Hydrochemical and Stable Isotope Characteristics of Lake Water and Groundwater in the Beiluhe Basin, Qinghai–Tibet Plateau
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.1.1. Physiography and Meteorology
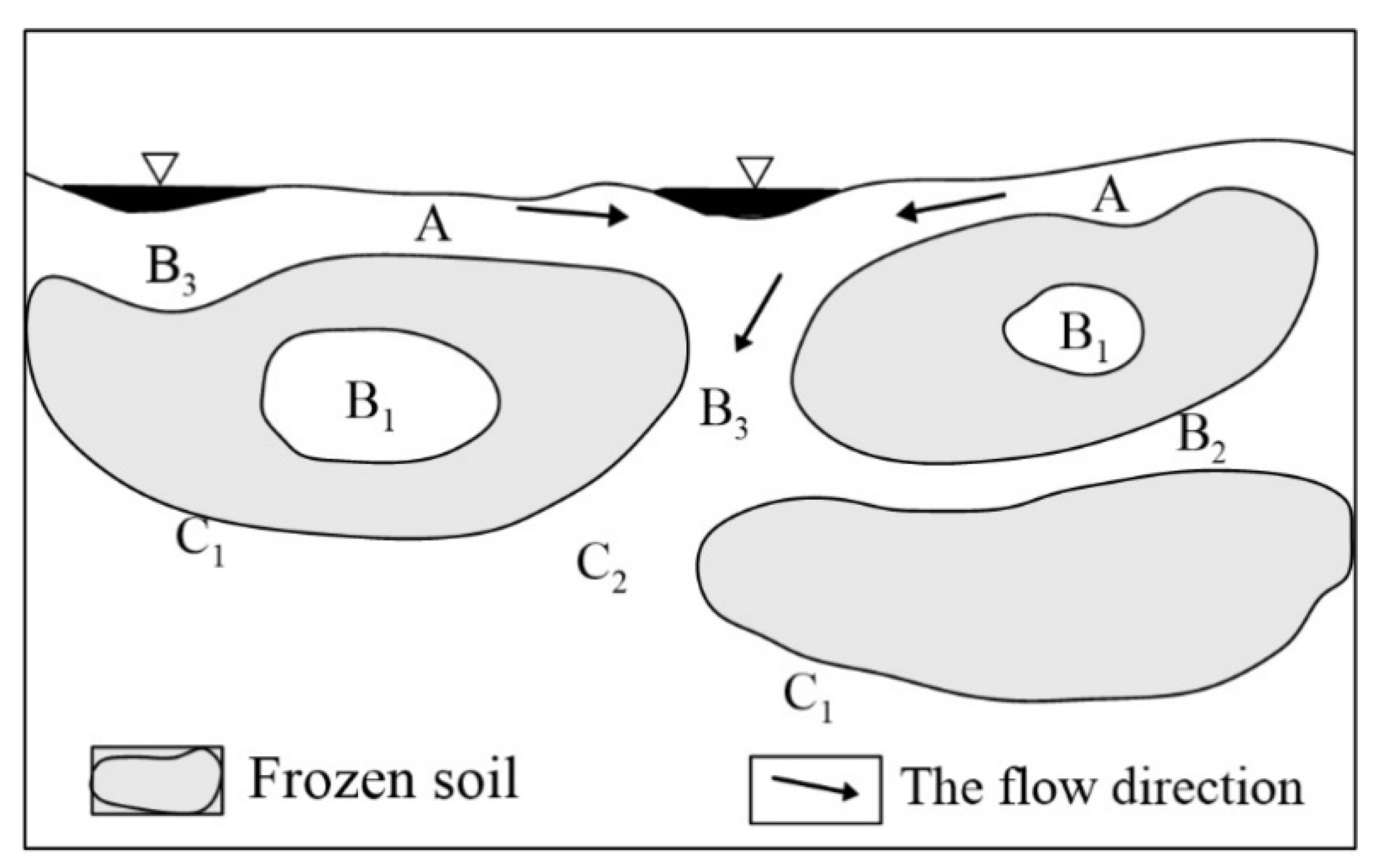
2.1.2. Local Geology and Hydrogeology
2.2. Sampling and Analysis
3. Results and Discussion
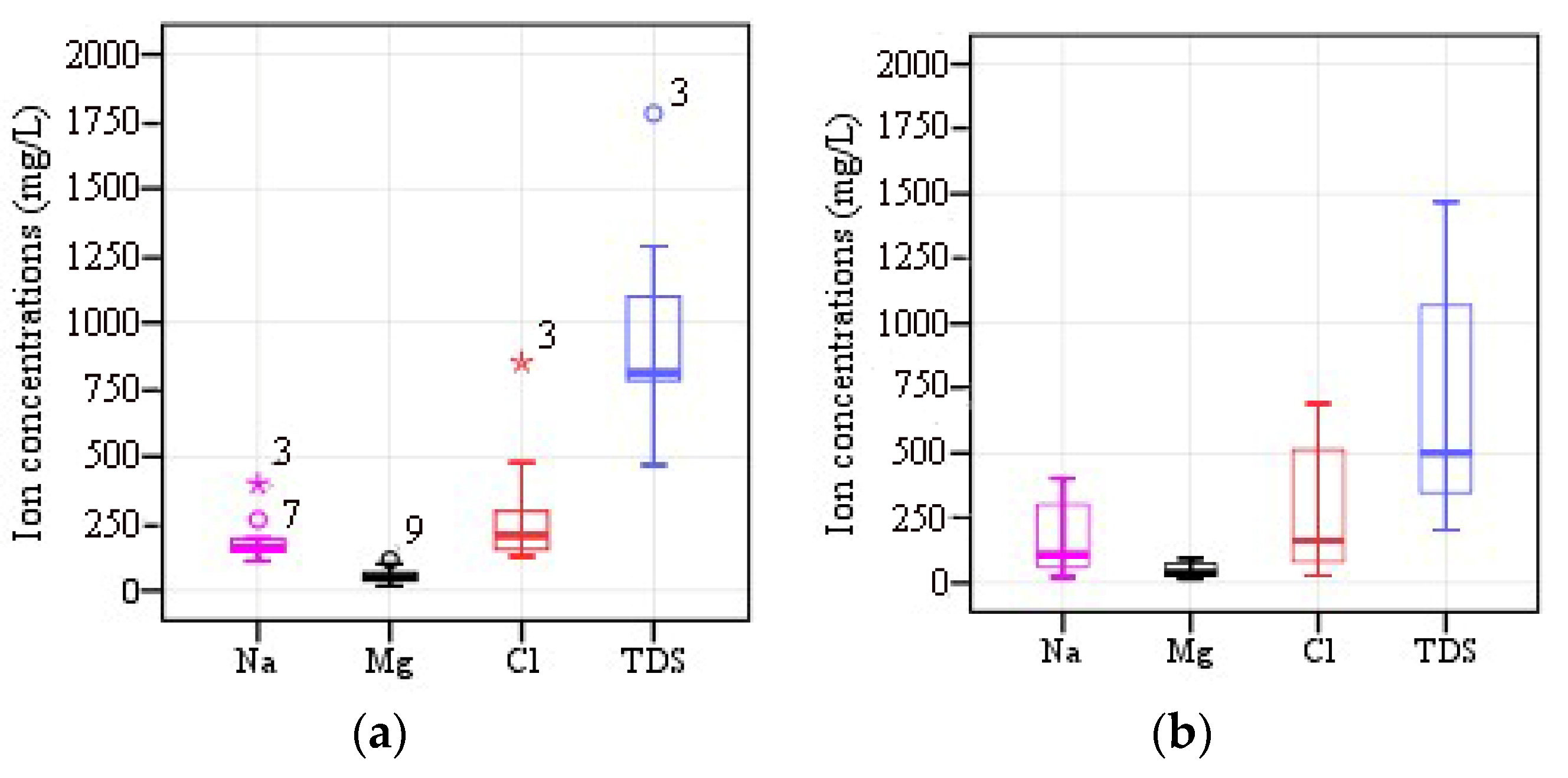
3.1. Major Ion Chemistry
3.2. Water Types
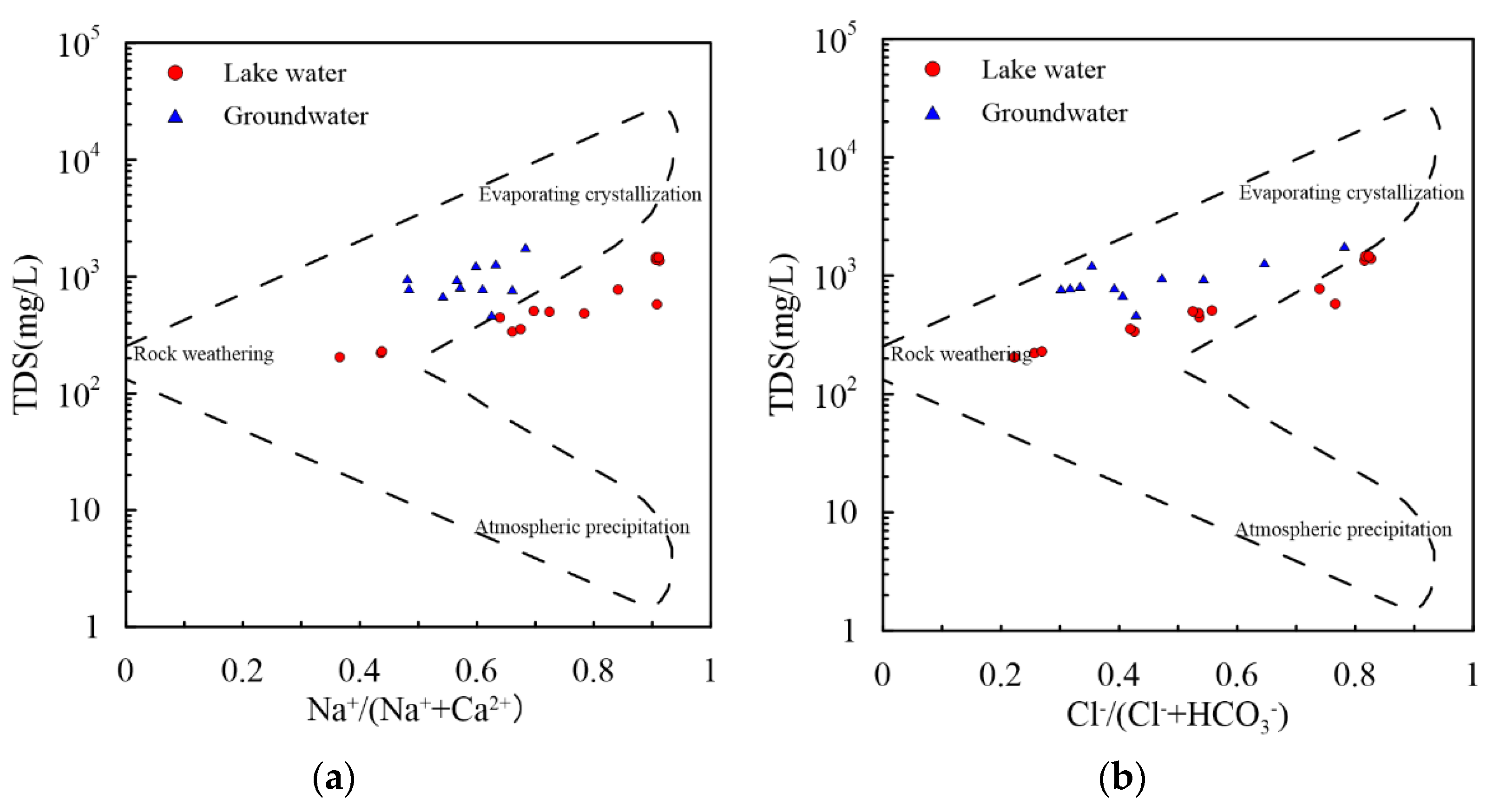
3.3. Processes Influence Water Chemistry
3.3.1. Na/Cl
3.3.2. (Ca + Mg)/HCO3
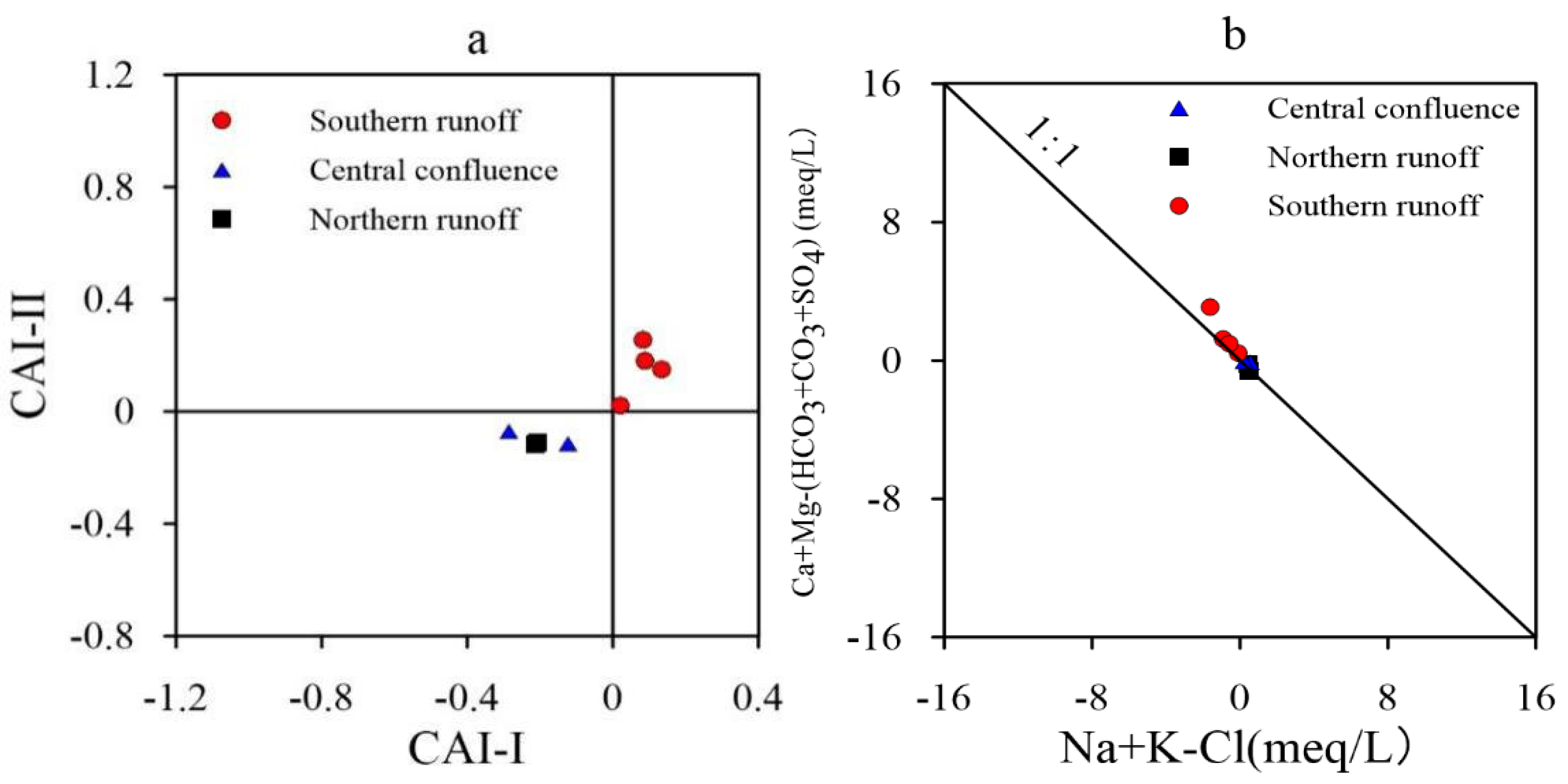
3.3.3. Ca/Mg and Ca/HCO3
3.3.4. HCO3/Cl
3.3.5. Ca/SO4
3.4. Stable Isotope Characteristics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ran, Y.; Li, X.; Cheng, G.-D. Climate warming over the past half century has led to thermal degradation of permafrost on the Qinghai–Tibet Plateau. Cryosphere 2018, 12, 595–608. [Google Scholar] [CrossRef]
- Qin, Y.; Lei, H.; Yang, D.; Gao, B.; Wang, Y.; Cong, Z.; Fan, W. Long-term change in the depth of seasonally frozen ground and its ecohydrological impacts in the Qilian Mountains, northeastern Tibetan Plateau. J. Hydrol. 2016, 542, 204–221. [Google Scholar] [CrossRef]
- Throckmorton, H.; Newman, B.D.; Heikoop, J.M.; Perkins, G.; Feng, X.; Graham, D.E.; O’Malley, D.; Vesselinov, V.V.; Young, J.; Wullschleger, S.D.; et al. Active layer hydrology in an arctic tundra ecosystem: Quantifying water sources and cycling using water stable isotopes. Hydrol. Process. 2016, 30, 4972–4986. [Google Scholar] [CrossRef]
- Niu, F.; Lin, Z.; Liu, H.; Lu, J. Characteristics of thermokarst lakes and their influence on permafrost in Qinghai–Tibet Plateau. Geomorphology 2011, 132, 222–233. [Google Scholar] [CrossRef]
- Polishchuk, Y.M.; Bogdanov, A.N.; Polishchuk, V.Y.; Manasypov, R.; Shirokova, L.S.; Kirpotin, S.N.; Pokrovsky, O. Size Distribution, Surface Coverage, Water, Carbon, and Metal Storage of Thermokarst Lakes in the Permafrost Zone of the Western Siberia Lowland. Water 2017, 9, 228. [Google Scholar] [CrossRef]
- Gao, Z.; Niu, F.; Lin, Z.; Luo, J.; Yin, G.; Wang, Y. Evaluation of thermokarst lake water balance in the Qinghai–Tibet Plateau via isotope tracers. Sci. Total Environ. 2018, 636, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Luo, J.; Niu, F. Development of a thermokarst lake and its effects on permafrost over nearly 10 yr in the Beiluhe Basin, Qinghai-Tibet Plateau. Geosphere 2016, 12, 632–643. [Google Scholar] [CrossRef]
- Fang, J.; Xu, A.; Yang, Y.; Zhang, Z. Hydrological changes and engineering implications of thermokarst lakes, Qinghai-Tibet Plateau. J. Cap. Norm. Univ. 2019, 40, 54–60. [Google Scholar]
- Painter, S.L.; Moulton, J.D.; Wilson, C.J. Modeling challenges for predicting hydrologic response to degrading permafrost. Hydrogeol. J. 2013, 21, 221–224. [Google Scholar] [CrossRef]
- Sefie, A.; Aris, A.Z.; Ramli, M.F.; Narany, T.S.; Shamsuddin, M.K.N.; Saadudin, S.B.; Zali, M.A. Hydrogeochemistry and groundwater quality assessment of the multilayered aquifer in Lower Kelantan Basin, Kelantan, Malaysia. Environ. Earth Sci. 2018, 77, 397. [Google Scholar] [CrossRef]
- Wang, L.; Dong, Y.; Xu, Z.; Qiao, X. Hydrochemical and isotopic characteristics of groundwater in the northeastern Tennger Desert, northern China. Hydrogeol. J. 2017, 25, 2363–2375. [Google Scholar] [CrossRef]
- Piper, A.M. A graphical procedure in the geochemical interpretation of water analysis. Eos Trans. Am. Geophys. Union 1944, 25, 914–928. [Google Scholar] [CrossRef]
- Gibbs, R.J. Mechanisms Controlling World Water Chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jin, D.; Wang, T.; Gao, M.; Yang, J.; Wang, Q. Hydrogeochemical processes and quality assessment of shallow groundwater in Chenqi coalfield, Inner Mongolia, China. Environ. Earth Sci. 2019, 78, 347. [Google Scholar] [CrossRef]
- Singh, A.K.; Mahato, M.K.; Neogi, B.; Singh, K.K.; Mahato, M.K. Quality Assessment of Mine Water in the Raniganj Coalfield Area, India. Mine Water Environ. 2010, 29, 248–262. [Google Scholar] [CrossRef]
- Huang, X.; Wang, G.; Liang, X.; Cui, L.; Ma, L.; Xu, Q. Hydrochemical and Stable Isotope (δD and δ18O) Characteristics of Groundwater and Hydrogeochemical Processes in the Ningtiaota Coalfield, Northwest China. Mine Water Environ. 2017, 37, 119–136. [Google Scholar] [CrossRef]
- Li, P.; Qian, H.; Wu, J.; Zhang, Y.; Zhang, H. Major ion chemistryof shallow groundwater in the Dongsheng Coalfield, OrdosBasin China. Mine Water Environ. 2013, 32, 195–206. [Google Scholar] [CrossRef]
- Li, P.; Wu, J.; Qian, H. Assessment of groundwater quality for irrigation purposes and identification of hydrogeochemical evolution mechanisms in Pengyang County, China. Environ. Earth Sci. 2012, 69, 2211–2225. [Google Scholar] [CrossRef]
- Cloutier, V.; Lefebvre, R.; Therrien, R.; Savard, M.M. Multivariate statistical analysis of geochemical data as indicative of the hydrogeochemical evolution of groundwater in a sedimentary rock aquifer system. J. Hydrol. 2008, 353, 294–313. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Liu, C.; Mostofa, K.M.G.; Zhao, Z.; Luo, R. Hydrogeochemistry and 13CDIC and 18O composition of three Chinese Tibetan Plateau lakes. Isot. Environ. Health Stud. 2017, 54, 89–105. [Google Scholar] [CrossRef]
- Gao, Z.; Lin, Z.; Niu, F.; Luo, J.; Liu, M.; Yin, G. Hydrochemistryand controllingmechanism of lakes in permafrost regions along the Qinghai-Tibet Engineering Corridor, China. Geomorphology 2017, 297, 159–169. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, Q.; Yun, H.; Jin, H.; Zhang, Z. Evaluation of the hydrological contributions of permafrost to the thermokarst lakes on the Qinghai–Tibet Plateau using stable isotopes. Glob. Planet. Chang. 2016, 140, 1–8. [Google Scholar] [CrossRef]
- Li, X.-Y.; Ma, Y.-J.; Huang, Y.-M.; Hu, X.; Wu, X.; Wang, P.; Li, G.-Y.; Zhang, S.-Y.; Wu, H.-W.; Jiang, Z.-Y.; et al. Evaporation and surface energy budget over the largest high-altitude saline lake on the Qinghai-Tibet Plateau. J. Geophys. Res. Atmos. 2016, 121, 10470–10485. [Google Scholar] [CrossRef]
- Cheng, G.; Jin, H. Permafrost and groundwater on the Qinghai-Tibet Plateau and in northeast China. Hydrogeol. J. 2013, 21, 5–23. [Google Scholar] [CrossRef]
- Guo, L.; Yu, Q.; You, Y.; Wang, X.; Yuan, C.; Li, X. Evaluation on the influences of lakes on the thermal regimes of nearby tower foundations along the Qinghai-Tibet Power Transmission Line. Appl. Therm. Eng. 2016, 102, 829–840. [Google Scholar] [CrossRef]
- Zhou, Y.; Guo, D.; Qiu, G.; Cheng, G.; Li, S. Geocryology in China; Science Press: Beijing, China, 2000; pp. 1–34. (In Chinese) [Google Scholar]
- Cheng, G.; Wu, I. Response of permafrost to climate change and their environmental significance, Qinghai-Tibet Plateau. J. Geophys. Res. 2007, 112, F02S03. [Google Scholar] [CrossRef]
- Ge, S.; McKenzie, J.; Voss, C.; Wu, Q. Exchange of groundwater and surface-water mediated by permafrost response to seasonal and long term air temperature variation. Geophys. Res. Lett. 2011, 38, L14402. [Google Scholar] [CrossRef]
- Zhou, P.; Wang, Z.; Zhang, J.; Yang, Z.; Li, X. Study on the hydrochemical characteristics of groundwater along the Taklimakan Desert Highway. Environ. Earth Sci. 2016, 75, 1378. [Google Scholar] [CrossRef]
- Wu, C.; Wu, X.; Qian, C.; Zhu, G. Hydrogeochemistry and groundwater quality assessment of high fluoride levels in the Yanchi endorheic region, northwest China. Appl. Geochem. 2018, 98, 404–417. [Google Scholar] [CrossRef]
- Li, P.; Zhang, Y.; Yang, N.; Jing, L.; Yu, P. Major Ion Chemistry and Quality Assessment of Groundwater in and Around a Mountainous Tourist Town of China. Expo. Health 2016, 8, 239–252. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, J.; Feng, J.; Wang, M.; Wu, G. Hydrogeochemical Characteristics and the Suitability of Groundwater in the Alluvial-Diluvial Plain of Southwest Shandong Province, China. Water 2019, 11, 1577. [Google Scholar] [CrossRef]
- Schoeller, H. Qualitative evaluation of groundwater resources. In Methods and Techniques of Groundwater Investigation and Development, Water Resource Series No. 33; UNESCO: Paris, France, 1967; pp. 44–52. [Google Scholar]
- Horita, J.; Wesolowski, D.J. Liquid-vapor fractionation of oxygen and hydrogen isotopes of water from the freezing to the critical temperature. Geochim. Cosmochim. Acta 1994, 58, 3425–3437. [Google Scholar] [CrossRef]
- Qian, H.; Dou, Y.; Li, X. The variation of stable isotopes of hydrogen and oxygen along the flow and its indication for river evaporation in dousitu river. Hydrogeol. Eng. Geol. 2007, 28, 107–112. [Google Scholar]
- Yang, Y.; Wu, Q.; Yun, H. Analysis of Stable isotope of precipitation and river water in permafrost area, Qinghai-Tibet Plateau, beiluhe basin. Adv. Water Sci. 2013, 24, 778–785. [Google Scholar]
- Gonfiantini, R. Environmental isotopes in lake studies. In Handbook of Environmental Isotope Geochemistry; Elsevier: Amsterdam, The Netherlands, 1986; pp. 113–168. [Google Scholar]
- Gibson, J.; Edwards, T.W.D. Regional water balance trends and evaporation-transpiration partitioning from a stable isotope survey of lakes in northern Canada. Glob. Biogeochem. Cycles 2002, 16, 10-1–10-14. [Google Scholar] [CrossRef]
- Gibson, J.; Birks, S.; Yi, Y. Stable isotope mass balance of lakes: A contemporary perspective. Quat. Sci. Rev. 2016, 131, 316–328. [Google Scholar] [CrossRef]











| Parameter | Groundwater | Lake Water | Precipitation | ||||
|---|---|---|---|---|---|---|---|
| Min | Max | Mean | Min | Max | Mean | ||
| K+ | 3.08 | 13.7 | 6.34 | 2.02 | 6.288 | 3.37 | 3.37 |
| Na+ | 111 | 397 | 187.36 | 22.5 | 400.25 | 132.06 | 132.06 |
| Ca2+ | 58.1 | 160 | 110.97 | 27.05 | 42.1 | 34.34 | 34.34 |
| Mg2+ | 18.2 | 115 | 56.16 | 15.1 | 92.63 | 41.19 | 41.19 |
| NH4+ | 0.031 | 0.26 | 0.18 | 0.03 L | 0.31 | 0.17 | 0.17 |
| Cl− | 128 | 851 | 284.73 | 28.4 | 680 | 213.27 | 213.27 |
| HCO3− | 293 | 781 | 507.55 | 170 | 256 | 216.38 | 216.38 |
| SO42− | 9.61 | 134 | 56.66 | 4.8 | 31.2 | 10.7 | 10.7 |
| F− | 0.28 | 0.76 | 0.45 | 0.2 | 0.45 | 0.27 | 0.27 |
| TDS | 468 | 1780 | 956.9 | 204 | 1420 | 563 | 563 |
| pH | 7.24 | 8.07 | 7.55 | 8.09 | 9.43 | 9 | 9 |
| Groundwater | Anhydrite | Aragonite | Calcite | Dolomite | Gypsum | Halite |
|---|---|---|---|---|---|---|
| ZK01 | −0.14 | 2.94 | 3.1 | 5.52 | 0.11 | −3.73 |
| ZK02 | 0.3 | 2.7 | 2.86 | 5.13 | 0.54 | −3.3 |
| ZK03 | 0.37 | 2.82 | 2.98 | 5.61 | 0.6 | −2.4 |
| ZK04 | 0.04 | 2.79 | 2.95 | 5.36 | 0.29 | −3.19 |
| ZK05 | 0.21 | 2.94 | 3.1 | 5.65 | 0.45 | −3.51 |
| ZK06 | 0.15 | 2.87 | 3.02 | 5.48 | 0.39 | −3.51 |
| ZK07 | 0.58 | 2.75 | 2.91 | 5.41 | 0.82 | −2.84 |
| ZK08 | 0.29 | 2.68 | 2.84 | 5.08 | 0.54 | −3.57 |
| ZK09 | 0.51 | 2.6 | 2.75 | 5.34 | 0.75 | −3.28 |
| ZK10 | 0.09 | 2.97 | 3.12 | 5.81 | 0.33 | −3.59 |
| ZK11 | −0.39 | 2.64 | 2.8 | 4.89 | -0.15 | −3.47 |
| Lake | Anhydrite | Aragonite | Calcite | Dolomite | Gypsum | Halite |
|---|---|---|---|---|---|---|
| L01 | −0.79 | 3.16 | 3.31 | 5.97 | −0.55 | −3.65 |
| L02 | −0.78 | 3.26 | 3.40 | 6.56 | −0.54 | −3.06 |
| L03 | −0.97 | 2.88 | 3.03 | 6.00 | −0.73 | −3.76 |
| L04 | −0.58 | 3.26 | 3.41 | 6.85 | −0.35 | −2.51 |
| L05 | −0.99 | 3.11 | 3.26 | 6.10 | −0.75 | −3.63 |
| L06 | −0.96 | 3.01 | 3.16 | 5.40 | −0.71 | −5.04 |
| L07 | −0.93 | 2.97 | 3.14 | 5.41 | −0.69 | −4.46 |
| L08 | −1.23 | 3.245 | 3.395 | 6.33 | −0.99 | −4.18 |
| Groundwater | Lake Water | |||
|---|---|---|---|---|
| Index | δ18O | δD | δ18O | δD |
| Minimum | −9.79 | −70.563 | −9.26 | −64.114 |
| Maximum | −4.574 | −43.951 | −4.218 | −36.372 |
| Mean | −8.453 | −60.644 | −6.01 | −45.718 |
| Lake | Calculated with 18O | Calculated with D |
|---|---|---|
| L01 | 0.52 | 1.09 |
| L02 | 0.56 | 1.11 |
| L03 | 0.58 | 1.24 |
| L04 | 0.68 | 1.35 |
| L05 | 0.29 | 0.47 |
| L06 | 0.17 | 0.27 |
| L07 | 0.24 | 0.28 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wang, W.; Wang, D.; Li, J.; Dong, J. Hydrochemical and Stable Isotope Characteristics of Lake Water and Groundwater in the Beiluhe Basin, Qinghai–Tibet Plateau. Water 2020, 12, 2269. https://doi.org/10.3390/w12082269
Li J, Wang W, Wang D, Li J, Dong J. Hydrochemical and Stable Isotope Characteristics of Lake Water and Groundwater in the Beiluhe Basin, Qinghai–Tibet Plateau. Water. 2020; 12(8):2269. https://doi.org/10.3390/w12082269
Chicago/Turabian StyleLi, Jinlong, Wei Wang, Dahao Wang, Jiaqi Li, and Jie Dong. 2020. "Hydrochemical and Stable Isotope Characteristics of Lake Water and Groundwater in the Beiluhe Basin, Qinghai–Tibet Plateau" Water 12, no. 8: 2269. https://doi.org/10.3390/w12082269
APA StyleLi, J., Wang, W., Wang, D., Li, J., & Dong, J. (2020). Hydrochemical and Stable Isotope Characteristics of Lake Water and Groundwater in the Beiluhe Basin, Qinghai–Tibet Plateau. Water, 12(8), 2269. https://doi.org/10.3390/w12082269




