Functionalized PET Waste Based Low-Cost Adsorbents for Adsorptive Removal of Cu(II) Ions from Aqueous Media
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Adsorbent Materials Preparation and Characterization
2.3. Adsorption Experiments
3. Results and Discussion
3.1. Adsorptive Characteristics of Functionalized PET Waste Materials
3.2. Modeling of Equilibrium Data
3.3. FTIR Spectra Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Morin-Crini, N. Conventional and non-conventional adsorbents for wastewater treatment. Environ. Chem. Lett. 2019, 17, 195–213. [Google Scholar] [CrossRef]
- Carvalho, J.; Araujo, J.; Castro, F. Alternative Low-cost Adsorbent for Water and Wastewater Decontamination Derived from Eggshell Waste: An Overview. Waste Biomass Valorization 2011, 2, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Ahsan, M.A.; Jabbari, V.; El-Gendy, A.A.; Curry, M.L.; Noveron, J.C. Ultrafast catalytic reduction of environmental pollutants in water via MOF-derived magnetic Ni and Cu nanoparticles encapsulated in porous carbon. Appl. Scurface Sci. 2019, 497, 143608. [Google Scholar] [CrossRef]
- Todaro, F.; Vitone, C.; Notarnicola, M. Stabilization and recycling of contaminated marine sediments. In E3S Web Conferences, Glasgow, UK, 28 June 2019; EDP Sciences: Ulis, France, 2019; Volume 92, p. 11004. [Google Scholar] [CrossRef]
- Frigione, M. Recycling of PET bottles as fine aggregate in concrete. Waste Manag. 2010, 30, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Acar, I.; Orbay, M. Aminoglycolysis of Waste Poly(Ethylene Terephthalate) with Diethanolamine and Evaluation of the Products as Polyurethane Surface Coating Materials. Polym. Eng. Sci. 2011, 51, 746–754. [Google Scholar] [CrossRef]
- Choudhary, K.; Sangwan, K.S.; Goyal, D. Environment and economic impacts assessment of PET waste recycling with conventional and renewable sources of energy. Procedia CIRP 2019, 80, 422–427. [Google Scholar] [CrossRef]
- Mendoza-Carrasco, R.; Cuerda-Correa, E.M.; Alexandre-Franco, M.F.; Fernandez-Gonzalez, C.; Gomez-Serrano, V. Preparation of High-Quality Activated Carbon from Polyethyleneterephthalate (PET) Bottle Waste Its Use in the Removal of Pollutants in Aqueous Solution. J. Environ. Manag. 2016, 181, 522–535. [Google Scholar]
- European Strategy for Plastics. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52013DC0123 (accessed on 4 September 2020).
- Shukla, S.R.; Harad, A.M.; Jawale, L.S. Recycling of waste PET into useful textile auxiliaries. Waste Manag. 2008, 28, 51–56. [Google Scholar] [CrossRef]
- Awaja, F.; Pavel, D. Recycling of PET. Eur. Polym. J. 2005, 41, 1453–1477. [Google Scholar] [CrossRef]
- Geyer, B.; Lorenz, G.; Kandelbauer, A. Recycling of poly(ethylene terephthalate)—A review focusing on chemical methods. Express Polym. Lett. 2016, 10, 559–586. [Google Scholar] [CrossRef]
- Kuczenski, B.; Geyer, R. Material flow analysis of polyethylene terephthalate in the US, 1996–2007. Resour. Conserv. Recycl. 2010, 54, 1161–1169. [Google Scholar] [CrossRef]
- Ingrao, C.; Giudice, A.L.; Tricase, C.; Rana, R.; Mbohwa, C.; Siracusa, V. Recycled-PET fibre based panels for building thermal insulation: Environmental impact and improvement potential assessment for a greener production. Sci. Total Environ. 2014, 493, 914–929. [Google Scholar] [CrossRef] [PubMed]
- Malik, N.; Kumar, P.; Shrivastava, S.; Ghosh, S.B. An overview on PET waste recycling for application in packaging. Int. J. Plast. Technol. 2017, 21, 1–24. [Google Scholar] [CrossRef]
- Naguib, H.M.; Zhang, X.H. Advanced recycled polyester based on PET and oleic acid. Polym. Test. 2018, 69, 450–455. [Google Scholar] [CrossRef]
- Rabindra, Z.L.; Padhan, K.; Sreeram, A. Production of a sustainable paving material through chemical recycling of waste PET into crumb rubber modified asphalt. J. Clean. Prod. 2018, 180, 682–688. [Google Scholar]
- Tavakoli, D.; Hashempour, M.; Heidari, A. Use of Waste Materials in Concrete: A review. Pertanika J. Sci. Technol. 2018, 26, 499–522. [Google Scholar]
- Fu, F.; Wang, Q. Removal of Heavy Metal Ions from Wastewaters: A Review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Wang, J.; Xu, L.; Cheng, C.; Meng, Y.; Li, A. Preparation of new chelating fiber with waste PET as adsorbent for fast removal of Cu2+ and Ni2+ from water: Kinetic and equilibrium adsorption studies. Chem. Eng. J. 2012, 193–194, 31–38. [Google Scholar] [CrossRef]
- Monier, M.; Abdel-Latif, D.A. Modification and characterization of PET fibers for fast removal of Hg(II), Cu(II) and Co(II) metal ions from aqueous solutions. J. Hazard. Mater. 2013, 250–251, 122–130. [Google Scholar] [CrossRef]
- Cojocariu, B.; Mocanu, A.M.; Bulgariu, D.; Nacu, G.; Bulgariu, L. Equilibrium isotherm study of metal ions adsorption on PET recycled fibers. In Proceedings of the 2017 E-Health and Bioengineering Conference (EHB), Sinaia, Romania, 22–24 June 2017; pp. 85–88. [Google Scholar]
- Cojocariu, B.; Mocanu, A.M.; Nacu, G.; Bulgariu, L. Possible utilization of PET waste as adsorbent for Orange G dye removal from aqueous media. Desalinization Water Treat. 2018, 104, 338–345. [Google Scholar] [CrossRef]
- Ungureanu, O.I.; Mocanu, A.M.; Bulgariu, L.A.U.R.A. Simple functionalization methods of PET waste using phenolic compounds. Bull. Polytech. Inst. Sect. Chem. Chem. Eng. 2018, 64, 9–19. [Google Scholar]
- Rangabhashiyam, S.; Anu, N.; Nandagopal Giri, M.S.; Selvaraju, N. Relevance of isotherm models in biosorption of pollutants by agricultural by-products. J. Environ. Chem. Eng. 2014, 2, 398–414. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Daana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A Rev. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef] [PubMed]
- Azizian, S.; Eris, S.; Wilson, L.D. Re-evaluation of the century-old Langmuir isotherm for modeling adsorption phenomena in solution. Chem. Phys. 2018, 513, 99–104. [Google Scholar] [CrossRef]
- Alandis, N.M.; Mekhamer, W.; Aldayel, O.; Hefne, J.A.A.; Alam, M. Adsorptive Applications of Montmorillonite Clay for the Removal of Ag(I) and Cu(II) from Aqueous Medium. J. Chem. 2019, 2019, 7129014. [Google Scholar] [CrossRef] [Green Version]
- Foroutan, R.; Esmaeili, H.; Abbasi, M.; Rezakazemi, M.; Mesbah, M. Adsorption behavior of Cu(II) and Co(II) using chemically modified marine algae. Environ. Technol. 2018, 39, 2792–2800. [Google Scholar] [CrossRef]
- Ahmad, Z.; Gao, B.; Mosa, A.; Yu, H.; Yin, X.; Bashir, A.; Ghoveisi, H.; Wang, S. Removal of Cu(II), Cd(II) and Pb(II) ions from aqueous solutions by biochars derived from potassium-rich biomass. J. Clean. Prod. 2018, 180, 437–449. [Google Scholar] [CrossRef]
- Liu, H.; Feng, S.; Zhang, N.; Du, X.; Liu, Y. Removal of Cu(II) ions from aqueous solution by activated carbon impregnated with humic acid. Front. Environ. Sci. Eng. 2014, 8, 329–336. [Google Scholar] [CrossRef]
- Ozcimen, D.; Ersoy-Mericboyu, A. Removal of copper from aqueous solutionsby adsorption onto chestnut shell and grapeseed activated carbons. J. Hazard. Mater. 2009, 168, 1118–1125. [Google Scholar] [CrossRef]
- Kalavathy, M.H.; Karthikeyan, T.; Rajgopal, S.; Miranda, L.R. Kinetic and isotherm studies of Cu (II) adsorption onto H3PO4-activated rubber wood sawdust. J. Colloid Interface Sci. 2005, 292, 354–362. [Google Scholar] [CrossRef]
- Li, X.; Xu, Q.; Han, G.; Zhu, W.; Chen, Z.; He, X.; Tian, X. Equilibrium and kineticstudies of copper (II) removal by three species of dead fungal biomasses. J. Hazard. Mater. 2009, 165, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Jianlong, W. Biosorption of copper (II) by chemically modified biomass of Saccharomyces cerevisiae. Process Biochem. 2002, 37, 847–850. [Google Scholar] [CrossRef]
- Vilar, V.J.P.; Botelho, C.M.S.; Pinheiro, J.P.S.; Domingos, R.F.; Boaventura, R.A.R. Copper removal by algal biomass: Biosorbents characterization and equilibrium modeling. J. Hazard. Mater. 2009, 163, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Aldrich, C. Adsorption of heavy metals by biomaterials derived from the marine alga Ecklonia maxima. Hydrometallurgy 2004, 73, 1–10. [Google Scholar] [CrossRef]
- Wartelle, L.H.; Marshall, W.E. Citric acid modified agricultural by-products as copper ion adsorbents. Adv. Environ. Res. 2000, 4, 1–7. [Google Scholar] [CrossRef]
- Chen, Z.; Hay, J.N.; Jenkins, M.J. The thermal analysis of poly(ethylene terephthalate) by FTIR spectroscopy. Thermochim. Acta 2013, 552, 123–130. [Google Scholar] [CrossRef]
- Ioakeimidis, C.; Fotopoulou, K.N.; Karapanagioti, H.K.; Geraga, M.; Zeri, C.; Papathanassiou, E.; Galgani, F.; Papatheodorou, G. The degradation potential of PET bottles in the marine environment: An ATR-FTIR based approach. Sci. Rep. 2016, 6, 23501. [Google Scholar] [CrossRef]
- Mecozzi, M.; Nisini, L. The differentiation of biodegradable and non-biodegradable polyethylene terephthalate (PET) samples by FTIR spectroscopy: A potential support for the structural differentiation of PET in environmental analysis. Infrared Phys. Technol. 2019, 101, 119–126. [Google Scholar] [CrossRef]
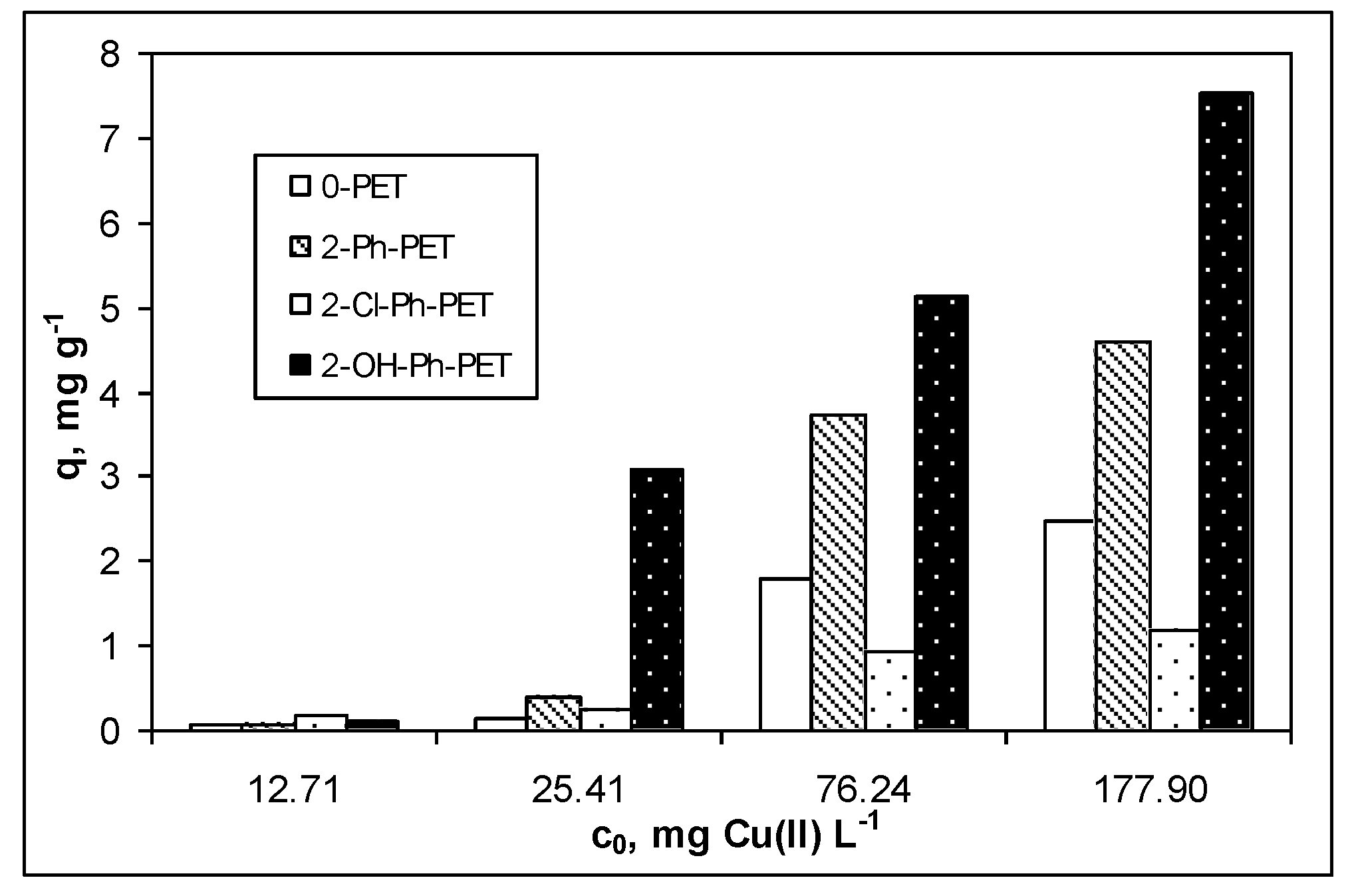
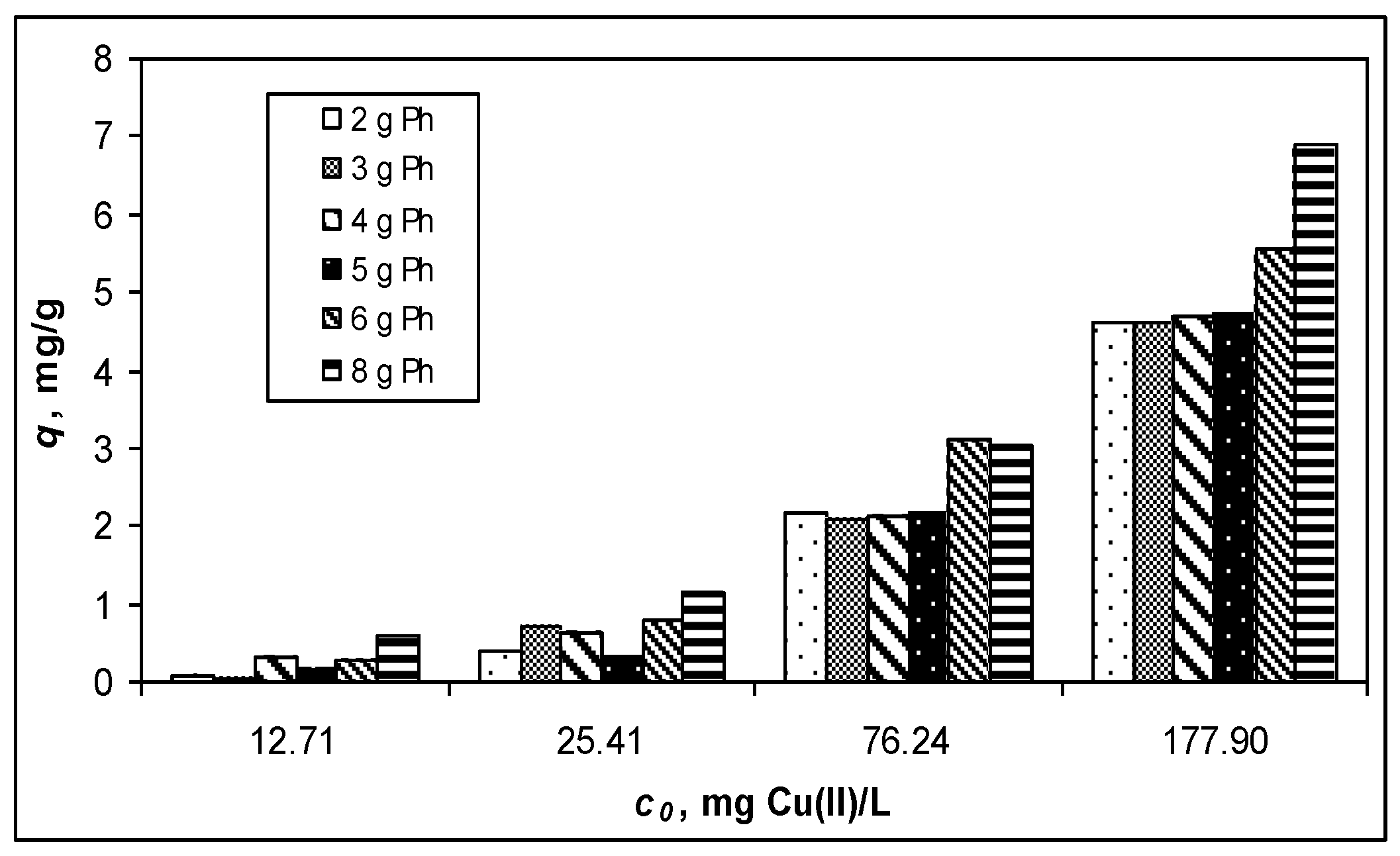
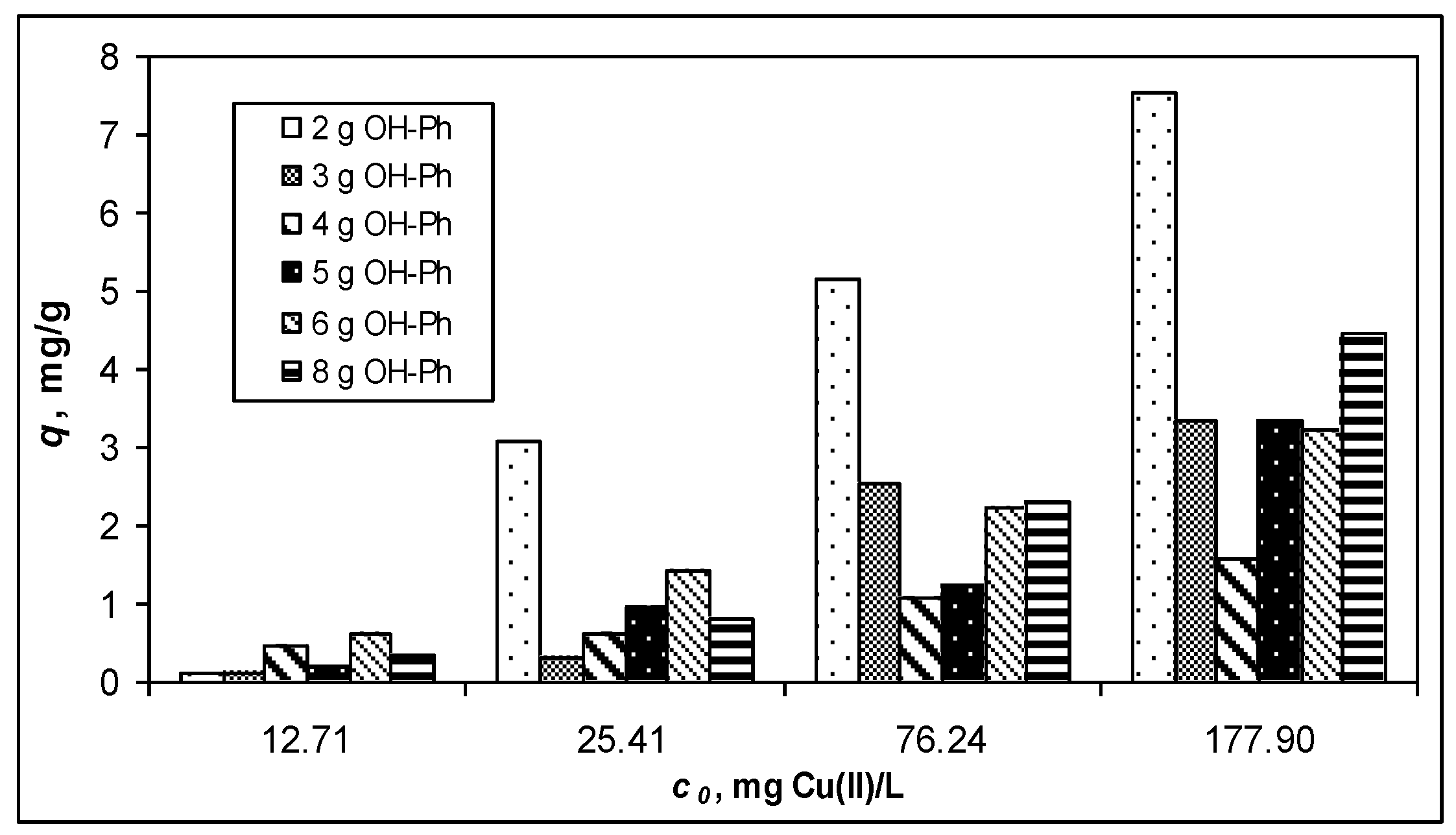
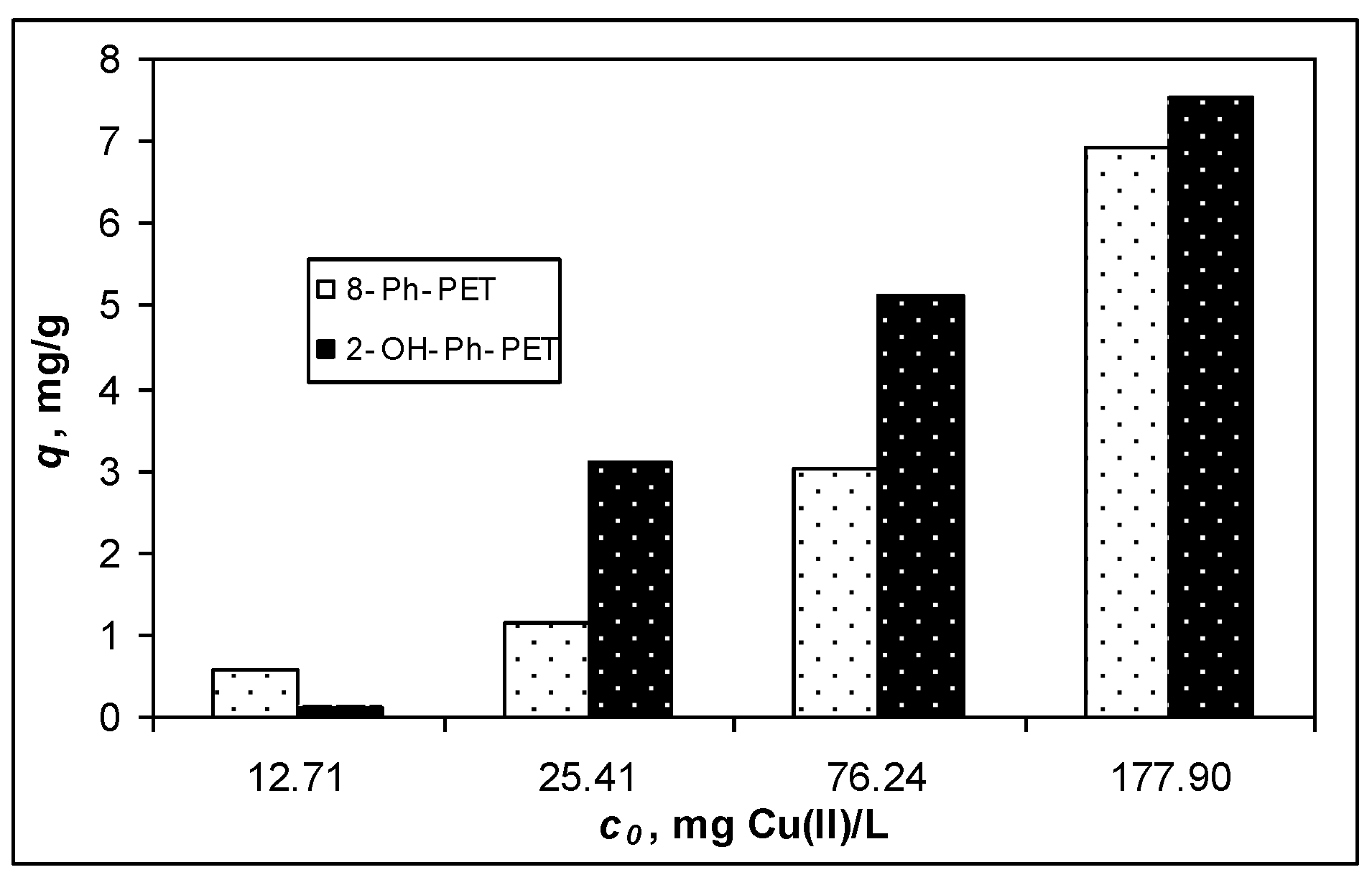


| Isotherm Model | Parameter | 8-Ph-PET | OH-Ph-PET |
|---|---|---|---|
| Langmuir model | R2 | 0.9945 | 0.9971 |
| qmax, mg g−1 | 12.80 | 61.73 | |
| KL, L g−1 | 0.0061 | 0.0009 | |
| Freundlich model | R2 | 0.9264 | 0.8777 |
| n | 1.22 | 1.09 | |
| KF, L g−1 | 0.1078 | 0.0164 |
| Adsorbent | pH | Adsorbent Dosage, g L−1 | Cu(II) | Reference |
|---|---|---|---|---|
| Montmorillonite clay | 6.0 | 5.0 | 32.26 | [28] |
| Bentonite clay | 6.0 | 5.0 | 10.16 | [29] |
| Cauliflower leaves biochar | 6.0 | 5.0 | 53.96 | [30] |
| Commercial activated carbon | 6.5 | 6.0 | 86.32 | [31] |
| Grape seed activated carbon impregnated with ZnCl2 | 5.0 | 25.0 | 48.78 | [32] |
| Activated carbon of rubber wood sawdust | 6.0 | 5.0 | 6.50 | [33] |
| Cladyspoium cladosporioides modified with formaldehyde | 6.0 | 2.0 | 7.74 | [34] |
| Saccharomyces cerevisiae modified with methanol | 5.0 | 1.0 | 4.00 | [35] |
| Algal waste | 5.3 | 1.0 | 15.89 | [36] |
| Ecklonia maxima modified with CaCl2 | 6.0 | - | 85.00 | [37] |
| Soya been hulls activated with NaOH | 6.0 | 10.0 | 91.51 | [38] |
| 8-Ph-PET | 6.5 | 8.0 | 12.80 | This study |
| 2-OH-Ph-PET | 6.5 | 8.0 | 61.73 | This study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ungureanu, O.I.; Bulgariu, D.; Mocanu, A.M.; Bulgariu, L. Functionalized PET Waste Based Low-Cost Adsorbents for Adsorptive Removal of Cu(II) Ions from Aqueous Media. Water 2020, 12, 2624. https://doi.org/10.3390/w12092624
Ungureanu OI, Bulgariu D, Mocanu AM, Bulgariu L. Functionalized PET Waste Based Low-Cost Adsorbents for Adsorptive Removal of Cu(II) Ions from Aqueous Media. Water. 2020; 12(9):2624. https://doi.org/10.3390/w12092624
Chicago/Turabian StyleUngureanu, Oana Ionela, Dumitru Bulgariu, Anca Mihaela Mocanu, and Laura Bulgariu. 2020. "Functionalized PET Waste Based Low-Cost Adsorbents for Adsorptive Removal of Cu(II) Ions from Aqueous Media" Water 12, no. 9: 2624. https://doi.org/10.3390/w12092624
APA StyleUngureanu, O. I., Bulgariu, D., Mocanu, A. M., & Bulgariu, L. (2020). Functionalized PET Waste Based Low-Cost Adsorbents for Adsorptive Removal of Cu(II) Ions from Aqueous Media. Water, 12(9), 2624. https://doi.org/10.3390/w12092624






