Insights on Dissolved Organic Matter Production Revealed by Removal of Charge-Transfer Interactions in Senescent Leaf Leachates
Abstract
1. Introduction
- (1)
- Soil HA displayed higher electron accepting capacity (EAC) and lower electron donating capacity (EDC) than aquatic DOM.
- (2)
- Lower wavelength absorption tracked changes in EAC in soil HA, to a greater degree than aquatic DOM.
- (3)
- Lower wavelength absorption appeared to track EDC during photodegradation of aquatic DOM, indicating that this absorption is influenced by oxidative removal of electron donors, such as phenols, more in aquatic environments than in soils.
2. Materials and Methods
2.1. Experimental Design
2.2. Optical Measurements
2.3. Borohydride Treatments
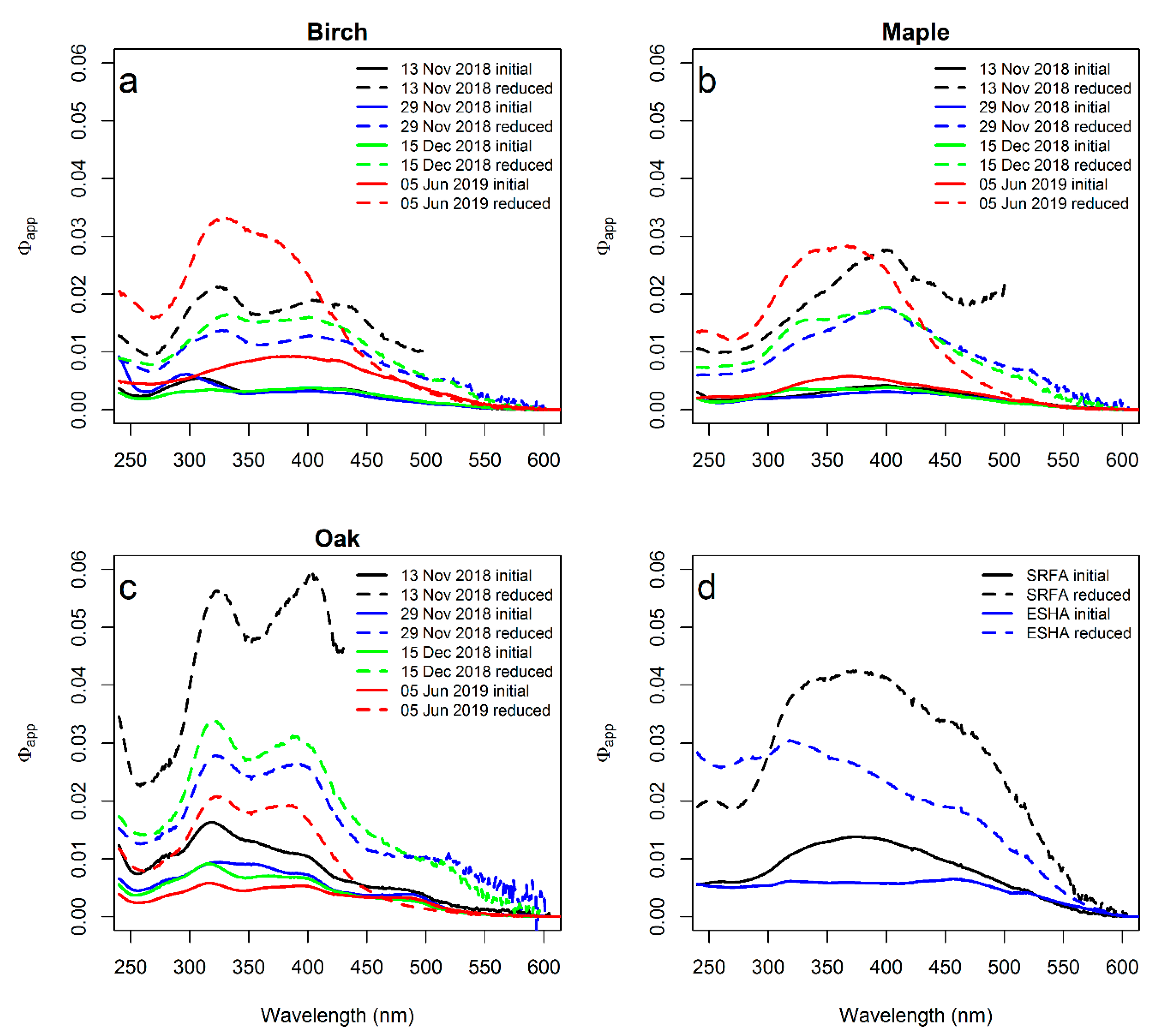
3. Results
3.1. Absorption Change after Borohydride Treatments
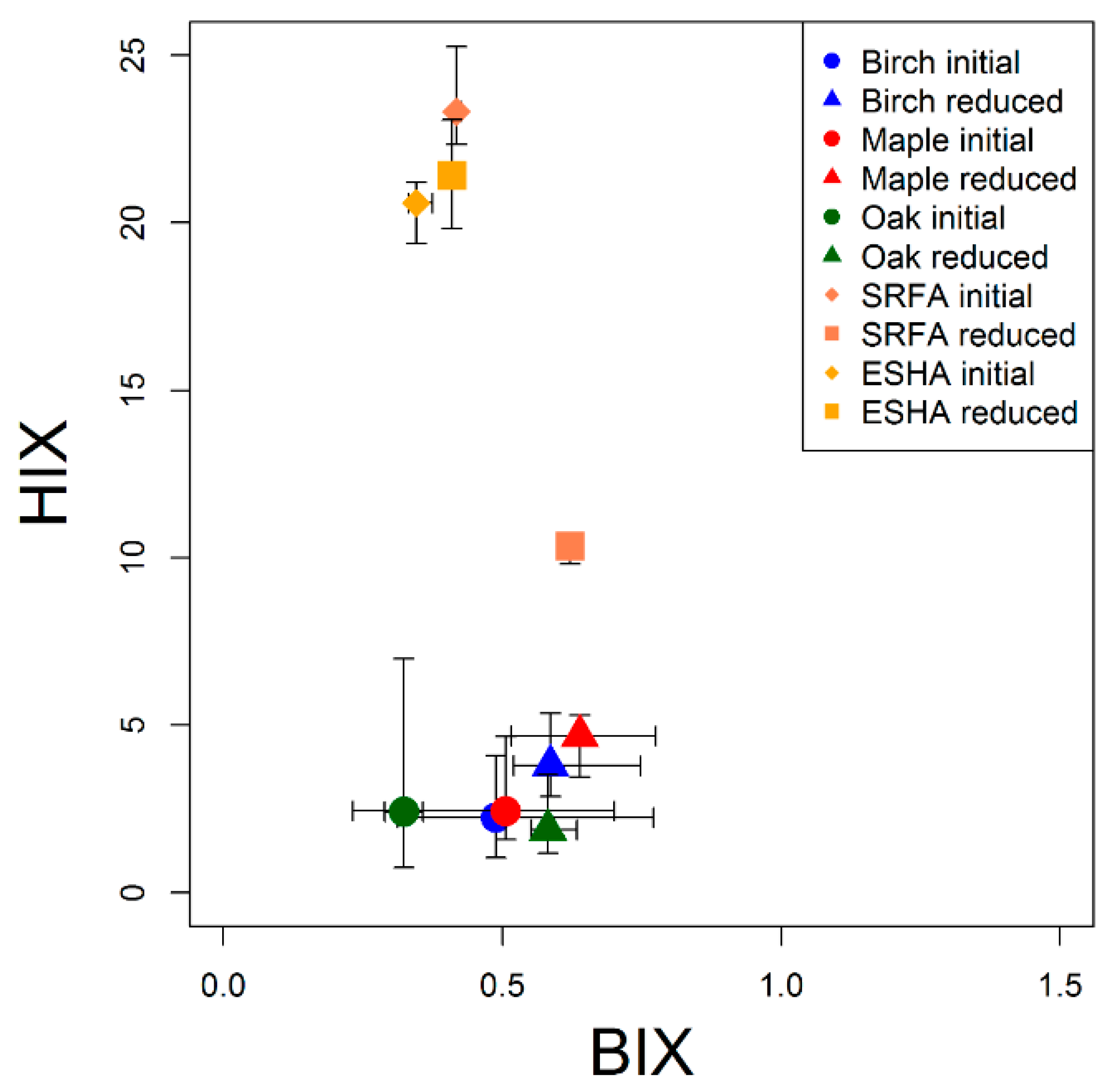
3.2. Fluorescence Response to Borohydride Treatment
4. Discussion
4.1. Absorption Response to Borohydride
4.2. Fluorescence Response to Borohydride
4.3. Tree Species Differences
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fisher, S.G.; Likens, G.E. Energy flow in Bear Brook, New Hampshire: An integrative approach to stream ecosystem metabolism. Ecol. Monogr. 1973, 43, 421–439. [Google Scholar] [CrossRef]
- Fellman, J.B.; Hood, E.; D’amore, D.V.; Edwards, R.T.; White, D. Seasonal changes in the chemical quality and biodegradability of dissolved organic matter exported from soils to streams in coastal temperate rainforest watersheds. Biogeochemistry 2009, 95, 277–293. [Google Scholar] [CrossRef]
- Inamdar, S.; Dhillon, G.; Singh, S.; Dutta, S.; Levia, D.; Scott, D.; McHale, P. Temporal variation in end-member chemistry and its influence on runoff mixing patterns in a forested, Piedmont catchment. Water Resour. Res. 2013, 49, 1828–1844. [Google Scholar] [CrossRef]
- Sharpless, C.M.; Blough, N.V. The importance of charge-transfer interactions in determining chromophoric dissolved organic matter (CDOM) optical and photochemical properties. Environ. Sci. Process. Impacts 2014, 16, 654–671. [Google Scholar] [CrossRef]
- McKnight, D.M.; Boyer, E.W.; Westerhoff, P.K.; Doran, P.T.; Kulbe, T.; Andersen, D.T. Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity. Limnol. Oceanogr. 2001, 46, 38–48. [Google Scholar] [CrossRef]
- Weishaar, J.L.; Aiken, G.R.; Bergamaschi, B.A.; Fram, M.S.; Fujii, R.; Mopper, K. Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ. Sci. Technol. 2003, 37, 4702–4708. [Google Scholar] [CrossRef]
- Helms, J.R.; Stubbins, A.; Ritchie, J.D.; Minor, E.C.; Kieber, D.J.; Mopper, K. Absorption spectral slopes and slope ratios as indicators of molecular weight, source, and photobleaching of chromophoric dissolved organic matter. Limnol. Oceanogr. 2008, 53, 955–969. [Google Scholar] [CrossRef]
- Del Vecchio, R.; Blough, N.V. On the origin of the optical properties of humic substances. Environ. Sci. Technol. 2004, 38, 3885–3891. [Google Scholar] [CrossRef]
- Ma, J.; Del Vecchio, R.; Golanoski, K.S.; Boyle, E.S.; Blough, N.V. Optical properties of humic substances and CDOM: Effects of borohydride reduction. Environ. Sci. Technol. 2010, 44, 5395–5402. [Google Scholar] [CrossRef]
- Andrew, A.A.; Del Vecchio, R.; Subramaniam, A.; Blough, N.V. Chromophoric dissolved organic matter (CDOM) in the Equatorial Atlantic Ocean: Optical properties and their relation to CDOM structure and source. Mar. Chem. 2013, 148, 33–43. [Google Scholar] [CrossRef]
- Andrew, A.A.; Del Vecchio, R.; Zhang, Y.; Subramaniam, A.; Blough, N.V. Are extracted materials truly representative of original samples? Impact of C18 extraction on CDOM optical and chemical properties. Front. Chem. 2016, 4, 4. [Google Scholar] [CrossRef]
- Cartisano, C.M.; Del Vecchio, R.; Bianca, M.R.; Blough, N.V. Investigating the sources and structure of chromophoric dissolved organic matter (CDOM) in the North Pacific Ocean (NPO) utilizing optical spectroscopy combined with solid phase extraction and borohydride reduction. Mar. Chem. 2018, 204, 20–35. [Google Scholar] [CrossRef]
- Del Vecchio, R.; Schendorf, T.M.; Blough, N.V. Contribution of quinones and ketones/aldehydes to the optical properties of humic substances (HS) and chromophoric dissolved organic matter (CDOM). Environ. Sci. Technol. 2017, 51, 13624–13632. [Google Scholar] [CrossRef]
- Osburn, C.L.; Kinsey, J.D.; Bianchi, T.S.; Shields, M.R. Formation of planktonic chromophoric dissolved organic matter in the ocean. Mar. Chem. 2019, 209, 1–13. [Google Scholar] [CrossRef]
- Schendorf, T.M.; Del Vecchio, R.; Bianca, M.; Blough, N.V. Combined effects of pH and borohydride reduction on optical properties of humic substances (HS): A comparison of optical models. Environ. Sci. Technol. 2019, 53, 6310–6319. [Google Scholar] [CrossRef]
- Schendorf, T.M.; Del Vecchio, R.; Koech, K.; Blough, N.V. A standard protocol for NaBH4 reduction of CDOM and HS. Limnol. Oceanogr. Methods 2016, 14, 414–423. [Google Scholar] [CrossRef]
- Kane, E.S.; Veverica, T.J.; Tfaily, M.M.; Lilleskov, E.A.; Meingast, K.M.; Kolka, R.K.; Chimner, R.A. Reduction-oxidation potential and dissolved organic matter composition in Northern Peat Soil: Interactive controls of water table position and plant functional groups. J. Geophys. Res. Biogeosci. 2019, 124. [Google Scholar] [CrossRef]
- Walpen, N.; Getzinger, G.J.; Schroth, M.H.; Sander, M. Electron-donating phenolic and electron-accepting quinone moieties in peat dissolved organic matter: Quantities and redox transformations in the context of peat biogeochemistry. Environ. Sci. Technol. 2018, 52, 5236–5245. [Google Scholar] [CrossRef]
- D’Andrilli, J.; Junker, J.R.; Smith, H.J.; Scholl, E.A.; Foreman, C.M. DOM composition alters ecosystem function during microbial processing of isolated sources. Biogeochemistry 2019, 142, 281–298. [Google Scholar] [CrossRef]
- Wantzen, K.M.; Junk, W.J. Aquatic-terrestrial linkages from streams to rivers: Biotic hot spots and hot moments. Arch. Hydrobiol. Suppl. 2006, 16, 595–611. [Google Scholar] [CrossRef]
- Qualls, R.G.; Haines, B.L. Geochemistry of dissolved organic nutrients in water percolating through a forest ecosystem. Soil Sci. Soc. J. 1991, 55, 1112–1123. [Google Scholar] [CrossRef]
- Sharpless, C.M.; Aeschbacher, M.; Page, S.E.; Wenk, J.; Sander, M.; McNeill, K. Photooxidation-induced changes in optical, electrochemical, and photochemical properties of humic substances. Environ. Sci. Technol. 2014, 48, 2688–2696. [Google Scholar] [CrossRef]
- Harfmann, J.L.; Guillemette, F.; Kaiser, K.; Spencer, R.G.; Chuang, C.Y.; Hernes, P.J. Convergence of terrestrial dissolved organic matter composition and the role of microbial buffering in aquatic ecosystems. J. Geophys. Res. Biogeosci. 2019, 124, 3125–3142. [Google Scholar] [CrossRef]
- McDowell, W.H.; Fisher, S.G. Autumnal processing of dissolved organic matter in a small woodland stream ecosystem. Ecology 1976, 57, 561–569. [Google Scholar] [CrossRef]
- McKnight, D.M.; Smith, R.L.; Harnish, R.A.; Miller, C.L.; Bencala, K.E. Seasonal relationships between planktonic microorganisms and dissolved organic material in an alpine stream. Biogeochemistry 1993, 21, 39–59. [Google Scholar] [CrossRef]
- Meyer, J.L.; Wallace, J.B.; Eggert, S.L. Leaf litter as a source of dissolved organic carbon in streams. Ecosystems 1998, 1, 240–249. [Google Scholar] [CrossRef]
- Green, S.A.; Blough, N.V. Optical absorption and fluorescence properties of chromophoric dissolved organic matter in natural waters. Limnol. Oceanogr. 1994, 39, 1903–1916. [Google Scholar] [CrossRef]
- Huguet, A.; Vacher, L.; Relexans, S.; Saubusse, S.; Froidefond, J.M.; Parlanti, E. Properties of fluorescent dissolved organic matter in the Gironde Estuary. Org. Geochem. 2009, 40, 706–719. [Google Scholar] [CrossRef]
- Zsolnay, A.; Baigar, E.; Jimenez, M.; Steinweg, B.; Saccomandi, F. Differentiating with fluorescence spectroscopy the sources of dissolved organic matter in soils subjected to drying. Chemosphere 1999, 38, 45–50. [Google Scholar] [CrossRef]
- Ohno, T. Fluorescence inner-filtering correction for determining the humification index of dissolved organic matter. Environ. Sci. Technol. 2002, 36, 742–746. [Google Scholar] [CrossRef]
- Velapoldi, R.A.; Tønnesen, H.H. Corrected emission spectra and quantum yields for a series of fluorescent compounds in the visible spectral region. J. Fluoresc. 2004, 14, 465–472. [Google Scholar] [CrossRef]
- Mueller, J.L.; Bidigare, R.R.; Trees, C.; Balch, W.M.; Dore, J.; Drapeau, D.T.; Karl, D.; Van Heukelem, L. Ocean Optics Protocols for Satellite Ocean Color Sensor Validation, Revision 5. Volume V: Biogeochemical and Bio-Optical Measurements and Data Analysis Protocols; Goddard Space Flight Space Center: Greenbelt, MD, USA, 2003.
- Aeschbacher, M.; Sander, M.; Schwarzenbach, R.P. Novel electrochemical approach to assess the redox properties of humic substances. Environ. Sci. Technol. 2010, 44, 87–93. [Google Scholar] [CrossRef]
- Aeschbacher, M.; Graf, C.; Schwarzenbach, R.P.; Sander, M. Antioxidant properties of humic substances. Environ. Sci. Technol. 2012, 46, 4916–4925. [Google Scholar] [CrossRef]
- Paerl, R.W.; Claudio, I.M.; Shields, M.R.; Bianchi, T.S.; Osburn, C.L. Dityrosine formation via reactive oxygen consumption yields increasingly recalcitrant humic-like fluorescent organic matter in the ocean. Limnol. Oceanogr. Lett. 2020. [Google Scholar] [CrossRef]
- Min, D.W.; Kim, K.; Lui, K.H.; Kim, B.; Kim, S.; Cho, J.; Choi, W. Abiotic formation of humic-like substances through freezing-accelerated reaction of phenolic compounds and nitrite. Environ. Sci. Technol. 2019, 53, 7410–7418. [Google Scholar] [CrossRef]
- Murphy, K.R.; Stedmon, C.A.; Wenig, P.; Bro, R. Open-Fluor–an online spectral library of auto-fluorescence by organic compounds in the environment. Anal. Methods-UK 2014, 6, 658–661. [Google Scholar] [CrossRef]
- Harrison, A.F. The inhibitory effect of oak leaf litter tannins on the growth of fungi, in relation to litter decomposition. Soil Biol. Biochem. 1971, 3, 167–172. [Google Scholar] [CrossRef]


| pH | DOC (mg L−1) | E2:E3 | BIX | HIX | S275–295 | S350–400 | |
|---|---|---|---|---|---|---|---|
| 13 November 2018 | |||||||
| Birch initial | 6.1 | 20.7 | 3.6 | 0.3 | 1.4 | 0.010 | 0.014 |
| Birch reduced | 4.4 | 0.5 | 2.9 | 0.017 | 0.020 | ||
| Maple initial | 5.2 | 11.1 | 3.9 | 0.7 | 1.6 | 0.012 | 0.015 |
| Maple reduced | 4.6 | 0.8 | 3.4 | 0.015 | 0.021 | ||
| Oak initial | 6.2 | 17.6 | 5.2 | 0.3 | 0.7 | 0.017 | 0.017 |
| Oak reduced | 6.9 | 0.6 | 1.2 | 0.027 | 0.025 | ||
| 29 November 2018 | |||||||
| Birch initial | 6.0 | 11.0 | 3.4 | 0.8 | 1.0 | 0.010 | 0.014 |
| Birch reduced | 4.1 | 0.5 | 3.9 | 0.016 | 0.019 | ||
| Maple initial | 5.6 | 14.3 | 3.6 | 0.6 | 1.7 | 0.012 | 0.015 |
| Maple reduced | 4.3 | 0.6 | 5.3 | 0.015 | 0.020 | ||
| Oak initial | 6.1 | 20.6 | 4.8 | 0.4 | 0.9 | 0.014 | 0.017 |
| Oak reduced | 5.4 | 0.6 | 1.3 | 0.023 | 0.021 | ||
| 15 December 2018 | |||||||
| Birch initial | 6.1 | 20.6 | 3.2 | 0.4 | 2.5 | 0.011 | 0.013 |
| Birch reduced | 4.2 | 0.5 | 5.4 | 0.018 | 0.018 | ||
| Maple initial | 4.8 | 14.0 | 3.3 | 0.2 | 1.8 | 0.013 | 0.014 |
| Maple reduced | 4.0 | 0.5 | 5.2 | 0.018 | 0.017 | ||
| Oak initial | 6.5 | 15.2 | 4.4 | 0.3 | 1.0 | 0.013 | 0.015 |
| Oak reduced | 5.8 | 0.6 | 1.5 | 0.022 | 0.021 | ||
| 5 June 2019 | |||||||
| Birch initial | 6.8 | 12.5 | 4.0 | 0.5 | 4.1 | 0.012 | 0.016 |
| Birch reduced | 5.3 | 0.7 | 3.0 | 0.021 | 0.016 | ||
| Maple initial | 6.1 | 17.6 | 4.0 | 0.5 | 4.7 | 0.015 | 0.019 |
| Maple reduced | 4.9 | 0.6 | 4.8 | 0.019 | 0.017 | ||
| Oak initial | 6.1 | 20.7 | 4.7 | 0.4 | 7.0 | 0.014 | 0.018 |
| Oak reduced | 6.4 | 0.6 | 3.5 | 0.021 | 0.018 | ||
| References | |||||||
| SRFA initial | -- | 10.2 | 4.6 | 0.4 | 23.3 | 0.012 | 0.019 |
| SRFA reduced | 6.8 | 0.6 | 10.3 | 0.018 | 0.023 | ||
| ESHA initial | -- | 3.2 | 2.3 | 0.6 | 1.5 | 0.007 | 0.009 |
| ESHA reduced | 2.8 | 0.5 | 5.4 | 0.009 | 0.010 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meingast, K.M.; Grunert, B.K.; Green, S.A.; Kane, E.S.; Khademimoshgenani, N. Insights on Dissolved Organic Matter Production Revealed by Removal of Charge-Transfer Interactions in Senescent Leaf Leachates. Water 2020, 12, 2356. https://doi.org/10.3390/w12092356
Meingast KM, Grunert BK, Green SA, Kane ES, Khademimoshgenani N. Insights on Dissolved Organic Matter Production Revealed by Removal of Charge-Transfer Interactions in Senescent Leaf Leachates. Water. 2020; 12(9):2356. https://doi.org/10.3390/w12092356
Chicago/Turabian StyleMeingast, Karl M., Brice K. Grunert, Sarah A. Green, Evan S. Kane, and Nastaran Khademimoshgenani. 2020. "Insights on Dissolved Organic Matter Production Revealed by Removal of Charge-Transfer Interactions in Senescent Leaf Leachates" Water 12, no. 9: 2356. https://doi.org/10.3390/w12092356
APA StyleMeingast, K. M., Grunert, B. K., Green, S. A., Kane, E. S., & Khademimoshgenani, N. (2020). Insights on Dissolved Organic Matter Production Revealed by Removal of Charge-Transfer Interactions in Senescent Leaf Leachates. Water, 12(9), 2356. https://doi.org/10.3390/w12092356






