Degradation of Hexacyanoferrate (III) from Gold Mining Wastewaters via UV-A/LED Photocatalysis Using Modified TiO2 P25
Abstract
1. Introduction
2. Materials and Methods
2.1. Catalyst Treatment
2.2. Catalyst Evaluation
- (1)
- Variation of the catalyst dose (0.1, 0.3, 0.5 and 0.7 g/L) to determine the best performing catalyst dose, at oxic conditions for an hour.
- (2)
- Comparison of reactions (during two hours) under anoxic and oxic conditions, using the best performing catalyst dose selected in the previous stage to select the best conditions for the following experiments: oxic (air) or anoxic (nitrogen).
- (3)
- Variation of the radiation intensity, by testing the power supplied by the LEDs (10, 20 and 30 W) during 3 h of reaction.
- (4)
- Variation of the initial concentration of the contaminant (50–100 ppm) during three hours of reaction.
2.3. Characterization
2.4. Estimation of the Electric Oxidation Efficiency (EEo)
3. Results and Discussion
3.1. Photolysis and Adsorption
3.2. Evaluation of Synthesized Materials
3.2.1. Catalyst Load
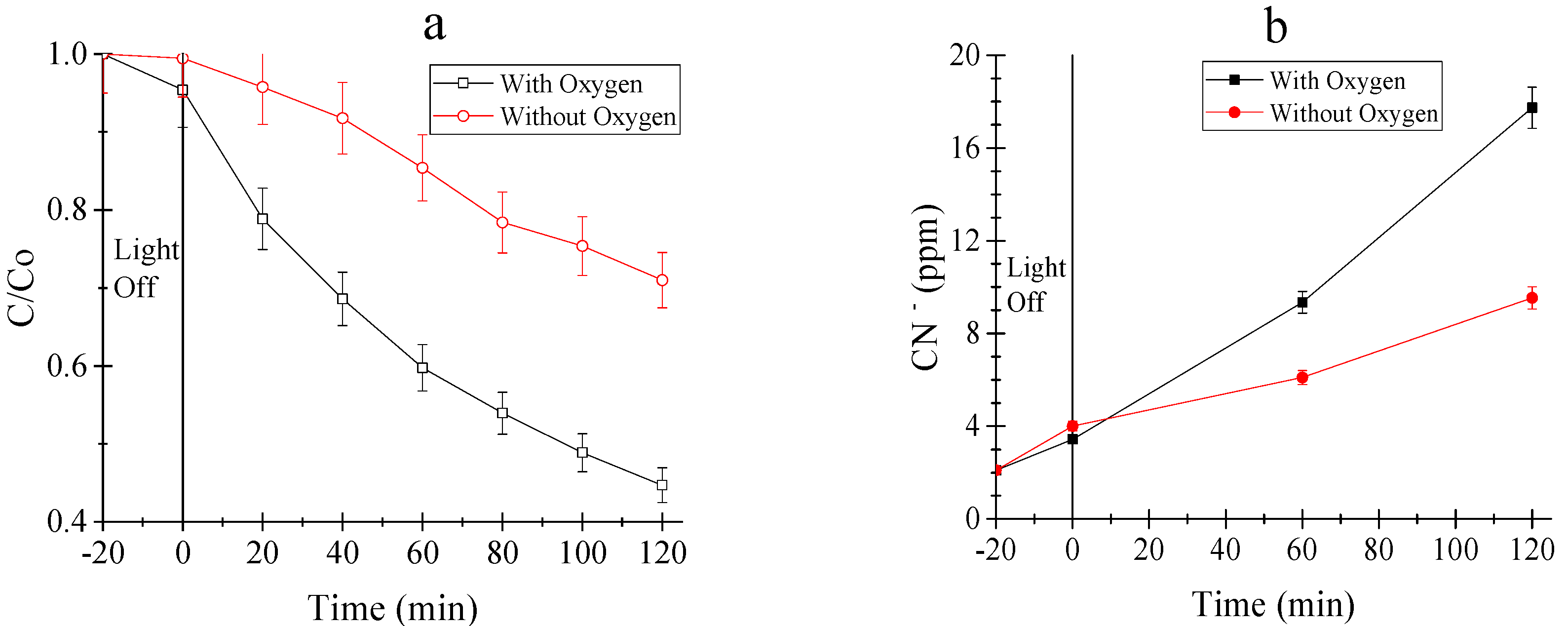
3.2.2. Tests Under Oxic and Anoxic Conditions
3.2.3. Effect of the Radiation Intensity
3.2.4. Comparison between Modified TiO2 and the Raw P25
3.2.5. Electric Oxidation Efficiency
3.3. Characterization of the Photocatalyst
3.3.1. Fourier-Transform Infrared Spectroscopy (FT-IR)
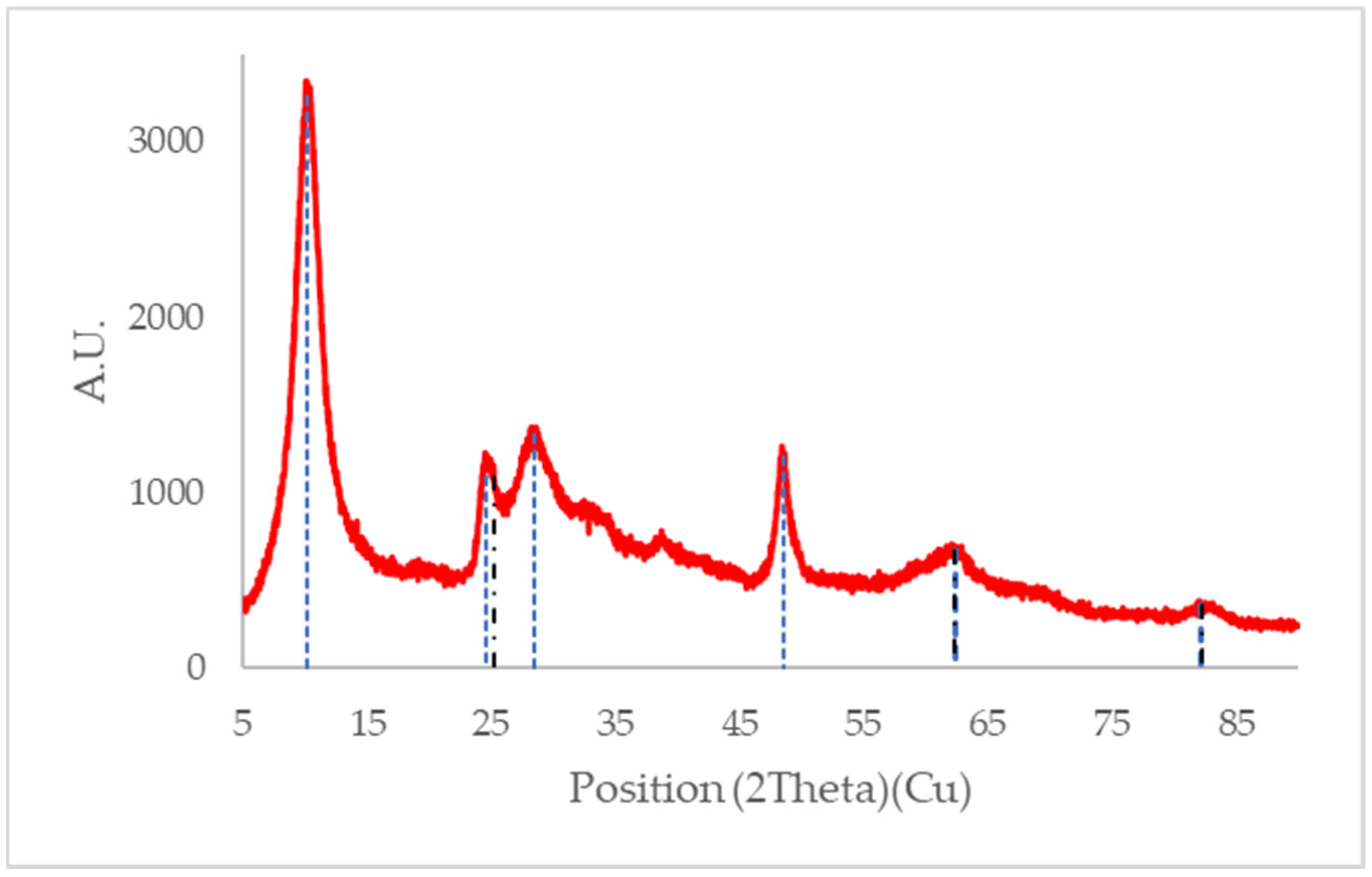
3.3.2. XRD Results
3.3.3. EDS Results
3.3.4. Surface Area Results
3.3.5. Bandgap Energy Estimation by DRS
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moran, R. El Cianuro en la Minería: Algunas Observaciones sobre la Química, Toxicidad y Análisis de las Aguas Asociadas con la Minería. Ecología 1999, 99, 23. [Google Scholar]
- Kuyucak, N.; Akcil, A. Cyanide and Removal Options from Effluents in Gold Mining and Metallurgical Processes. Miner. Eng. 2013, 50–51, 13–29. [Google Scholar] [CrossRef]
- López-Vásquez, A.F.; Colina-Márquez, J.A.; Machuca-Martínez, F. Multivariable analysis of 2,4-d herbicide photocatalytic degradation. DYNA 2011, 78, 119–125. [Google Scholar]
- Pichat, P. A brief survey of the practicality of using photocatalysis to purify the ambient air (indoors or outdoors) or air effluents. Appl. Catal. B 2019, 245, 770–776. [Google Scholar] [CrossRef]
- Lozano-Morales, S.; Morales, G.; Lopez Zavala, M.; Arce, A.; Machuca, F. Photocatalytic Treatment of Paracetamol Using TiO2 Nanotubes: Effect of pH. Processes 2019, 7, 319. [Google Scholar] [CrossRef]
- Arce-Sarria, A.; Machuca-Martínez, F.; Bustillo-Lecompte, C.; Hernández-Ramírez, A.; Colina-Márquez, J. Degradation and Loss of Antibacterial Activity of Commercial Amoxicillin with TiO2/WO3-Assisted Solar Photocatalysis. Catalysts 2018, 8, 222. [Google Scholar] [CrossRef]
- Sulka, G.D.; Brzózka, A.; Liu, L. Fabrication of diameter-modulated and ultrathin porous nanowires in anodic aluminum oxide templates. Electrochim. Acta 2011, 56, 4972–4979. [Google Scholar] [CrossRef]
- Soto Rodriguez, P.E.D.; Olivares, F.; Gómez-Ruiz, S.; Cabrera, G.; Villalonga, R.; Segura del Río, R. Functionalized carbon nanotubes decorated with fluorine-doped titanium dioxide nanoparticles on silicon substrate as template for titanium dioxide film photo-anode grown by chemical vapour deposition. Thin Solid Film. 2018, 656, 30–36. [Google Scholar] [CrossRef]
- Wang, H.; Guo, Z.; Wang, S.; Liu, W. One-dimensional titania nanostructures: Synthesis and applications in dye-sensitized solar cells. Thin Solid Film. 2014, 558, 1–19. [Google Scholar] [CrossRef]
- Byrappa, K.; Adschiri, T. Hydrothermal technology for nanotechnology. Prog. Cryst. Growth Charact. Mater. 2007, 53, 117–166. [Google Scholar] [CrossRef]
- Wang, Y.; He, Y.; Lai, Q.; Fan, M. Review of the progress in preparing nano TiO2: An important environmental engineering material. J. Environ. Sci. 2014, 26, 2139–2177. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.L.; Lim, S.; Ong, H.C.; Chong, W.T. A critical review on the recent progress of synthesizing techniques and fabrication of TiO2-based nanotubes photocatalysts. Appl. Catal. A 2014, 481, 127–142. [Google Scholar] [CrossRef]
- Kasuga, T.; Hiramatsu, M.; Hoson, A.; Sekino, T.; Niihara, K. Titania Nanotubes Prepared by Chemical Processing. Adv. Mater. 1999, 11, 1307–1311. [Google Scholar] [CrossRef]
- Suzuki, Y.; Yoshikawa, S. Synthesis and Thermal Analyses of TiO2-Derived Nanotubes Prepared by the Hydrothermal Method. J. Mater. Res. 2004, 19, 982–985. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Libera, J.A.; Yoshimura, M. Hydrothermal synthesis of multiwall carbon nanotubes. J. Mater. Res. 2000, 15, 2591–2594. [Google Scholar] [CrossRef]
- Nguyen Phan, T.-D.; Pham, H.-D.; Viet Cuong, T.; Jung Kim, E.; Kim, S.; Woo Shin, E. A simple hydrothermal preparation of TiO2 nanomaterials using concentrated hydrochloric acid. J. Cryst. Growth 2009, 312, 79–85. [Google Scholar] [CrossRef]
- Yoshimura, M.; Byrappa, K. Hydrothermal Processing of Materials: Past, Present and Future. J. Mater. Sci. 2007, 43, 2085–2103. [Google Scholar] [CrossRef]
- Dolat, D.; Quici, N.; Kusiak-Nejman, E.; Morawski, A.W.; Li Puma, G. One-step, hydrothermal synthesis of nitrogen, carbon co-doped titanium dioxide (N,CTiO2) photocatalysts. Effect of alcohol degree and chain length as carbon dopant precursors on photocatalytic activity and catalyst deactivation. Appl. Catal. B 2012, 115–116, 81–89. [Google Scholar] [CrossRef]
- Nguyen-Phan, T.-D.; Pham, V.H.; Chung, J.S.; Chhowalla, M.; Asefa, T.; Kim, W.-J.; Shin, E.W. Photocatalytic performance of Sn-doped TiO2/reduced graphene oxide composite materials. Appl. Catal. A 2014, 473, 21–30. [Google Scholar] [CrossRef]
- Li, Z.; Hou, B.; Xu, Y.; Wu, D.; Sun, Y.; Hu, W.; Deng, F. Comparative study of sol–gel-hydrothermal and sol–gel synthesis of titania–silica composite nanoparticles. J. Solid State Chem. 2005, 178, 1395–1405. [Google Scholar] [CrossRef]
- Andersson, M.; Österlund, L.; Ljungström, S.; Palmqvist, A. Preparation of Nanosize Anatase and Rutile TiO2 by Hydrothermal Treatment of Microemulsions and Their Activity for Photocatalytic Wet Oxidation of Phenol. J. Phys. Chem. B 2002, 106, 10674–10679. [Google Scholar] [CrossRef]
- Liu, N.; Chen, X.; Zhang, J.; Schwank, J.W. A review on TiO2-based nanotubes synthesized via hydrothermal method: Formation mechanism, structure modification, and photocatalytic applications. Catal. Today 2014, 225, 34–51. [Google Scholar] [CrossRef]
- Huang, K.-C.; Chien, S.-H. Improved visible-light-driven photocatalytic activity of rutile/titania-nanotube composites prepared by microwave-assisted hydrothermal process. Appl. Catal. B 2013, 140–141, 283–288. [Google Scholar] [CrossRef]
- Camposeco, R.; Castillo, S.; Mejía-Centeno, I.; Navarrete, J.; Gomez, R. Effect of the Ti/Na molar ratio on the acidity and the structure of TiO2 nanostructures: Nanotubes, nanofibers and nanowires. Mater. Charact. 2014, 90, 113–120. [Google Scholar] [CrossRef]
- Van Grieken, R.; Aguado, J.; López-Muñoz, M.-J.; Marugán, J. Photocatalytic degradation of iron–cyanocomplexes by TiO2 based catalysts. Appl. Catal. B 2005, 55, 201–211. [Google Scholar] [CrossRef]
- Betancourt-Buitrago, L.A.; Hernandez-Ramirez, A.; Colina-Marquez, J.A.; Bustillo-Lecompte, C.F.; Rehmann, L.; Machuca-Martinez, F. Recent Developments in the Photocatalytic Treatment of Cyanide Wastewater: An Approach to Remediation and Recovery of Metals. Processes 2019, 7, 225. [Google Scholar] [CrossRef]
- Betancourt-Buitrago, L.A.; Ossa-Echeverry, O.E.; Rodriguez-Vallejo, J.C.; Barraza, J.M.; Marriaga, N.; Machuca-Martínez, F. Anoxic photocatalytic treatment of synthetic mining wastewater using TiO2 and scavengers for complexed cyanide recovery. Photochem. Photobiol. Sci. 2019, 18, 853–862. [Google Scholar] [CrossRef]
- Caicedo, D.F.; Brum, I.A.S.; Buitrago, L.A.B. Photocatalytic degradation of ferricyanide as synthetic gold mining wastewater using TiO2 assisted by H2O2. REM-Int. Eng. J. 2020, 73, 99–107. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, S.W.; Lee, G.M.; Lee, B.-T.; Yun, S.-T.; Kim, S.-O. Monitoring of TiO2-catalytic UV-LED photo-oxidation of cyanide contained in mine wastewater and leachate. Chemosphere 2016, 143, 106–114. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, L.; Liu, R.; Gao, Z.; Yang, X.; Tu, Z.; Yang, F.; Ye, Z.; Cui, L.; Xu, C.; et al. Hydrothermal synthesis of N-doped TiO2 nanowires and N-doped graphene heterostructures with enhanced photocatalytic properties. J. Alloy. Compd. 2016, 656, 24–32. [Google Scholar] [CrossRef]
- Procek, M.; Stolarczyk, A.; Pustelny, T.; Maciak, E. A Study of a QCM Sensor Based on TiO₂ Nanostructures for the Detection of NO₂ and Explosives Vapours in Air. Sensors 2015, 15, 9563–9581. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.; Cheng, Z.; Warren, R.; Song, G.; Shen, J.; Lin, L. Highly Efficient Photocatalysts for Surface Hybridization of TiO2 Nanofibers with Carbon Films. ChemPlusChem 2015, 80, 827–831. [Google Scholar] [CrossRef] [PubMed]
- Mozia, S.; Borowiak-Paleń, E.; Przepiórski, J.; Grzmil, B.; Tsumura, T.; Toyoda, M.; Grzechulska-Damszel, J.; Morawski, A.W. Physico-chemical properties and possible photocatalytic applications of titanate nanotubes synthesized via hydrothermal method. J. Phys. Chem. Solids 2010, 71, 263–272. [Google Scholar] [CrossRef]
- Mozia, S. Application of temperature modified titanate nanotubes for removal of an azo dye from water in a hybrid photocatalysis-MD process. Catal. Today 2010, 156, 198–207. [Google Scholar] [CrossRef]
- Betancourt-Buitrago, L.A.; Vásquez, C.; Veitia, L.; Ossa-Echeverry, O.; Rodriguez-Vallejo, J.; Barraza-Burgos, J.; Marriaga-Cabrales, N.; Machuca-Martínez, F. An approach to utilize the artificial high power LED UV-A radiation in photoreactors for the degradation of methylene blue. Photochem. Photobiol. Sci. 2017, 16, 79–85. [Google Scholar] [CrossRef]
- Blanco, J.; Malato, S.; Peral, J.; Sánchez, B.; Cardona, I. Diseño de reactores para fotocatálisis: Evaluación comparativa de las distintas opciones. In Eliminación de Contaminantes por Fotocatálisis Heterogénea; Blesa, M., Ed.; CYTED: Buenos Aires, Argentina, 2001; p. 253. [Google Scholar]
- Rice, E.W.; Bridgewater, L.; American Public Health; American Water Works; Water Environment. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Osathaphan, K.; Ruengruehan, K.; Yngard, R.; Sharma, V. Photocatalytic degradation of Ni(II)-Cyano and Co(III)-cyano complexes. Water Air Soil Pollut. 2013, 224, 1647. [Google Scholar] [CrossRef]
- Samarghandi, M.; Yang, J.-K.; Giahi, O.; Shirzad-Siboni, M. Photocatalytic reduction of hexavalent chromium with illuminated amorphous FeOOH. Environ. Technol. 2014, 36, 1–30. [Google Scholar] [CrossRef]
- Daneshvar, N.; Aleboyeh, A.; Khataee, A.R. The evaluation of electrical energy per order (EEo) for photooxidative decolorization of four textile dye solutions by the kinetic model. Chemosphere 2005, 59, 761–767. [Google Scholar] [CrossRef]
- Shirzad-Siboni, M.; Farrokhi, M.; Darvishi Cheshmeh Soltani, R.; Khataee, A.; Tajassosi, S. Photocatalytic Reduction of Hexavalent Chromium over ZnO Nanorods Immobilized on Kaolin. Ind. Eng. Chem. Res. 2014, 53, 1079–1087. [Google Scholar] [CrossRef]
- Barakat, M.; Chen, Y.T.; Huang, C.P. Removal of toxic cyanide and Cu(II) Ions from water by illuminated TiO. Appl. Catal. B 2004, 53, 13–20. [Google Scholar] [CrossRef]
- Colina-Márquez, J.; Machuca, F.; Li Puma, G. Radiation Absorption and Optimization of Solar Photocatalytic Reactors for Environmental Applications. Environ. Sci. Technol. 2010, 44, 5112–5120. [Google Scholar] [CrossRef] [PubMed]
- Mueses, M.A.; Machuca-Martinez, F.; Li Puma, G. Effective quantum yield and reaction rate model for evaluation of photocatalytic degradation of water contaminants in heterogeneous pilot-scale solar photoreactors. Chem. Eng. J. 2013, 215–216, 937–947. [Google Scholar] [CrossRef]
- Osathaphan, K.; Chucherdwatanasak, B.; Rachdawong, P.; Sharma, V.K. Photocatalytic oxidation of cyanide in aqueous titanium dioxide suspensions: Effect of ethylenediaminetetraacetate. Sol. Energy 2008, 82, 1031–1036. [Google Scholar] [CrossRef]
- Yang, J.-K.; Lee, S.-M.; Farrokhi, M.; Giahi, O.; Shirzad-Siboni, M. Photocatalytic removal of Cr(VI) with illuminated TiO2. Desalin. Water Treat. 2012, 46, 1–6. [Google Scholar] [CrossRef]
- Ku, Y.; Jung, I.-L. Photocatalytic reduction of Cr(VI) in aqueous solutions by UV irradiation with the presence of titanium dioxide. Water Res. 2001, 35, 135–142. [Google Scholar] [CrossRef]
- Thennarasu, S.; Rajasekar, K.; Balkis Ameen, K. Hydrothermal temperature as a morphological control factor: Preparation, characterization and photocatalytic activity of titanate nanotubes and nanoribbons. J. Mol. Struct. 2013, 1049, 446–457. [Google Scholar] [CrossRef]
- White, L.; Koo, Y.; Yun, Y.; Sankar, J. TiO2 Deposition on AZ31 Magnesium Alloy Using Plasma Electrolytic Oxidation. J. Nanomater. 2013, 2013, 319437. [Google Scholar] [CrossRef]
- Turki, A.; Kochkar, H.; Guillard, C.; Berhault, G.; Ghorbel, A. Effect of Na content and thermal treatment of titanate nanotubes on the photocatalytic degradation of formic acid. Appl. Catal. B 2013, 138–139, 401–415. [Google Scholar] [CrossRef]
- Sikhwivhilu, L.; Sinha Ray, S.; Coville, N. Influence of bases on hydrothermal synthesis of titanate nanostructures. Appl. Phys. A 2009, 94, 963–973. [Google Scholar] [CrossRef]
- Fen, L.B.; Han, T.K.; Nee, N.M.; Ang, B.C.; Johan, M.R. Physico-chemical properties of titania nanotubes synthesized via hydrothermal and annealing treatment. Appl. Surf. Sci. 2011, 258, 431–435. [Google Scholar] [CrossRef]
- Camposeco, R.; Castillo, S.; Navarrete, J.; Gomez, R. Synthesis, characterization and photocatalytic activity of TiO2 nanostructures: Nanotubes, nanofibers, nanowires and nanoparticles. Catal. Today 2016, 266, 90–101. [Google Scholar] [CrossRef]
- López, R.; Gomez, R. Band-Gap Energy Estimation from Diffuse Reflectance Measurements on Sol–Gel and Commercial TiO2: A Comparative Study. J. Sol-Gel Sci. Technol. 2011, 61, 1–7. [Google Scholar] [CrossRef]

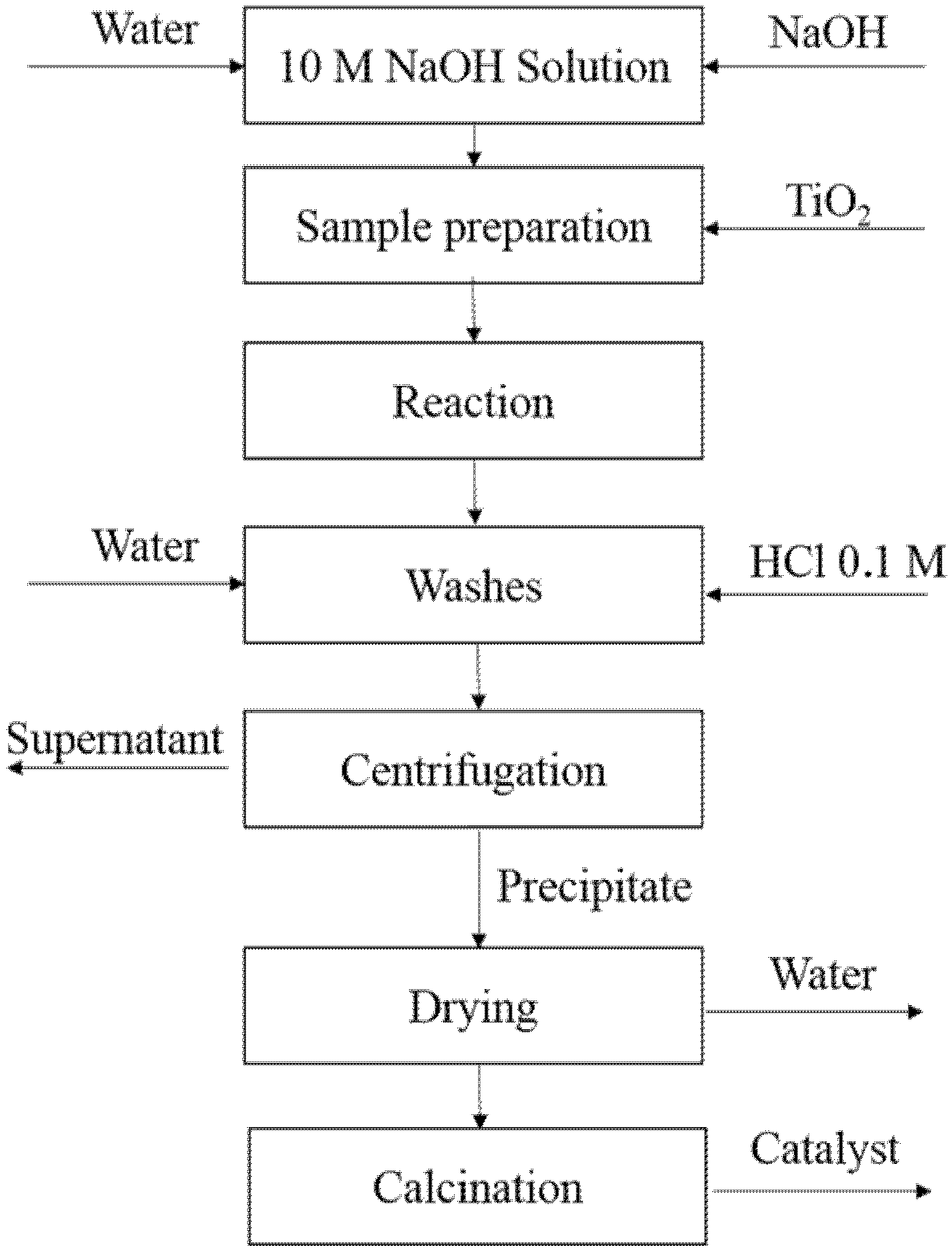









| Labels | Reaction Temperature (°C) | Reaction Time (h) | Calcination Temperature (°C) |
|---|---|---|---|
| SL400 | 120 (S) | 24 (L) | 400 |
| SL500 | 120 (S) | 24 (L) | 500 |
| SH400 | 120 (S) | 72 (H) | 400 |
| SH500 | 120 (S) | 72 (H) | 500 |
| LL400 | 180 (L) | 24 (L) | 400 |
| LL500 | 180 (L) | 24 (L) | 500 |
| LH400 | 180 (L) | 72 (H) | 400 |
| LH500 | 180 (L) | 72 (H) | 500 |
| Calcination Temperature | 400 °C | 500 °C | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Synth. Time | 24 (L) | 72 (H) | 24 (L) | 72 (H) | |||||
| Synthesis Temp. | |||||||||
| SL400 | SH400 | SL500 | SH500 | ||||||
| 120 °C (S) | 53 | 51 | 50 | 50 | 49 | 48 | 47 | 49 | |
| LL400 | LH400 | LL500 | LH500 | ||||||
| 180 °C (L) | 50 | 48 | 48 | 50 | 43 | 44 | 51 | 50 | |
| Source of Variation | SS | df | MS | F | p-Value |
|---|---|---|---|---|---|
| A: Calcination Temperature | 225.625 | 1 | 225.625 | 10.62 | 0.0116 |
| B: Synthesis time | 50.625 | 1 | 50.625 | 2.38 | 0.1613 |
| C: Synthesis temperature | 105.625 | 1 | 105.625 | 4.97 | 0.0563 |
| AB | 180.625 | 1 | 180.625 | 8.50 | 0.0194 |
| AC | 0.5625 | 1 | 0.5625 | 0.26 | 0.6208 |
| BC | 225.625 | 1 | 225.625 | 10.62 | 0.0116 |
| Blocks | 0.0625 | 1 | 0.0625 | 0.03 | 0.8681 |
| Total error | 17.0 | 8 | 2.125 | ||
| Total (corr.) | 964.375 | 15 |
| Initial Concentration (ppm) | Catalyst Type | Apparent Reaction Rate Constant |
|---|---|---|
| 100 | P25 | 0.1924 |
| 100 | SL400 | 0.1679 |
| 50 | P25 | 0.211 |
| 50 | SL400 | 0.1977 |
| Catalyst Type | Voltage (V)/Amperage (A) | Initial Concentration of Contaminant | EEo (kWh/m3) |
|---|---|---|---|
| P25 | 30/0.8 | 100 | 7.98 |
| SL400 | 30/0.8 | 100 | 9.15 |
| P25 | 30/0.8 | 50 | 7.28 |
| SL400 | 30/0.8 | 50 | 7.77 |
| Spectra | C (%) | O (%) | Na (%) | Ti (%) | Total (%) |
|---|---|---|---|---|---|
| 1 | - | 34.87 | 5.13 | 60.00 | 100 |
| 2 | 4.62 | 44.52 | 7.37 | 43.49 | 100 |
| 3 | 4.56 | 43.86 | 6.22 | 45.36 | 100 |
| 4 | 3.18 | 47.63 | 7.69 | 41.50 | 100 |
| 5 | 5.67 | 46.00 | 7.02 | 41.31 | 100 |
| Max | 5.67 | 47.63 | 7.69 | 60.00 | |
| Min | 3.18 | 34.87 | 5.13 | 41.31 | |
| Average | 3.61 | 43.38 | 6.69 | 46.33 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arce-Sarria, A.; Aldana-Villegas, K.M.; Betancourt-Buitrago, L.A.; Colina-Márquez, J.Á.; Machuca-Martínez, F.; Mueses, M.A. Degradation of Hexacyanoferrate (III) from Gold Mining Wastewaters via UV-A/LED Photocatalysis Using Modified TiO2 P25. Water 2020, 12, 2531. https://doi.org/10.3390/w12092531
Arce-Sarria A, Aldana-Villegas KM, Betancourt-Buitrago LA, Colina-Márquez JÁ, Machuca-Martínez F, Mueses MA. Degradation of Hexacyanoferrate (III) from Gold Mining Wastewaters via UV-A/LED Photocatalysis Using Modified TiO2 P25. Water. 2020; 12(9):2531. https://doi.org/10.3390/w12092531
Chicago/Turabian StyleArce-Sarria, Augusto, Kevin Mauricio Aldana-Villegas, Luis Andres Betancourt-Buitrago, Jose Ángel Colina-Márquez, Fiderman Machuca-Martínez, and Miguel Angel Mueses. 2020. "Degradation of Hexacyanoferrate (III) from Gold Mining Wastewaters via UV-A/LED Photocatalysis Using Modified TiO2 P25" Water 12, no. 9: 2531. https://doi.org/10.3390/w12092531
APA StyleArce-Sarria, A., Aldana-Villegas, K. M., Betancourt-Buitrago, L. A., Colina-Márquez, J. Á., Machuca-Martínez, F., & Mueses, M. A. (2020). Degradation of Hexacyanoferrate (III) from Gold Mining Wastewaters via UV-A/LED Photocatalysis Using Modified TiO2 P25. Water, 12(9), 2531. https://doi.org/10.3390/w12092531








