance between parallel rows of “Green Wall Panel” photobioreactors was 1 m [50].
The area requirements presented here are higher than those reported by Boelee et al. (2012) [34] in his theoretical analysis of nutrient removal using a microalgal biofilm. In this analysis, the presumed area requirement would be 2.1 m2 per PE for a microalgal biofilm system removing N and P. However, our study indicates that the sufficient area requirement would range between 2.6 and 3.2 m2 per PE in the implementation alternative B (Figure 2—scheme B). This difference could be due to our usage of real data measured during the experiments as opposed to the theoretical analysis performed by Boelee et al. (2012) [34].
The feasibility of microalgal biofilms in WWT is closely linked to the required area for removal of P from wastewater. Reduction of the necessary area stemming from a higher efficiency of nutrient removal would result in a more accessible application of microalgal biofilms in WWT technologies. Environmental conditions (“e.g.,” solar irradiance, temperature) and technical design parameters of a microalgal biofilm WWT system are important factors for effective P removal [31,32]. Hydraulic retention time (in hours) and cultivation area (footprint) of a microalgal biofilm system (in m2) are the main design parameters related to the efficacy of treatment processes and operation of a WWT system as a whole [51]. Our previous study described a reduction of nearly 98% P concentration after a period of 8 to 18 hours depending on light conditions, while the hydraulic retention time of the wastewater in the biofilm system was 24 hours [33]. The variance in the amount of time necessary for P reduction and the total hydraulic retention time of the wastewater in the microalgal biofilm system opens possibilities for higher efficiency with respect to the optimization of hydraulic retention time. Similarly, Shilton et al. (2012) [52] emphasize that maximization of P accumulation can potentially result in a reduction of the area requirement for P uptake from high rate ponds, widespread systems for wastewater treatment using microalgae in liquid suspension.
Another possible approach to increasing biofilm system efficiency consists in reduction of the footprint area. One option involves a three-dimensional structure. Gou et al. (2018) [51] suggests that an algal-bacterial biofilm reactor filled with a three-dimensional biofilm carrier made from polyethylene would reduce the footprint by nearly 80% compared to flat biofilm systems [24,33]. However, a biofilm reactor with a three-dimensional carrier turned out to be 50% less efficient in terms of P removal compared to a biofilm system using a flat slab of concrete [27]. Another significant drawback of the three-dimensional system would issue from difficulties in biomass harvesting, a crucial feature in P recovery.
The largest area requirement is related to scheme B in which the microalgal biofilm is featured in connection with the effluent from the activated sludge stage. However, construction of microalgal biofilm WWT systems with an area of around 1 ha (10,000 m2) seems unrealistic. The algal turf scrubber systems (ATS) for nutrient removal occupy the area of approximately 1 ha (10,000 m2) and have been put into practice in several regions of the USA [53,54].
Integration of biofilm systems into small-scale WWTPs seems realistic, especially since the potential adverse effects of P release from small wastewater sources are often underestimated or entirely neglected [55]. The required area, ranging from 115 m2 to 145 m2, calculated for small-scale WWT plants (Figure 2—scheme C) seems to be a viable alternative for construction and integration of biofilm systems into small-scale WWTPs. Intensification and optimization of WWT processes using a microalgal biofilm would be feasible mainly in small WWT facilities, in contrast to the high area requirement for WWTPs with the capacity of approximately 3500 PE.
An important aspect concerning implementation and operation of microalgal biofilm systems in WWTPs is the realistic possibility of P recovery in terms of the P stored in the produced microalgal biofilm biomass. The daily biomass production of 230 kg per ha presented herein is nearly identical to other attached algae-based systems. Pizarro et al. (2006) [56] established the biomass production of 220 kg per ha per day for ATS. Similarly, Boelee et al. (2011) [25] determined the biomass production of 200 kg per ha per day for a microalgal biofilm system on the laboratory scale. The observed P recovery potential of the microalgal biofilm system amounted to one half of the ATS P recovery potential identified by Pizarro et al. (2006) [56]. Using microalgal biomass with captured P as a biofertilizer was demonstrated for algal biomass harvested from ATS [20]. Considering the fact that biofilm biomass and ATS biomass are comparable in terms of P content, we suppose that microalgal biofilm biomass might indeed be put to use as a bio-fertilizer in a manner similar to ATS biomass.
The necessity of developing new methods for P recovery is emphasized in a study which presents a detailed analysis of P flows in European countries [7]. The study points out large P losses in Europe’s environment along with relatively low P recycling. Van Dijk et al. (2016) [7] report that in 2005, i.e. the year they examined, the P emissions into the hydrosphere were nearly 207 Gg of P. The high release of P into the hydrosphere, and wastewater in particular, points towards the potential quantities of P available for recycling. Hence, the use of microalgal biofilms for P recovery can be regarded as a promising technology. Moreover, using phototrophic microalgae for P removal is related to releasing of O2 into the atmosphere and simultaneously to capturing CO2 from the atmosphere.
5. Conclusions
In summary, the present study proposes theoretical scenarios for microalgal biofilm integration into medium (3 500 PE) and small (50 PE) wastewater treatment plants including a comparison of area requirements. The microalgal biofilm is suggested as a post-treatment system for the removal of P residues after conventional wastewater treatment and as the tertiary treatment stage for P removal in conventional wastewater treatment facilities. The determined footprint area for the proposed scenarios ranges between 2.3 to 3.2 m2. In terms of footprint area, the geotextile-based microalgal biofilm WWT system is more advantageous than a concrete-based microalgal biofilm system for P removal. The smallest footprint area was established for integration of the biofilm into a small-scale WWTP (50 PE). Therefore, the integration of biofilm systems into WWT processes seems feasible mainly in small-scale WWTPs. The microalgal biofilm system integrated as tertiary treatment stage displayed a high P recovery potential, amounting to about 1132 kg P per year. The connection of conventional treatment technologies with autotrophic microalgae assemblages in biofilm systems appears to be an effective solution to both eutrophication of surface waters and the impending shortages of P for agricultural use.
Author Contributions
Conceptualization, K.S.; methodology, K.S.; formal analysis, K.S. and J.D.; investigation, K.S. and D.V.; writing—original draft preparation, K.S., J.D. and D.V.; writing—review and editing, K.S. and J.D.; visualization, K.S. and J.D.; supervision, J.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Education, Youth and Sports of the Czech Republic under the OP RDE grant number CZ.02.1.01/0.0/0.0/16_026/0008413 ‘Strategic Partnership for Environmental Technologies and Energy Production’.
Acknowledgments
This work was supported by the Ministry of Education, Youth and Sports of the Czech Republic under the OP RDE grant number CZ.02.1.01/0.0/0.0/16_026/0008413 ‘Strategic Partnership for Environmental Technologies and Energy Production’.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Geng, J.; Niu, X.; Jin, X.; Wang, X.; Gu, X.; Edwards, M.; Glindemann, D. Simultaneous Monitoring of Phosphine and of Phosphorus Species in Taihu Lake Sediments and Phosphine Emission from Lake Sediments. Biogeochemistry 2005, 76, 283–298. [Google Scholar] [CrossRef]
- Roels, J.; Verstraete, W. Biological formation of volatile phosphorus compounds. Bioresour. Technol. 2001, 79, 243–250. [Google Scholar] [CrossRef]
- Desmidt, E.; Ghyselbrecht, K.; Zhang, Y.; Pinoy, L.; Van der Bruggen, B.; Verstraete, W.; Rabaey, K.; Meesschaert, B. Global Phosphorus Scarcity and Full-Scale P-Recovery Techniques: A Review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 336–384. [Google Scholar] [CrossRef]
- Jones, B. Recycled insular phosphates and coated grains: Case study from Little Cayman, British West Indies. Sedimentology 2020, 67/4, 1844–1878. [Google Scholar] [CrossRef]
- Liu, Y.; Villalba, G.; Ayres, R.U.; Schroder, H. Global Phosphorus Flows and Environmental Impacts from a Consumption Perspective. J. Ind. Ecol. 2008, 12, 229–247. [Google Scholar] [CrossRef]
- Schlesinger, W.H. Biogeochemistry: An Analysis of Global Change, 3rd ed.; Elsevier/Academic Press: New York, NY, USA, 2012. [Google Scholar]
- Van Dijk, K.C.; Lesschen, J.P.; Oenema, O. Phosphorus flows and balances of the European Union Member States. Sci. Total Environ. 2016, 542, 1078–1093. [Google Scholar] [CrossRef]
- Heffer, P.; Prud’homme, M. Fertilizer Outlook 2014–2018; International Fertilizer Industry Association (IFA): Paris, France, 2014; p. 9. [Google Scholar]
- Peng, L.; Dai, H.; Wu, Y.; Peng, Y.; Lu, X. A Comprehensive Review of the Available Media and Approaches for Phosphorus Recovery from Wastewater. Water Air Soil Pollut. 2018, 229, 1–28. [Google Scholar] [CrossRef]
- Mantzavinos, D.; Kalogerakis, N. Treatment of olive mill effluents: Part I. Organic matter degradation by chemical and biological processes—An overview. Environ. Int. 2005, 31, 289–295. [Google Scholar] [CrossRef]
- De-Bashan, L.E.; Bashan, Y. Recent advances in removing phosphorus from wastewater and its future use as fertilizer. Water Res. 2004, 38, 4222–4246. [Google Scholar] [CrossRef]
- Rawat, I.; Kumar, R.R.; Mutanda, T.; Bux, F. Dual role of microalgae: Phycoremediation of domestic wastewater and biomass production for sustainable biofuels production. Appl. Energy 2011, 88, 3411–3424. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, Z.; Zhu, L.; Ye, T.; Zuo, J.; Li, X.; Xiao, B.; Jin, S. Vertical-algal-biofilm enhanced raceway pond for cost effective wastewater treatment and value-added products production. Water Res. 2018, 139, 144–157. [Google Scholar]
- Liu, J.; Wu, Y.; Wu, C.; Muylaert, K.; Vyverman, W.; Yu, H.; Muňoz, R.; Rittmann, B. Advanced nutrient removal from surface water by a consortium of attached microalgae and bacteria: A review. Bioresour. Technol. 2017, 241, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Whitton, R.; Ometto, F.; Pidou, M.; Jarvis, P.; Villa, R.; Jefferson, B. Microalgae for municipal wastewater nutrient remediation: Mechanisms, reactors and outlook for tertiary treatment. Environ. Technol. Rev. 2015, 4, 133–148. [Google Scholar] [CrossRef]
- Molinuevo-Salces, B.; Riano, B.; Hernández, D.; Garcia-Gonzáles, M.C. Microalgae and Wastewater Treatment: Advantages and Disadvantages. In Microalgae Biotechnology for Development of Biofuel and Wastewater Treatment; Alam, M.A., Wang, Z., Eds.; Springer: Singapore, 2019; pp. 505–533. [Google Scholar]
- Liu, J.; Vyverman, W. Differences in nutrient uptake capacity of the benthic filamentous algae Cladophora sp., Klebsormidium sp. and Pseudanabaena sp. under varying N/P conditions. Bioresour. Technol. 2015, 179, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Kesaano, M.; Sims, R.C. Algal biofilm based technology for wastewater treatment. Algal Res. 2014, 5, 231–240. [Google Scholar] [CrossRef]
- Gross, M.; Jarboe, D.; Wen, Z. Biofilm-based algal cultivation systems. Appl. Microbiol. Biotechnol. 2015, 99, 5781–5789. [Google Scholar] [CrossRef]
- Mulbry, W.; Westhead, E.K.; Pizarro, C.; Sikora, L. Recycling of manure nutrients: Use of algal biomass from dairy manure treatment as a slow release fertilizer. Bioresour. Technol. 2005, 96, 451–458. [Google Scholar] [CrossRef]
- Mulbry, W.; Kondrad, S.; Pizarro, C. Biofertilizers from Algal Treatment of Dairy and Swine Manure Effluents: Characterization of Algal Biomass as a Slow Release Fertilizer. J. Veg. Sci. 2007, 12, 107–125. [Google Scholar] [CrossRef]
- Callow, M.E.; Algal, B. Biofilms: Recent Advances in Their Study and Control; Evans, L.V., Ed.; Harwood Academic Publishers: Amsterdam, The Netherlands, 2000; pp. 196–218. [Google Scholar]
- Sabater, S.; Guasch, H.; Romaní, A.; Muñoz, I. The effect of biological factors on the efficiency of river biofilms in improving water quality. Hydrobiologia 2002, 469, 149–156. [Google Scholar] [CrossRef]
- Posadas, E.; García-Encina, P.A.; Soltau, A.; Domínguez, A.; Díaz, I. Carbon and nutrient removal from centrates and domestic wastewater using algal-bacterial biofilm bioreactors. Bioresour. Technol. 2013, 139, 50–58. [Google Scholar] [CrossRef]
- Boelee, N.C.; Temmink, H.; Janssen, M.; Buisman, C.J.N.; Wijffels, R.H. Nitrogen and phosphorus removal from municipal wastewater effluent using microalgal biofilms. Water Res. 2011, 45, 5925–5933. [Google Scholar] [PubMed]
- Liu, T.; Wang, J.; Hu, Q.; Cheng, P.; Ji, B.; Liu, J.; Chen, Y.; Zhang, W.; Chen, X.; Chen, L.; et al. Attached cultivation technology of microalgae for efficient biomass feedstock production. Bioresour. Technol. 2013, 127, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Sukačová, K.; Kočí, R.; Žídková, M.; Vítěz, T.; Trtílek, M. Novel insight into the process of nutrients removal using an algal biofilm: The evaluation of mechanism and efficiency. Int. J. Phytoremediation 2017, 19, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Podola, B.; Melkonian, M. Application of a prototype-scale Twin-Layer photobioreactor for effective N and P removal from different process stages of municipal wastewater by immobilized microalgae. Bioresour. Technol. 2014, 154, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Zippel, B.; Rijstenbil, J.; Neu, T.R. A flow-lane incubator for studying freshwater and marine phototrophic biofilms. J. Microbiol. Methods 2007, 70, 336–345. [Google Scholar] [CrossRef]
- Mantzorou, A.; Ververidis, F. Microalgal biofilms: A further step over current microalgal cultivation techniques. Sci. Total Environ. 2019, 651, 3187–3201. [Google Scholar] [CrossRef]
- Guzzon, A.; Bohn, A.; Diociaiuti, M.; Albertano, P. Cultured phototrophic biofilms for phosphorus removal in wastewater treatment. Water Res. 2008, 42, 4357–4367. [Google Scholar] [CrossRef]
- Boelee, N.C.; Janssen, M.; Temmink, H.; Shrestha, R.; Buisman, C.J.N.; Wijffels, R.H. Nutrient removal and biomass production in an outdoor pilot-scale phototrophic biofilm reactor for effluent polishing. Appl. Biochem. Biotechnol. 2014, 172, 405–422. [Google Scholar] [CrossRef]
- Sukačová, K.; Trtílek, M.; Rataj, T. Phosphorus removal using a microalgal biofilm in a new biofilm photobioreactor for tertiary wastewater treatment. Water Res. 2015, 71, 55–63. [Google Scholar]
- Boelee, N.C.; Temmink, H.; Janssen, M.; Buisman, C.J.N.; Wijffels, R.H. Scenario analysis of nutrient removal from municipal wastewater by microalgal biofilms. Water 2012, 4, 460–473. [Google Scholar] [CrossRef]
- Craggs, R.; Sutherland, D.; Campbell, H. Hectare-scale demonstration of high rate algal ponds for enhanced wastewater treatment and biofuel production. J. Appl. Phycol. 2012, 24, 329–337. [Google Scholar] [CrossRef]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota, 2. Teil: Oscillatoriales. In Süsswasserflora von Mitteleuropa; Büdel, B., Gärtner, G., Krienitz, L., Schagerl, M., Eds.; Elsevier: München, Germany, 2005; Volume 18/2, pp. 1–759. [Google Scholar]
- Ettl, H.; Gärtner, G. Syllabus der Boden-, Luft- und Flechtenalgen; Gustav Fischer Verlag: Stuttgart, Germany, 1995; pp. 1–721. [Google Scholar]
- Lewis, M.L.; Wurtsbaugh, W.A.; Paerl, H.W. Rationale for Control of Anthropogenic Nitrogen and Phosphorus to reduce Euthrophication of Inland Waters. Environ. Sci. Technol. 2011, 45, 10300–10305. [Google Scholar] [CrossRef] [PubMed]
- Water Environment Federation. Nutrient Removal. In WEF Manual of Practise No.34; WEF Press: Alexandria, VA, USA, 2010; p. 450. [Google Scholar]
- De Assis, L.R.; Calijuri, M.L.; Assemany, P.P.; Berg, E.C.; Febroni, L.V.; Bartolomeu, T.A. Evaluation of the performance of different materials to support the attached growth of algal biomass. Algal Res. 2019, 39, 1–8. [Google Scholar] [CrossRef]
- Johnson, M.; Wen, Z. Development of an attached microalgal growth system for biofuel production. Appl. Microbiol. Biotechnol. 2010, 85, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, P.; Proulx, D.; Lessard, P.; Vincent, W.F.; Noüe, J. Nitrogen and phosphorus removal by high latitude mat-forming cyanobacteria for potential use in tertiary wastewater treatment. J. Appl. Phycol. 2000, 12, 105–112. [Google Scholar] [CrossRef]
- Doria, E.; Longoni, P.; Scibilia, L.; Iazzi, N.; Cella, R.; Nielsen, E. Isolation and characterization of a Scenedesmus acutus strain to be used for bioremediation of urban wastewater. J. Appl. Phycol. 2012, 24, 375–383. [Google Scholar] [CrossRef]
- Renuka, N.; Sood, A.; Ratha, S.K.; Prasanna, R.; Ahluwalia, A.S. Evaluation of microalgal consortia for treatment of primary treated sewage effluent and biomass production. J. Appl. Phycol. 2013, 25, 1529–1537. [Google Scholar] [CrossRef]
- Pittman, J.K.; Dean, A.P.; Osundeko, O. The potential of sustainable algal biofuel production using wastewater resources. Bioresour. Technol. 2011, 102, 17–25. [Google Scholar] [CrossRef]
- Kangas, P.; Mulbry, W. Nutrient removal from agricultural drainage water using algal turf scrubbers and solar power. Bioresour. Technol. 2014, 152, 484–489. [Google Scholar] [CrossRef]
- Zamalloa, C.; Boon, N.; Verstraete, W. Decentralized two-stage sewage treatment by chemical-biological flocculation combined with microalgae biofilm for nutrient immobilization in a roof installed parallel plate reactor. Bioresour. Technol. 2013, 130, 152–160. [Google Scholar] [CrossRef]
- Silviera, O.E.; Moura, D.; Rieger, A.; Machado, L.E.; Lutterbeck, A.C. Performance of an integrated system combining microalgae and vertical flow constructed wetlands for urban wastewater treatment. Environ. Sci. Pollut. Res. 2017, 24, 20469–20478. [Google Scholar] [CrossRef]
- Gross, M.; Wesley, H.; Clayton, M.; Wen, Z. Development of a rotating algal biofilm growth system for attached microalgae growth with in situ biomass harvest. Bioresour. Technol. 2013, 150, 195–201. [Google Scholar] [CrossRef]
- Tredici, M.R.; Bassi, N.; Prussi, M.; Biondi, N.; Rodolfi, L.; Zittelli, C.G.; Sampietro, G. Energy balance of algal biomass in a closed reactor achieving a high Net Energy Ratio. Appl. Energy 2015, 154, 1103–1111. [Google Scholar] [CrossRef]
- Gou, Y.; Yang, J.; Fang, F.; Guo, J.; Ma, H. Feasibility of using a novel algal-bacterial biofilm reactor for efficient domestic wastewater treatment. Environ. Technol. 2020, 41, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Shilton, A.N.; Powell, N.; Guieysse, B. Plant based phosphorus recovery from wastewater via algae and macrophytes. Curr. Opin. Biotechnol. 2012, 23, 884–889. [Google Scholar] [CrossRef]
- Adey, W.H.; Laughinghouse, H.D.; Miller, J.B. Algal Turf Scrubbers (ATS) Floways on the Gret Wicomico River, Chesapeake Bay: Productivity, Algal community structure, substrate and chemistry. J. Phycol. 2013, 49, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Adey, W.H.; Kangas, P.C.; Mulbry, W. Algal Turf Scrubbing: Cleaning surface waters with solar energy while producing a biofuel. Bioscience 2011, 61, 434–441. [Google Scholar] [CrossRef]
- Bunce, T.J.; Ndam, E.; Ofiteru, D.I.; Moore, A.; Graham, D.W. A Review of Phosphorus Removal Technologies and Their Applicability to Small-Scale Domestic Wastewater Treatment Systems. Front. Environ. Sci. 2018, 6, 1–15. [Google Scholar] [CrossRef]
- Pizarro, C.; Mulbry, W.; Blersch, D.; Kangas, P. An economic assessment of algal turf scrubber technology for treatment of dairy manure effluent. Ecol. Eng. 2006, 26, 321–327. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).