Multivariate Statistical Approach to Study Spatiotemporal Variations in Water Quality of aHimalayan Urban Fresh Water Lake
Abstract
1. Introduction
2. Material and Methods
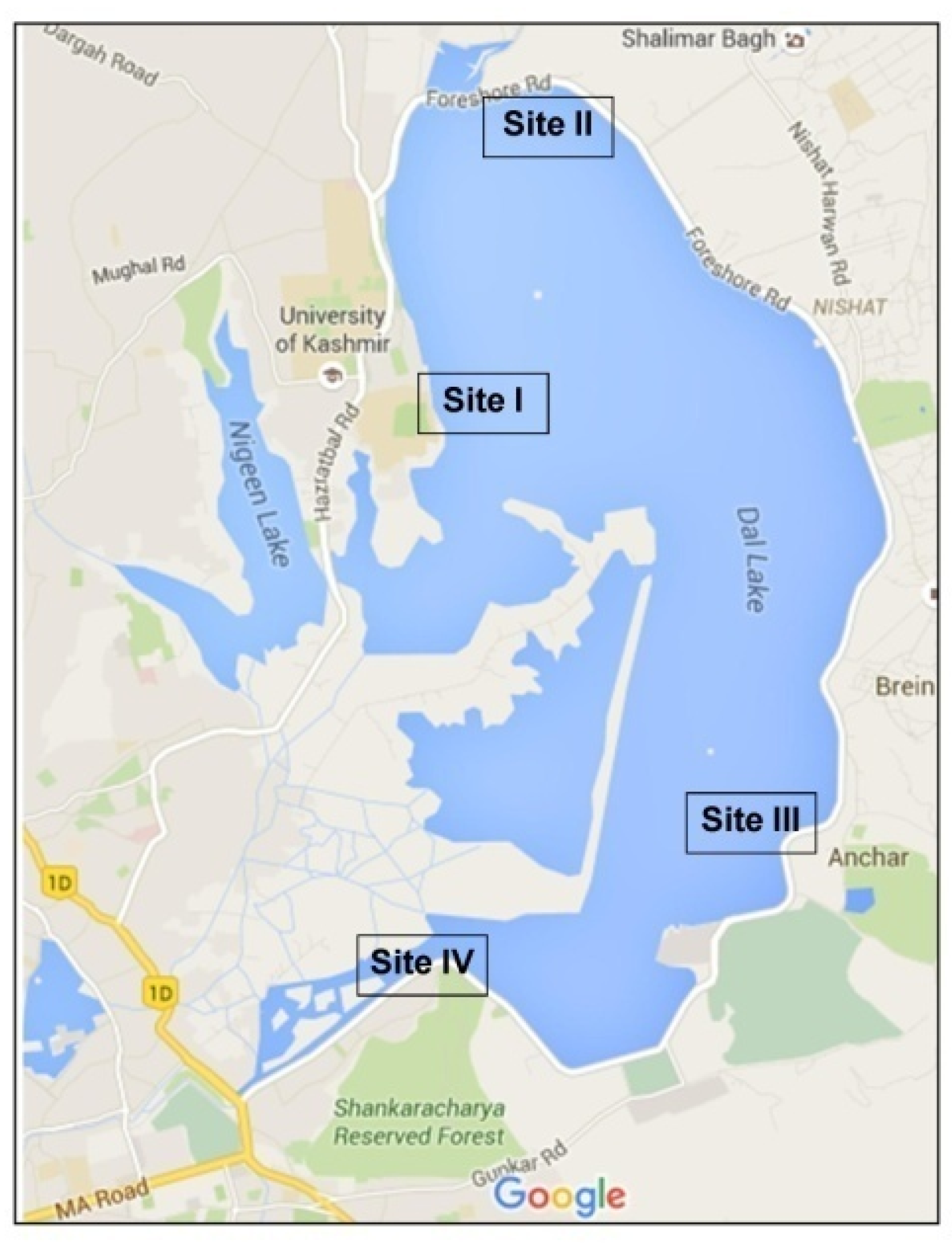
2.1. Study Area
2.2. Methodology
2.3. Statistical Analysis
2.4. Water Quality Index (WQI)
3. Results and Discussion
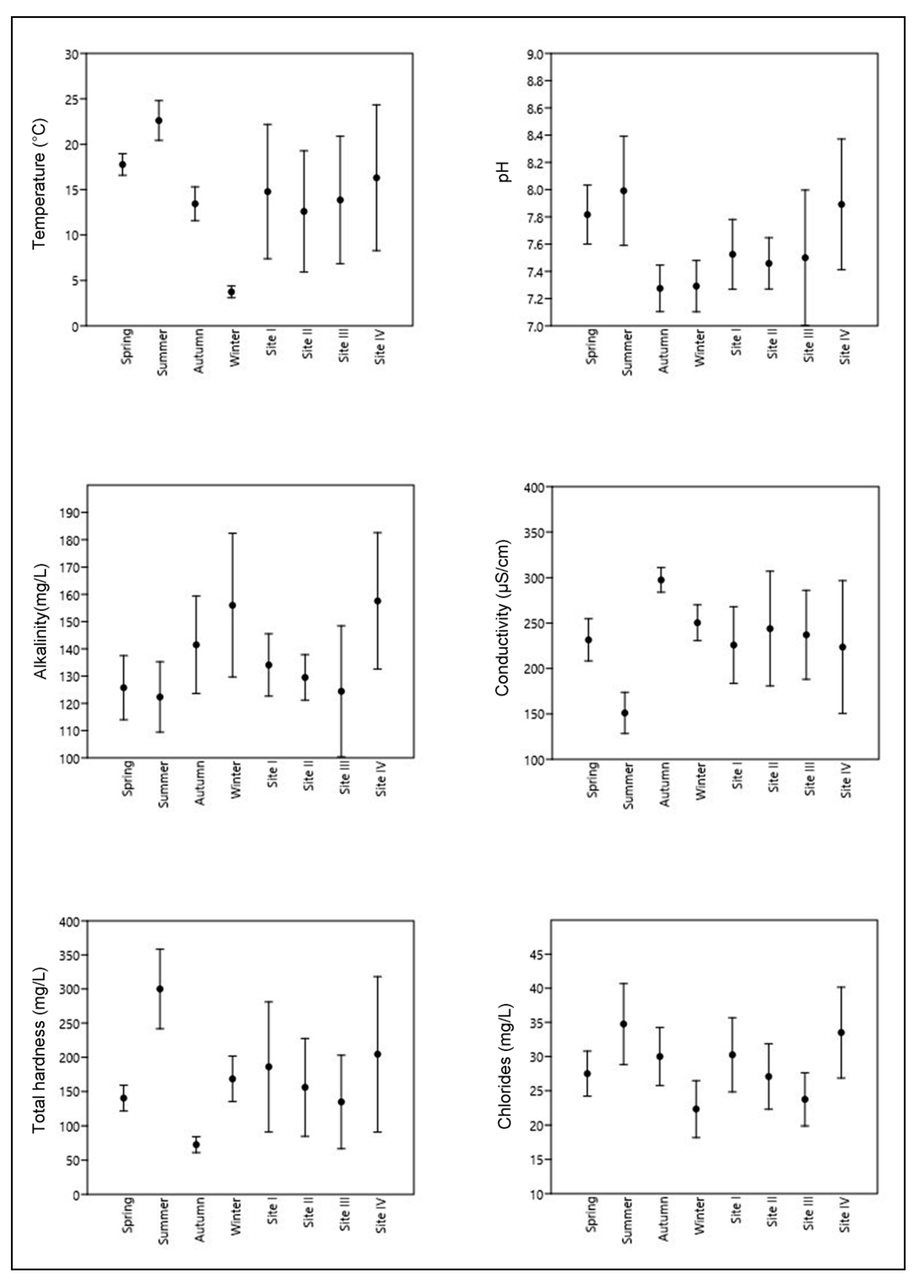
3.1. Physicochemical Analysis
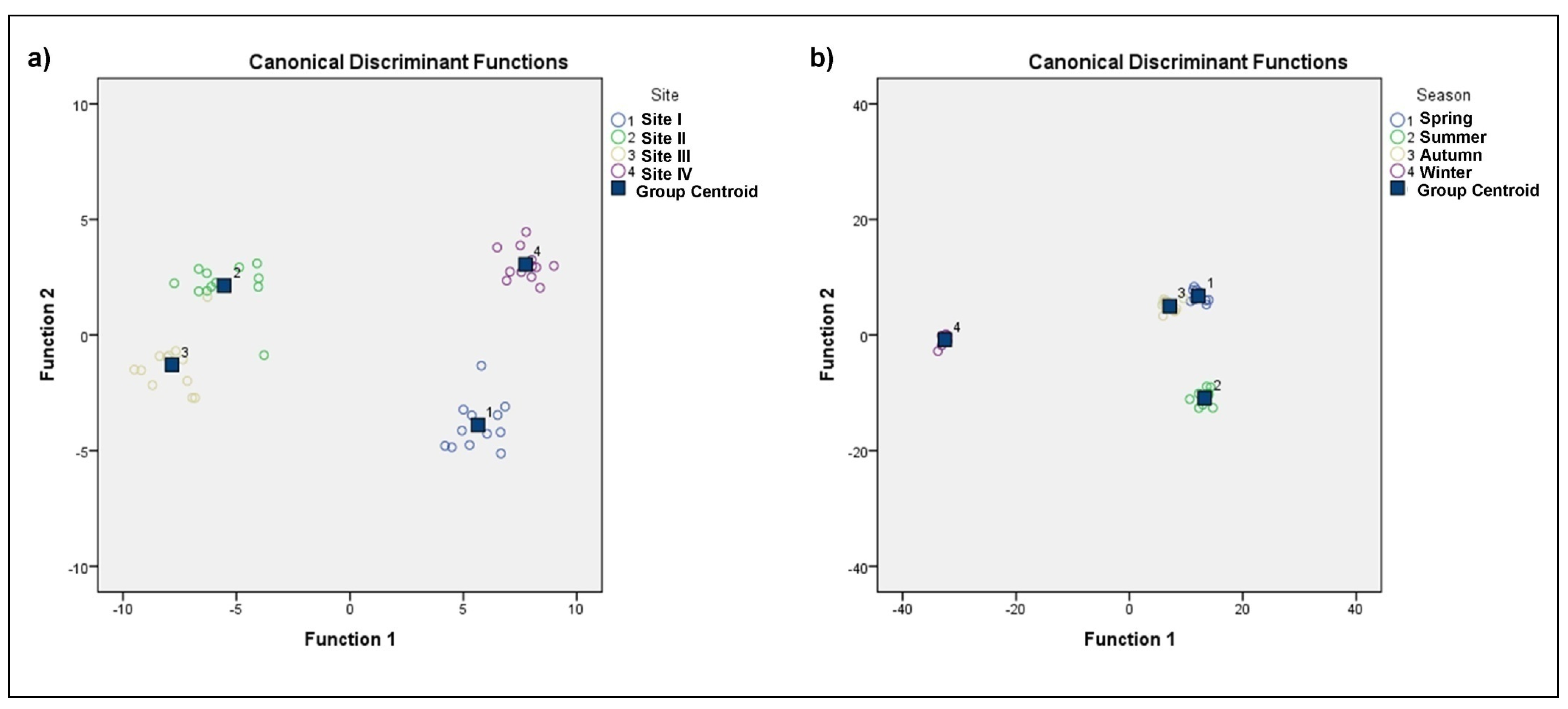
3.2. Discriminant Analysis
3.3. Water Quality Index
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zutshi, D.; Vass, K. Limnological Studies on Dal Lake, Srinagar. III. Biological Features. Proc. Indian Natl. Sci. Sect. B 1982, 48, 234–241. [Google Scholar]
- Ul Solim, S.; Wanganeo, A. Excessive phosphorus loading to Dal Lake, India: Implications for managing shallow eutrophic lakes in urbanized watersheds. Int. Rev. Hydrobiol. 2008, 93, 148–166. [Google Scholar] [CrossRef]
- Micklin, P. The Aral sea disaster. Annu. Rev. Earth Planet. Sci. 2007, 35, 47–72. [Google Scholar] [CrossRef]
- Hussein, H. Politics of the Dead Sea Canal: A historical review of the evolving discourses, interests, and plans. Water Int. 2017, 42, 527–542. [Google Scholar] [CrossRef]
- Qadri, H.; Yousuf, A. Dal Lake ecosystem: Conservation strategies and problems. In Proceedings of the TAAL 2007: The 12th World Lake Conference, Jaipur, India, 28 October–2 November 2007; pp. 1453–1457. [Google Scholar]
- Lawrence, W.R. The Valley of Kashmir; H. Frowde: London, UK, 1895. [Google Scholar]
- Yu, F.C.; Fang, G.H.; Ru, X.W. Eutrophication, health risk assessment and spatial analysis of water quality in Gucheng Lake, China. Environ. Earth Sci. 2010, 59, 1741–1748. [Google Scholar] [CrossRef]
- Bora, M.; Goswami, D.C. Water quality assessment in terms of water quality index (WQI): Case study of the Kolong River, Assam, India. Appl. Water Sci. 2016, 7, 3125–3135. [Google Scholar] [CrossRef]
- Fink, G.; Alcamo, J.; Flörke, M.; Reder, K. Phosphorus loadings to the world’s largest lakes: Sources and trends. Glob. Biogeochem. Cycles 2018, 32, 617–634. [Google Scholar] [CrossRef]
- Zhao, Y.; Xia, X.H.; Yang, Z.F.; Wang, F. Assessment of water quality in Baiyangdian Lake using multivariate statistical techniques. Procedia Environ. Sci. 2012, 13, 1213–1226. [Google Scholar] [CrossRef]
- Niemi, G.J.; DeVore, P.; Detenbeck, N.; Taylor, D.; Lima, A.; Pastor, J.; Yount, J.D.; Naiman, R.J. Overview of case studies on recovery of aquatic systems from disturbance. Environ. Manag. 1990, 14, 571–587. [Google Scholar] [CrossRef]
- Kazi, T.; Arain, M.; Jamali, M.K.; Jalbani, N.; Afridi, H.; Sarfraz, R.; Baig, J.; Shah, A.Q. Assessment of water quality of polluted lake using multivariate statistical techniques: A case study. Ecotoxicol. Environ. Saf. 2009, 72, 301–309. [Google Scholar] [CrossRef]
- Herrera-Silveira, J.A.; Morales-Ojeda, S.M. Evaluation of the health status of a coastal ecosystem in southeast Mexico: Assessment of water quality, phytoplankton and submerged aquatic vegetation. Mar. Pollut. Bull. 2009, 59, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Eaton, A.D.; Clesceri, L.S.; Rice, E.W.; Greenberg, A.E.; Franson, M. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association, American Waterworks Association, Water Environmental Federation: Washington, DC, USA, 2005; pp. 1–88. [Google Scholar]
- Trivedy, R.; Goel, P. Chemical and Biological Methods for Water Pollution Studies; Environmental Publications: Portland, OR, USA, 1984. [Google Scholar]
- US-EPA. Methods for Chemical Analysis of Water and Wastes; Environmental Protection Agency: Washington, DC, USA, 1991.
- Singh, K.P.; Malik, A.; Mohan, D.; Sinha, S. Multivariate statistical techniques for the evaluation of spatial and temporal variations in water quality of Gomti River (India)—A case study. Water Res. 2004, 38, 3980–3992. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.M.; McClelland, N.I.; Deininger, R.; Tozer, R.G. A water quality index-do we dare? water sewage works. Ojahio Mcred 1970, 117, 339–343. [Google Scholar]
- Bhat, S.A.; Pandit, A.K. Surface Water Quality Assessment of Wular Lake, A Ramsar Site in Kashmir Himalaya, Using Discriminant Analysis and WQI. J. Ecosyst. 2014, 2014, 1–18. [Google Scholar] [CrossRef]
- Brown, R.M.; McClelland, N.I.; Deininger, R.A.; O’Connor, M.F. A Water Quality Index—Crashing the Psychological Barrier. In Indicators of Environmental Quality; Springer: New York, NY, USA, 1972; pp. 173–182. [Google Scholar] [CrossRef]
- Garg, R.; Rao, R.; Uchchariya, D.; Shukla, G.; Saksena, D. Seasonal variations in water quality and major threats to Ramsagar reservoir, India. Afr. J. Environ. Sci. Technol. 2010, 4, 61–76. [Google Scholar]
- Lu, Q.; He, Z.L.; Graetz, D.A.; Stoffella, P.J.; Yang, X. Phytoremediation to remove nutrients and improve eutrophic stormwaters using water lettuce (Pistia stratiotes L.). Environ. Sci. Pollut. Res. 2010, 17, 84–96. [Google Scholar] [CrossRef]
- Jeelani, G.; Shah, A.Q. Geochemical characteristics of water and sediment from the Dal Lake, Kashmir Himalaya: Constraints on weathering and anthropogenic activity. Environ. Geol. 2006, 50, 12–23. [Google Scholar] [CrossRef]
- Rashid, I.; Romshoo, S.A.; Amin, M.; Khanday, S.A.; Chauhan, P. Linking human-biophysical interactions with the trophic status of Dal Lake, Kashmir Himalaya, India. Limnologica 2017, 62, 84–96. [Google Scholar] [CrossRef]
- Solanki, V.R.; Hussain, M.M.; Raja, S.S. Water quality assessment of Lake Pandu Bodhan, Andhra Pradesh State, India. Environ. Monit. Assess. 2010, 163, 411–419. [Google Scholar] [CrossRef]
- Najar, I.A.; Khan, A.B. Assessment of seasonal variation in water quality of Dal Lake (Kashmir, India) using multivariate statistical techniques. In Proceedings of the Water Pollution XI, Southampton, UK, 10 July 2012. [Google Scholar]
- Khan, M.Y.A.; Hu, H.; Tian, F.; Wen, J. Monitoring the spatio-temporal impact of small tributaries on the hydrochemical characteristics of Ramganga River, Ganges Basin, India. Int. J. River Basin Manag. 2020, 18, 231–241. [Google Scholar] [CrossRef]
- Varol, M.; Gökot, B.; Bekleyen, A.; Şen, B. Spatial and temporal variations in surface water quality of the dam reservoirs in the Tigris River basin, Turkey. Catena 2012, 92, 11–21. [Google Scholar] [CrossRef]
- Emerenciano, M.G.C.; Martínez-Córdova, L.R.; Martínez-Porchas, M.; Miranda-Baeza, A. Biofloc technology (BFT): A tool for water quality management in aquaculture. In Water Quality; Tutu, H., Ed.; IntechOpen: Rijeka, Croatia, 2017; pp. 92–109. [Google Scholar]
- Sharip, Z.; Suratman, S. Formulating specific water quality criteria for lakes: A Malaysian perspective. In Water Quality; Tutu, H., Ed.; IntechOpen: Rijeka, Croatia, 2017; pp. 293–313. [Google Scholar]
- Ruman, M.; Polkowska, Ż.; Zygmunt, B. Processes and the Resulting Water Quality in the Medium-Size Turawa Storage Reservoir after 60-Year Usage. In Water Quality; Tutu, H., Ed.; IntechOpen: Rijeka, Croatia, 2017; pp. 377–400. [Google Scholar]
- Igbinosa, E.; Okoh, A. Impact of discharge wastewater effluents on the physico-chemical qualities of a receiving watershed in a typical rural community. Int. J. Environ. Sci. Technol. 2009, 6, 175–182. [Google Scholar] [CrossRef]
- Edokpayi, J.N.; Odiyo, J.O.; Durowoju, O.S. Impact of wastewater on surface water quality in developing countries: A case study of South Africa. In Water Quality; Tutu, H., Ed.; IntechOpen: Rijeka, Croatia, 2017; pp. 401–416. [Google Scholar]
- Mohammad, A.H.; Jung, H.C.; Odeh, T.; Bhuiyan, C.; Hussein, H. Understanding the impact of droughts in the Yarmouk Basin, Jordan: Monitoring droughts through meteorological and hydrological drought indices. Arab. J. Geosci. 2018, 11, 103. [Google Scholar] [CrossRef]
- Odeh, T.; Mohammad, A.H.; Hussein, H.; Ismail, M.; Almomani, T. Over-pumping of groundwater in Irbid governorate, northern Jordan: A conceptual model to analyze the effects of urbanization and agricultural activities on groundwater levels and salinity. Environ. Earth Sci. 2019, 78, 40. [Google Scholar] [CrossRef]
- Riad, P.; Graefe, S.; Hussein, H.; Buerkert, A. Landscape transformation processes in two large and two small cities in Egypt and Jordan over the last five decades using remote sensing data. Landsc. Urban.Plan. 2020, 197, 103766. [Google Scholar] [CrossRef]

| Parameter | BIS/ICMR Standard (Vs) | Unit Weight (Wn) |
|---|---|---|
| pH | 8.5 | 0.210471 |
| Alkalinity | 120 | 0.014908 |
| Conductivity | 300 | 0.005963 |
| Chlorides | 250 | 0.007156 |
| Hardness | 300 | 0.005963 |
| DO | 5 | 0.3578 |
| BOD | 5 | 0.3578 |
| Nitrate-N | 45 | 0.039756 |
| ΣWn | 0.999817 | |
| Standardized Canonical Discriminant Function Coefficients | |||
|---|---|---|---|
| Functions | |||
| 1 | 2 | 3 | |
| Water Temperature | −3.031 | 1.188 | 5.123 |
| pH | 1.725 | 2.490 | 0.832 |
| Alkalinity | 2.421 | 0.947 | 1.737 |
| Hardness | −0.696 | 0.891 | −1.135 |
| Chlorides | −1.813 | 0.241 | −0.512 |
| BOD | −0.267 | −1.107 | −0.052 |
| Nitrate | −0.977 | 1.179 | −3.231 |
| Ammonia | 2.162 | −0.046 | −0.834 |
| Total Phosphorous | 2.478 | 1.214 | −0.196 |
| Orthophosphorous | 3.615 | −4.431 | 0.433 |
| Eigenvalues | |||
| Eigenvalue | 50.181 | 8.360 | 2.282 |
| % of Variance | 82.5 | 13.7 | 3.8 |
| Cumulative% | 82.5 | 96.2 | 100.0 |
| Canonical Correlation | 0.990 | 0.945 | 0.834 |
| Wilks’ Lambda | |||
| Wilks’ Lambda | 0.001 | 0.033 | 0.305 |
| Chi-square | 294.411 | 136.997 | 47.539 |
| Df | 30 | 18 | 8 |
| Sig. | 0.000 | 0.000 | 0.000 |
| Standardized Canonical Discriminant Function Coefficients | |||
|---|---|---|---|
| Functions | |||
| 1 | 2 | 3 | |
| Water Temperature | 2.406 | 0.526 | 0.544 |
| Alkalinity | −2.589 | 0.537 | 0.553 |
| Conductivity | 0.319 | 0.844 | 0.126 |
| Hardness | −2.135 | −1.385 | 0.728 |
| DO | −0.172 | 1.374 | 1.028 |
| BOD | 1.643 | 0.467 | 0.132 |
| Nitrate | 3.164 | 0.328 | 0.089 |
| Total Phosphorous | −0.916 | 0.857 | −1.077 |
| Eigenvalues | |||
| Eigenvalue | 391.282 | 51.633 | 13.136 |
| % of Variance | 85.8 | 11.3 | 2.9 |
| Cumulative% | 85.8 | 97.1 | 100.0 |
| Canonical Correlation | 0.999 | 0.990 | 0.964 |
| Wilks’ Lambda | |||
| Wilks’ Lambda | 0.000 | 0.001 | 0.071 |
| Chi-square | 515.946 | 271.095 | 108.598 |
| Df | 24 | 14 | 6 |
| Sig. | 0.000 | 0.000 | 0.000 |
| Parameter | Spring | Summer | Autumn | Winter | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vn | Qn | QnWn | Vn | Qn | QnWn | Vn | Qn | QnWn | Vn | Qn | QnWn | |
| pH | 7.77 | 51.33333 | 10.80416 | 7.73 | 48.66667 | 10.2429 | 7.36 | 24 | 5.051294 | 7.23 | 15.33333 | 3.227216 |
| Alkalinity | 122.3 | 101.9167 | 1.519408 | 132 | 110 | 1.639917 | 130.3 | 108.5833 | 1.618797 | 151.67 | 126.3917 | 1.884289 |
| Conductivity | 204 | 27.2 | 0.064881 | 176.3 | 58.76667 | 0.350445 | 284.3 | 94.76667 | 0.565125 | 238.67 | 79.55667 | 0.474423 |
| Chlorides | 30.7 | 12.28 | 0.087876 | 36.7 | 14.68 | 0.10505 | 31 | 12.4 | 0.088734 | 22.67 | 9.068 | 0.064891 |
| Hardness | 130.7 | 43.56667 | 0.259803 | 326.6 | 108.8667 | 0.649208 | 86.3 | 28.76667 | 0.171545 | 201 | 67 | 0.399543 |
| DO | 8.6 | 62.5 | 22.3625 | 5.13 | 98.64583 | 35.29548 | 5.96 | 90 | 32.202 | 8.13 | 67.39583 | 24.11423 |
| BOD | 12.5 | 250 | 89.45 | 21.5 | 430 | 153.854 | 24.3 | 486 | 173.8908 | 10.16 | 203.2 | 72.70496 |
| Nitrate-N | 1.38 | 3.066667 | 0.121917 | 1.43 | 3.177778 | 0.126334 | 1.23 | 2.733333 | 0.108665 | 0.919 | 2.042222 | 0.08119 |
| ΣWnQn | 124.6705 | ΣWnQn | 202.2633 | ΣWnQn | 213.697 | ΣWnQn | 102.9507 | |||||
| WQI | 125.1412 | WQI | 202.3003 | WQI | 213.736 | WQI | 102.9696 | |||||
| Average WQI = 161 | ||||||||||||
| Parameter | Spring | Summer | Autumn | Winter | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vn | Qn | QnWn | Vn | Qn | QnWn | Vn | Qn | QnWn | Vn | Qn | QnWn | |
| pH | 7.57 | 38 | 7.997882 | 7.63 | 42 | 8.839765 | 7.2 | 13.33333 | 2.806275 | 7.43 | 28.66667 | 6.03349 |
| Alkalinity | 130.3 | 108.5833 | 1.618797 | 125.67 | 104.725 | 1.561275 | 141.67 | 118.0583 | 1.760053 | 120.3 | 100.25 | 1.49456 |
| Conductivity | 264.3 | 35.24 | 0.084059 | 140.3 | 46.76667 | 0.278885 | 289.67 | 96.55667 | 0.5758 | 281.3 | 93.76667 | 0.559162 |
| Chlorides | 28.3 | 11.32 | 0.081006 | 31 | 12.4 | 0.088734 | 29 | 11.6 | 0.08301 | 20 | 8 | 0.057248 |
| Hardness | 143.3 | 47.76667 | 0.284849 | 255 | 85 | 0.506883 | 63 | 21 | 0.12523 | 162.3 | 54.1 | 0.322616 |
| DO | 8.07 | 68.02083 | 24.33785 | 11 | 37.5 | 13.4175 | 6.6 | 83.33333 | 29.81667 | 8.27 | 65.9375 | 23.59244 |
| BOD | 8 | 160 | 57.248 | 21.6 | 432 | 154.5696 | 14.5 | 290 | 103.762 | 6.5 | 130 | 46.514 |
| Nitrate-N | 1.57 | 3.488889 | 0.138703 | 1.63 | 3.622222 | 0.144003 | 1.43 | 3.177778 | 0.126334 | 0.737 | 1.637778 | 0.065111 |
| ΣWnQn | 91.79115 | ΣWnQn | 179.4066 | ΣWnQn | 139.0554 | ΣWnQn | 78.63863 | |||||
| WQI | 92.13767 | WQI | 179.4395 | WQI | 139.0808 | WQI | 78.65301 | |||||
| Average WQI =122 | ||||||||||||
| Parameter | Spring | Summer | Autumn | Winter | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vn | Qn | QnWn | Vn | Qn | QnWn | Vn | Qn | QnWn | Vn | Qn | QnWn | |
| pH | 7.83 | 55.33333 | 11.64604 | 8.07 | 71.33333 | 15.01357 | 7.06 | 4 | 0.841882 | 7.03 | 2 | 0.420941 |
| Alkalinity | 110 | 91.66667 | 1.366597 | 101.3 | 84.41667 | 1.258512 | 125 | 104.1667 | 1.552951 | 161.3 | 134.4167 | 2.003928 |
| Conductivity | 236 | 31.46667 | 0.075058 | 166.3 | 55.43333 | 0.330567 | 297.67 | 99.22333 | 0.591702 | 248.3 | 82.76667 | 0.493565 |
| Chlorides | 23.67 | 9.468 | 0.067753 | 28.3 | 11.32 | 0.081006 | 24.67 | 9.868 | 0.070615 | 18.3 | 7.32 | 0.052382 |
| Hardness | 119.67 | 39.89 | 0.237877 | 239.67 | 79.89 | 0.476411 | 60.66 | 20.22 | 0.120579 | 119.3 | 39.76667 | 0.237142 |
| DO | 8.5 | 63.54167 | 22.73521 | 5.4 | 95.83333 | 34.28917 | 6.4 | 85.41667 | 30.56208 | 8 | 68.75 | 24.59875 |
| BOD | 8 | 160 | 57.248 | 11.27 | 225.4 | 80.64812 | 13.3 | 266 | 95.1748 | 7.6 | 152 | 54.3856 |
| Nitrate-N | 1.318 | 2.928889 | 0.11644 | 1.35 | 3 | 0.119267 | 1.27 | 2.822222 | 0.112199 | 0.79 | 1.755556 | 0.069793 |
| ΣWnQn | 93.49297 | ΣWnQn | 132.2166 | ΣWnQn | 129.0268 | ΣWnQn | 82.2621 | |||||
| WQI | 93.84591 | WQI | 132.2408 | WQI | 129.0504 | WQI | 82.27715 | |||||
| Average WQI =109 | ||||||||||||
| Parameter | Spring | Summer | Autumn | Winter | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vn | Qn | QnWn | Vn | Qn | QnWn | Vn | Qn | QnWn | Vn | Qn | QnWn | |
| pH | 8.1 | 73.33333 | 15.43451 | 8.53 | 102 | 21.468 | 7.47 | 31.33333 | 6.594745 | 7.47 | 31.33333 | 6.594745 |
| Alkalinity | 140.3 | 116.9167 | 1.743033 | 130.3 | 108.5833 | 1.618797 | 169 | 140.8333 | 2.09959 | 190.67 | 158.8917 | 2.36881 |
| Conductivity | 221.67 | 29.556 | 0.070501 | 121.3 | 40.43333 | 0.241117 | 318 | 106 | 0.632113 | 233.3 | 77.76667 | 0.463749 |
| Chlorides | 27.3 | 10.92 | 0.078144 | 43 | 17.2 | 0.123083 | 35.3 | 14.12 | 0.101043 | 28.3 | 11.32 | 0.081006 |
| Hardness | 167.83 | 55.94333 | 0.333609 | 378.67 | 126.2233 | 0.752712 | 80.3 | 26.76667 | 0.159619 | 191.3 | 63.76667 | 0.380262 |
| DO | 7.77 | 71.14583 | 25.45598 | 5.6 | 93.75 | 33.54375 | 5.47 | 95.10417 | 34.02827 | 7.53 | 73.64583 | 26.35048 |
| BOD | 13 | 260 | 93.028 | 14 | 280 | 100.184 | 18.5 | 370 | 132.386 | 13 | 260 | 93.028 |
| Nitrate-N | 1.54 | 3.422222 | 0.136052 | 1.62 | 3.6 | 0.14312 | 1.35 | 3 | 0.119267 | 0.97 | 2.155556 | 0.085695 |
| ΣWnQn | 136.2798 | ΣWnQn | 158.0746 | ΣWnQn | 176.1206 | ΣWnQn | 129.3527 | |||||
| WQI | 136.7943 | WQI | 158.1035 | WQI | 176.1529 | WQI | 129.3764 | |||||
| Average WQI =150 | ||||||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, T.; Gupta, G.; Sharma, A.; Kaur, B.; Alsahli, A.A.; Ahmad, P. Multivariate Statistical Approach to Study Spatiotemporal Variations in Water Quality of aHimalayan Urban Fresh Water Lake. Water 2020, 12, 2365. https://doi.org/10.3390/w12092365
Ahmad T, Gupta G, Sharma A, Kaur B, Alsahli AA, Ahmad P. Multivariate Statistical Approach to Study Spatiotemporal Variations in Water Quality of aHimalayan Urban Fresh Water Lake. Water. 2020; 12(9):2365. https://doi.org/10.3390/w12092365
Chicago/Turabian StyleAhmad, Tawseef, Gaganjot Gupta, Anshula Sharma, Baljinder Kaur, Abdulaziz Abdullah Alsahli, and Parvaiz Ahmad. 2020. "Multivariate Statistical Approach to Study Spatiotemporal Variations in Water Quality of aHimalayan Urban Fresh Water Lake" Water 12, no. 9: 2365. https://doi.org/10.3390/w12092365
APA StyleAhmad, T., Gupta, G., Sharma, A., Kaur, B., Alsahli, A. A., & Ahmad, P. (2020). Multivariate Statistical Approach to Study Spatiotemporal Variations in Water Quality of aHimalayan Urban Fresh Water Lake. Water, 12(9), 2365. https://doi.org/10.3390/w12092365







