Effect of Current and Initial pH on Electrocoagulation in Treating the Distillery Spent Wash with Very High Pollutant Content
Abstract
1. Introduction
2. Materials and Methods
2.1. Distillery Spent Wash
2.2. Experimental Set-Up
2.3. Experimental Design and Procedures
2.4. Analysis
3. Development of Simple Mechanistic Models
3.1. Route 1
3.2. Route 2
4. Results and Discussions
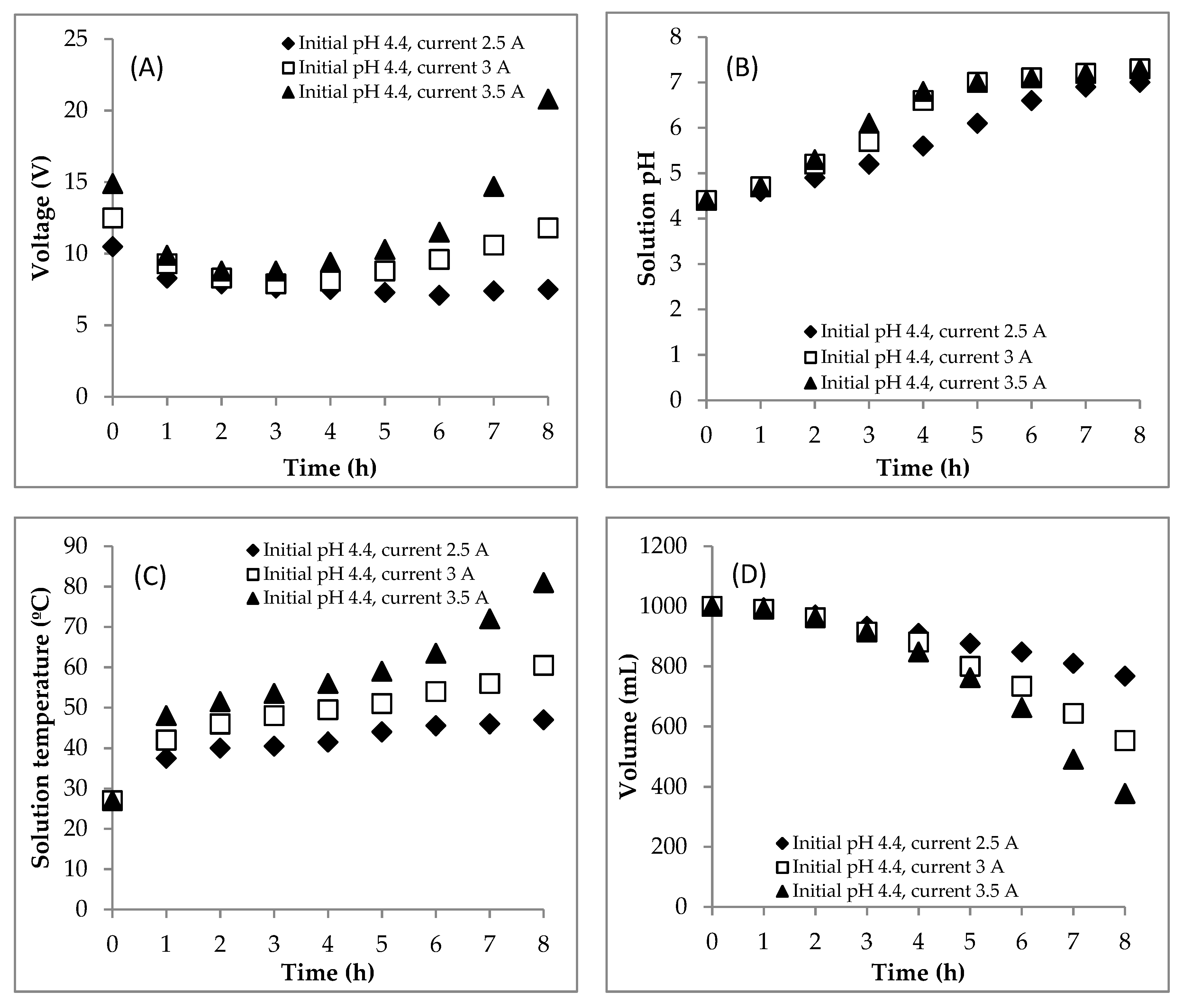
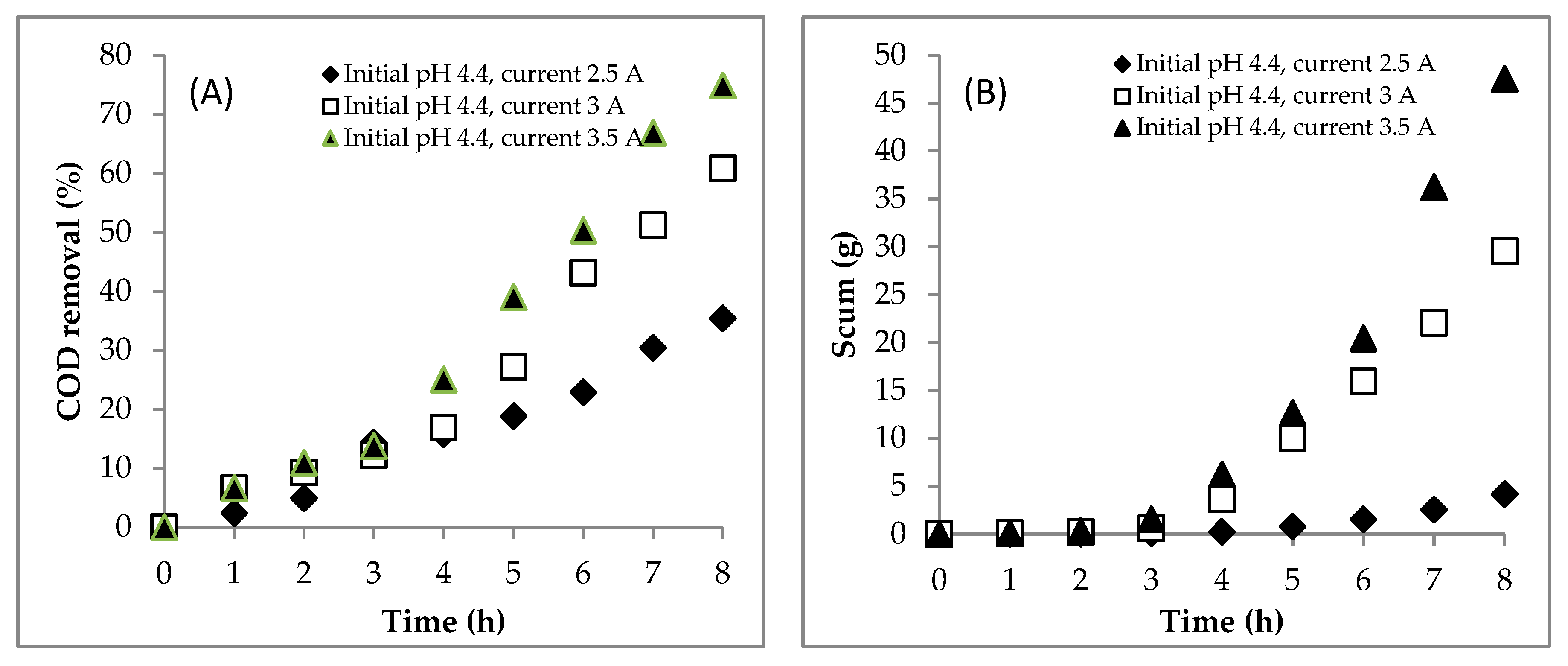
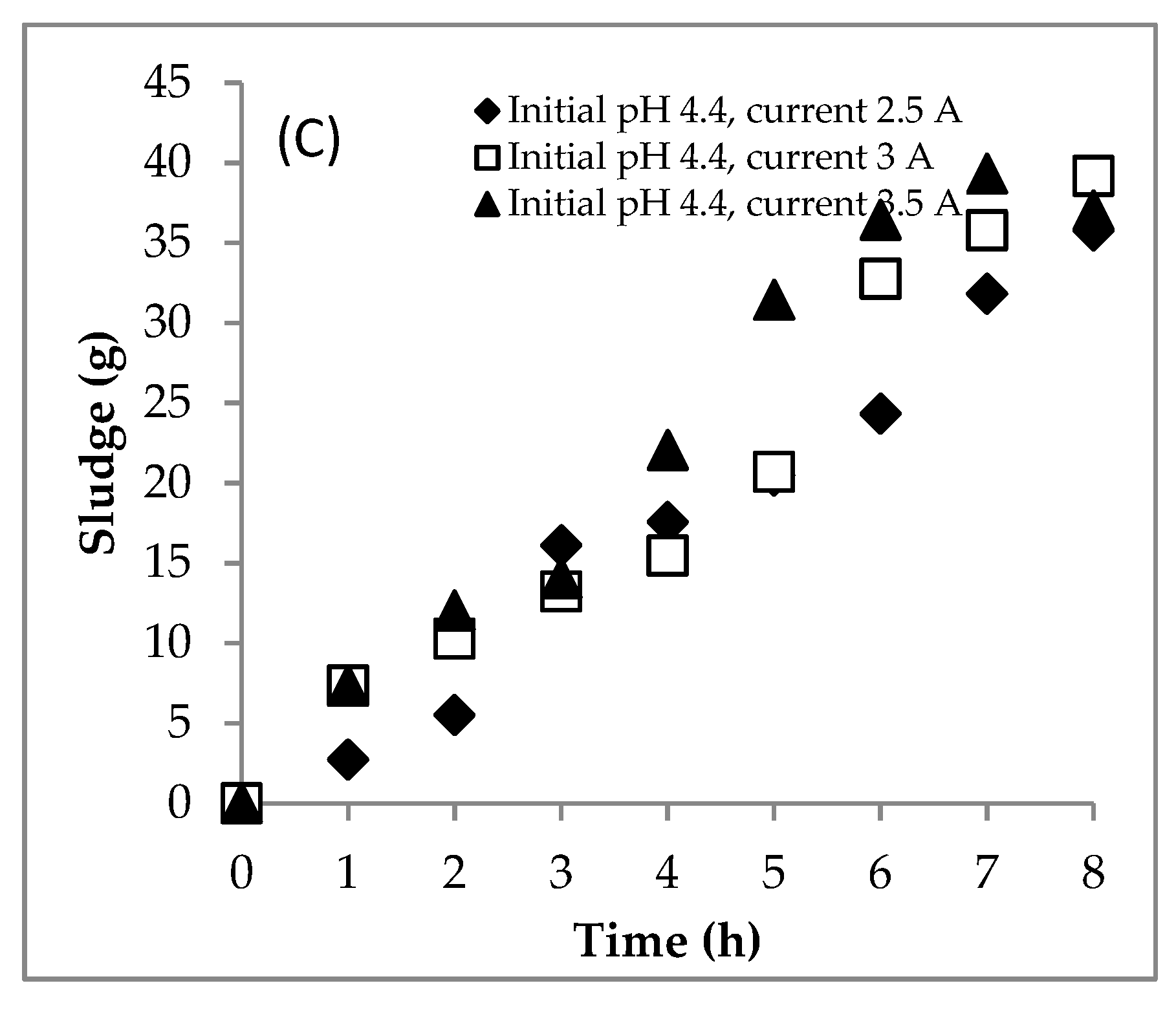
4.1. Section 1: Effect of Electrical Current
4.1.1. Experiment
4.1.2. Modeling
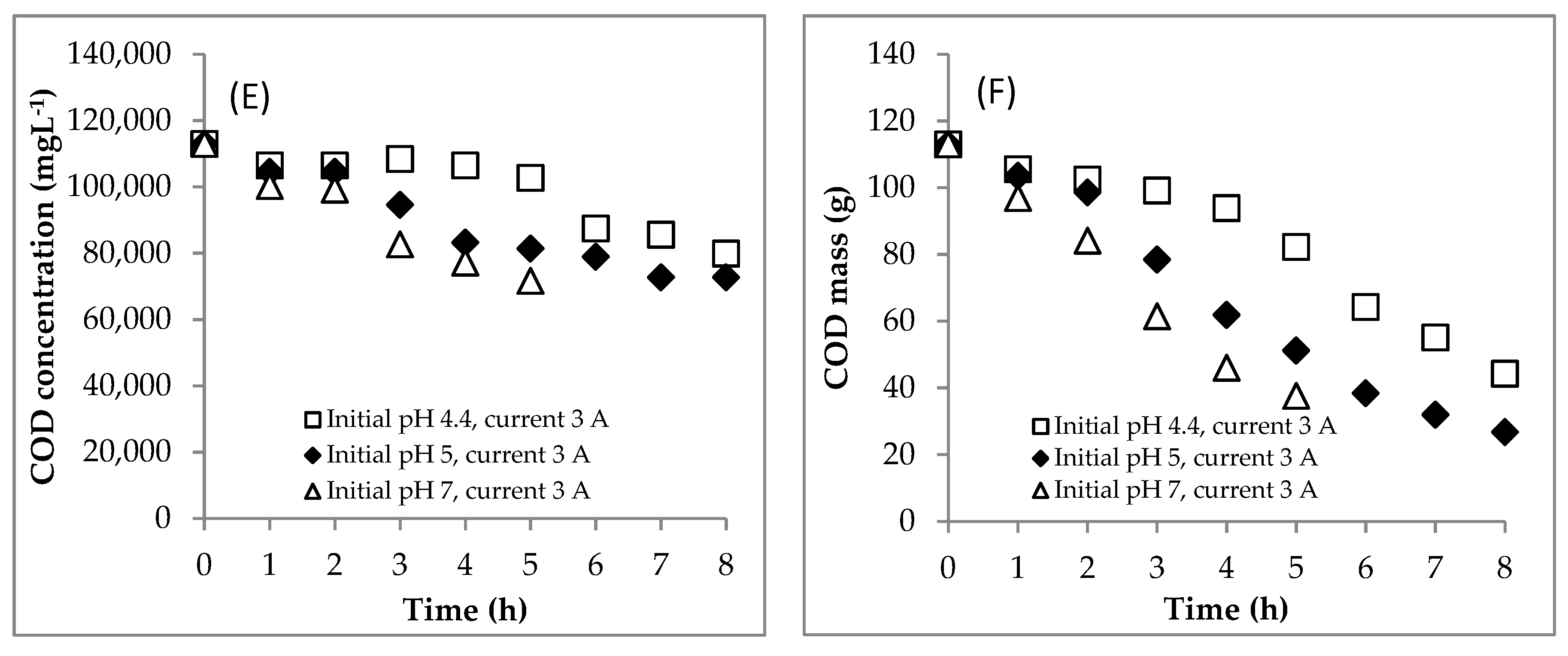
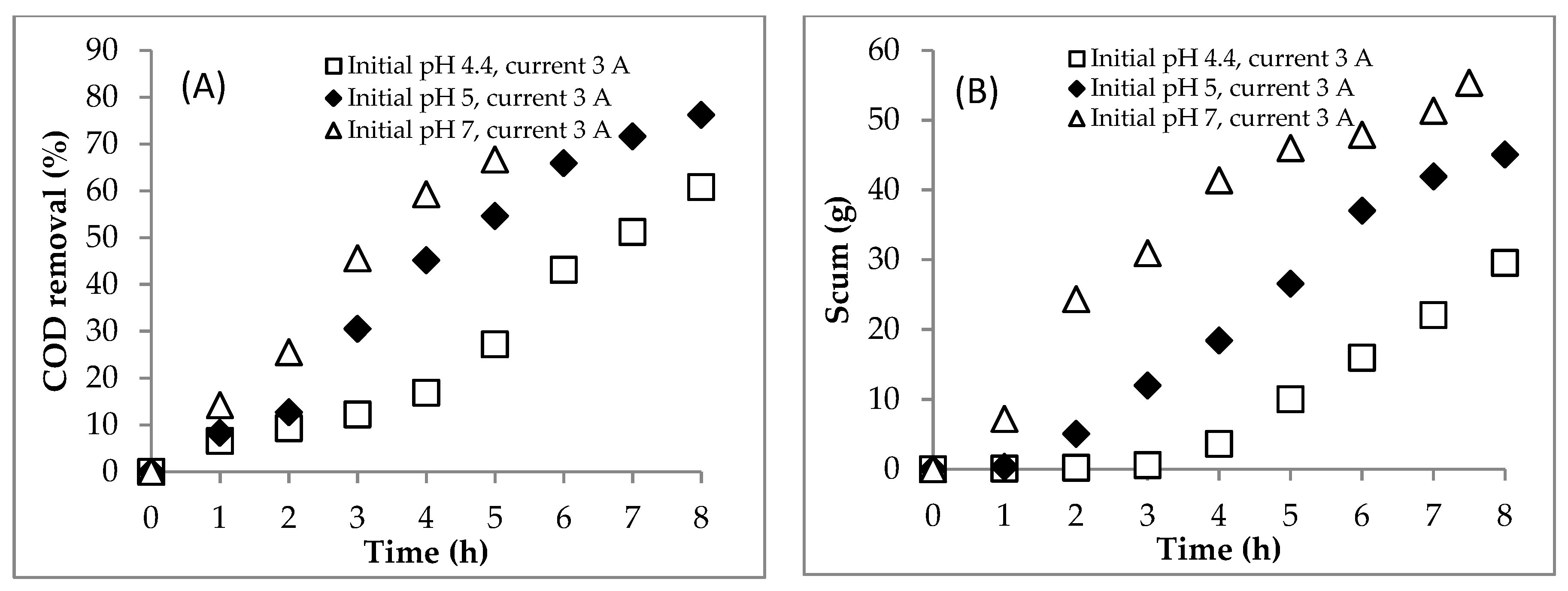
4.2. Section 2: Effect of Initial pH
4.2.1. Experiment
4.2.2. Modeling
4.3. Summary of the EC for Treating the DSW
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclatures
| = mass of COD (g) | |
| = mass of scum (g) | |
| = mass of sludge (g) | |
| = the kinetic constant for sludge formation (h−1) | |
| = the kinetic constant for scum formation (h−1) | |
| = measured data (g) | |
| = predicted data (g) | |
| = average of measured data (g) | |
| SSE | = Sum of Squared Error |
| = the coefficient of determination of the model | |
| = current (A) | |
| = electrolysis time (s) | |
| = active surface of electrode (cm2) | |
| = current density (A cm−2) | |
| = charge loading (C g-COD−1) | |
| = inter-electrode distance (cm) |
References
- Yavuz, Y. EC and EF processes for the treatment of alcohol distillery wastewater. Sep. Purif. Technol. 2007, 53, 135–140. [Google Scholar] [CrossRef]
- Khandegar, V.; Saroha, A.K. Electrocoagulation of distillery spentwash for complete organic reduction. Int. J. Chem. Tech. Res. 2013, 5, 712–718. [Google Scholar]
- Asaithambi, P.; Sajjadi, B.; Aziz, A.; Raman, A.; Daud, W.; Bin, W.M.A. Performance evaluation of hybrid electrocoagulation process parameters for the treatment of distillery industrial effluent. Process Saf. Environ. Prot. 2016, 104, 406–412. [Google Scholar] [CrossRef]
- Reis, C.E.R.; Hu, B. Vinasse from Sugarcane Ethanol Production: Better Treatment or Better Utilization? Front. Energy Res. 2017, 5. [Google Scholar] [CrossRef]
- Prajapati, A.K.; Chaudhari, P.K. Physicochemical Treatment of Distillery Wastewater—A Review. Chem. Eng. Commun. 2015, 202, 1098–1117. [Google Scholar] [CrossRef]
- Budiyono; Syaichurrozi, I.; Sumardiono, S. Effect of total solid content to biogas production rate from vinasse. Int. J. Eng. Trans. B Appl. 2014, 27, 177–184. [Google Scholar]
- Kharayat, Y. Distillery wastewater: Bioremediation approaches. J. Integr. Environ. Sci. 2012, 9, 69–91. [Google Scholar] [CrossRef]
- Syaichurrozi, I.; Sarto, S.; Sediawan, W.B.; Hidayat, M. Mechanistic model of electrocoagulation process for treating vinasse waste: Effect of initial pH. J. Environ. Chem. Eng. 2020, 8, 103756. [Google Scholar] [CrossRef]
- Asaithambi, P.; Susree, M.; Saravanathamizhan, R.; Matheswaran, M. Ozone assisted electrocoagulation for the treatment of distillery effluent. Desalination. 2012, 297, 1–7. [Google Scholar] [CrossRef]
- Aziz, A.R.A.; Asaithambi, P.; Daud, W.M.A.B.W. Combination of electrocoagulation with advanced oxidation processes for the treatment of distillery industrial effluent. Process Saf. Environ. Prot. 2016, 99, 227–235. [Google Scholar] [CrossRef]
- Sharma, P.; Joshi, H. Utilization of electrocoagulation-treated spent wash sludge in making building blocks. Int. J. Environ. Sci. Technol. 2016, 13, 349–358. [Google Scholar] [CrossRef]
- Hashim, K.S.; AlKhaddar, R.; Shaw, A.; Kot, P.; Al-Jumeily, D.; Alwash, R.; Aljefery, M.H. Electrocoagulation as an eco-friendly river water treatment method. In Advances in Water Resources Engineering and Management; Springer: Singapore, 2020; pp. 219–235. [Google Scholar]
- Garcia-Segura, S.; Eiband, M.M.S.G.; de Melo, J.V.; Martínez-Huitle, C.A. Electrocoagulation and advanced electrocoagulation processes: A general review about the fundamentals, emerging applications and its association with other technologies. J. Electroanal. Chem. 2017, 801, 267–299. [Google Scholar] [CrossRef]
- Syaichurrozi, I.; Sarto, S.; Sediawan, W.B.; Hidayat, M. Mechanistic models of electrocoagulation kinetics of pollutant removal in vinasse waste: Effect of voltage. J. Water Process Eng. 2020, 36, 101312. [Google Scholar] [CrossRef]
- Darmadi, D.; Lubis, M.R.; Hizir, H.; Chairunnisak, A.; Arifin, B. Comparison of Palm Oil Mill Effluent Electrocoagulation by Using Fe-Fe and Al-Al Electrodes: Box-Behnken Design. ASEAN J. Chem. Eng. 2018, 18, 30–43. [Google Scholar]
- Largitte, L.; Pasquier, R. A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon. Chem. Eng. Res. Des. 2016, 109, 495–504. [Google Scholar] [CrossRef]
- Ouaissa, Y.A.; Chabani, M.; Amrane, A.; Bensmaili, A. Removal of tetracycline by electrocoagulation: Kinetic and isotherm modeling through adsorption. J. Environ. Chem. Eng. 2014, 2, 177–184. [Google Scholar] [CrossRef]
- Mamelkina, M.A.; Vasilyev, F.; Tuunila, R.; Sillanpaa, M.; Hakkinen, A. Investigation of the parameters affecting the treatment of mining waters by electrocoagulation. J. Water Process. Eng. 2019, 32, 100929. [Google Scholar] [CrossRef]
- Sumardiono, S.; Syaichurrozi, I.; Budiyono; Sasongko, S.B. The Effect of COD/N Ratios and pH Control to Biogas Production from Vinasse. Int. J. Biochem. Res. Rev. 2013, 3, 401–413. [Google Scholar] [CrossRef]
- Naspolini, B.F.; Machado, A.C.D.O.; Junior, W.B.C.; Freire, D.M.G.; Cammarota, M.C. Bioconversion of Sugarcane Vinasse into High-Added Value Products and Energy. BioMed Res. Int. 2017, 2017. [Google Scholar] [CrossRef]
- García-García, I.; Bonilla-Venceslada, J.L.; Jimenez-Pena, P.R.; Ramos-Gomez, E. Biodegradation of phenol compounds in vinasse using aspergillus terreus and geotrichum candidum. War. Res. 1997, 31, 2005–2011. [Google Scholar] [CrossRef]
- Syaichurrozi, I. Review—Biogas Technology to Treat Bioethanol Vinasse. Waste Technol. 2016, 4, 16–23. [Google Scholar] [CrossRef]
- David, C.; Arivazhagan, M.; Tuvakara, F. Decolorization of distillery spent wash effluent byelectrooxidation (EC and EF) and Fenton processes: A comparative study. Ecotoxicol. Environ. Saf. 2015, 121, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Secula, M.S.; Creţescu, I.; Petrescu, S. An experimental study of indigo carmine removal from aqueous solution by electrocoagulation. Desalination. 2011, 277, 227–235. [Google Scholar] [CrossRef]
- Elazzouzi, M.; Haboubi, K.; Elyoubi, M.S. Electrocoagulation flocculation as a low-costprocess for pollutants removal from urbanwastewater. Chem. Eng. Res. Des. 2017, 117, 614–626. [Google Scholar] [CrossRef]
- Kobya, M.; Can, O.T.; Bayramoglu, M. Treatment of textile wastewaters by electrocoagulation using iron and aluminum electrodes. J. Hazard. Mat. 2003, B100, 163–178. [Google Scholar] [CrossRef]
- Kim, K.-J.; Baek, K.; Ji, S.; Cheong, Y.; Yim, G.; Jang, A. Study on electrocoagulation parameters (current density, pH, and electrode distance) for removal of fluoride from groundwater. Environ. Earth Sci. 2016, 75, 45. [Google Scholar] [CrossRef]
- Giwa, S.O.; Polat, K.; Hapoglu, H. The Effects of Operating Parameters on Temperature and Electrode Dissolution in Electrocoagulation Treatment of Petrochemical Wastewater. Int. J. Eng. Res. Technol. 2012, 1, 1–9. [Google Scholar]
- Brahmi, K.; Bouguerra, W.; Hamrouni, B.; Elaloui, E.; Loungou, M.; Tlili, Z. Investigation of electrocoagulation reactor design parameters effect on the removal of cadmium from synthetic and phosphate industrial wastewater. Arab. J. Chem. 2019, 12, 1848–1859. [Google Scholar]
- Rezaei, H.; Narooie, M.R.; Khosravi, R.; Mohammadi, M.J.; Sharafi, H.; Biglari, H. Humic Acid Removal by Electrocoagulation Process from Natural Aqueous Environments. Int. J. Electrochem. Sci. 2018, 13, 2379–2389. [Google Scholar] [CrossRef]
- Moreno-Casillas, H.A.; Cocke, D.L.; Gomes, J.A.G.; Morkovsky, P.; Parga, J.R.; Peterson, E. Electrocoagulation mechanism for COD removal. Sep. Purif. Technol. 2007, 56, 204–211. [Google Scholar] [CrossRef]
- Khandegar, V.; Saroha, A.K. Electrochemical treatment of distillery spent wash using aluminum and iron electrodes. Chi. J. Chem. Eng. 2012, 20, 439–443. [Google Scholar] [CrossRef]
- Khandegar, V.; Saroh, A.K. Treatment of distillery spentwash by electrocoagulation. J. Clean Energy Technol. 2014, 2, 244–247. [Google Scholar] [CrossRef]






| Parameters | Value | Unit | Methods |
|---|---|---|---|
| Color | Dark brown | - | Organoleptic method |
| COD | 112,948.5 | mgL−1 | SNI 06-6989.15-2004 |
| pH | 4.4 | - | A digital pH meter |
| Volatile fatty acid | 19,291.24 | mg-Acetic acid L−1 | Steam distillation |
| Total nitrogen | 420 | mgL−1 | Total Kjeldahl Nitrogen |
| Total solid | 92,624.25 | mgL−1 | APHA 22nd 2012 |
| Potassium | 985 | mgL−1 | SNI 06-6989.25-2005 |
| Chloride | 1108.6 | mgL−1 | APHA 22nd 2012 |
| Conductivity | 27,287.68 | μScm−1 | An electrical conductivity meter |
| Rate | Based on Route 1 (Model 1) | Based on Route 2 (Model 2) |
|---|---|---|
| 2.5 A | 3 A | 3.5 A | |
|---|---|---|---|
| Model 1 (based on route 1) | |||
| · (h−1) | 0.0490 | 0.0908 | 0.1270 |
| · (h−1) | 0.0249 | 0.1497 | 0.2002 |
| SSE | 2.03 × 10−4 | 3.4×10−3 | 8.8×10−3 |
| R2 | 0.93 | 0.85 | 0.82 |
| Model 2 (based on route 2) | |||
| · (h−1) | 0.0452 | 0.0571 | 0.0701 |
| · (h−1) | 0.0043 | 0.0374 | 0.0660 |
| SSE | 2.60×10−4 | 4.6×10−3 | 1.32×10−2 |
| R2 | 0.80 | 0.79 | 0.76 |
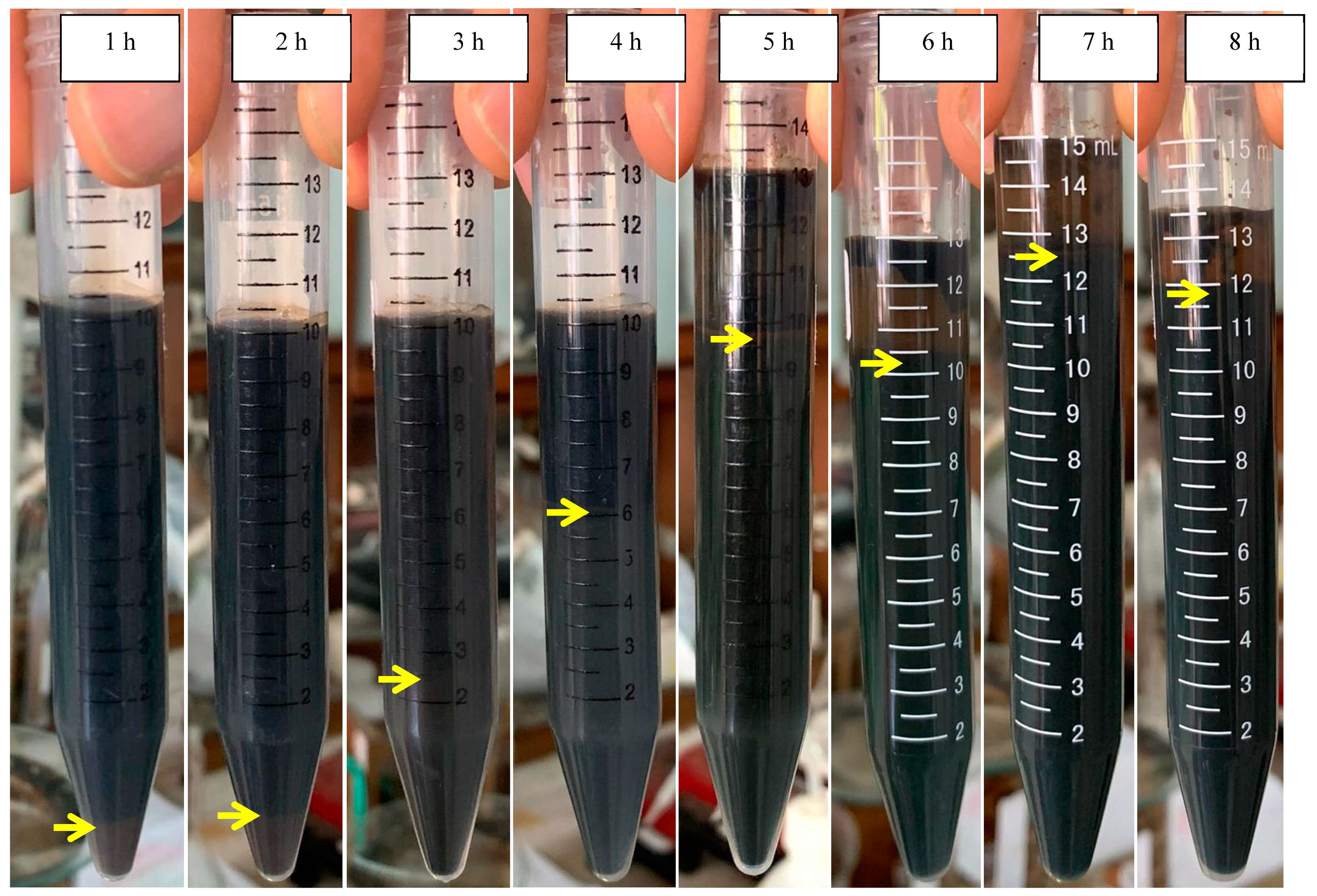
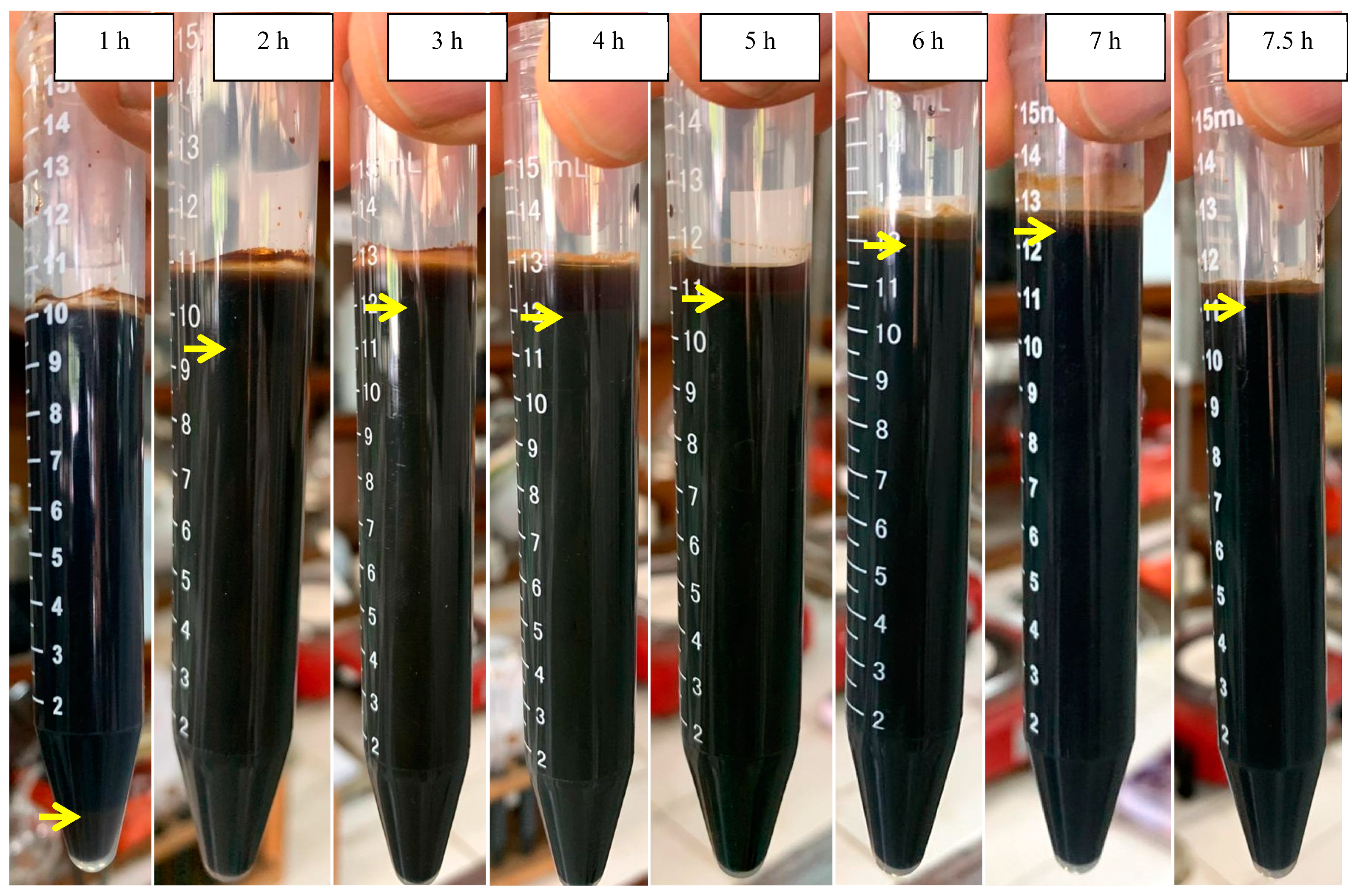
| Time (h) | Sludge at the Initial pH of 5.0 (%) | Sludge at the Initial pH of 7.0 (%) |
|---|---|---|
| 0 | 0 | 0 |
| 1 | 10 | 10 |
| 2 | 10 | 86.4 |
| 3 | 25 | 92.3 |
| 4 | 63 | 92.3 |
| 5 | 74.6 | 95.7 |
| 6 | 79.2 | 96 |
| 7 | 84.7 | 100 |
| 7.5 | - | 100 |
| 8 | 88.9 | - |
| pH 4.4 | pH 5.0 | pH 7.0 | |
|---|---|---|---|
| Model 1 (based on route 1) | |||
| · (h−1) | 0.0908 | 0.1575 | 0.1629 |
| · (h−1) | 0.1497 | 0.2089 | 0.4558 |
| SSE | 3.4×10−3 | 3×10−3 | 0.5869 |
| R2 | 0.85 | 0.90 | 0.86 |
| Model 2 (based on route 2) | |||
| · (h−1) | 0.0571 | 0.0843 | 0.0844 |
| · (h−1) | 0.0374 | 0.0793 | 0.1292 |
| SSE | 4.6×10−3 | 4.7×10−3 | 2.43×10−2 |
| R2 | 0.79 | 0.92 | 0.98 |
| Initial COD (mg L−1) | Initial Volume (mL) | Initial pH | Temperature (°C) | Current (A) | Agitation Speed (rpm) | Current Density (mA cm−2) | Electrodes | Electrode Active Surface (cm2) | d (cm) | t (h) | Charge Loading (C g-COD−1) | COD Removal Efficiency (%) | COD Removal (%) per Charge Loading (C g-COD−1) | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 112,948.5 | 1000 | 4.4 | 27 | 3.5 | 500 | 55.03 | Fe-Fe | 63.6 | 5.5 | 8 | 892.42 | 74.9 | 0.0839 | This study |
| 112,948.5 | 1000 | 7 | 30 | 3 | 500 | 47.17 | Fe-Fe | 63.6 | 5.5 | 5 | 478.10 | 66.7 | 0.1395 | This study |
| 120,000 | 300 | 3 | - | - | 500 | 187.5 | Fe-Fe | 16 | 3 | 2 | 600.00 | 52.4 | 0.0873 | [32] |
| 100,160 | 1000 | 6 | Room | 2.15 | 200 | - | Fe-Fe | 63.6 | 5.5 | 1 | 77.28 | 19.9 | 0.2571 | [14] |
| 100,160 | 1000 | 6 | Room | 3.99 | 200 | - | Fe-Fe | 63.6 | 5.5 | 1 | 143.41 | 51.7 | 0.3603 | [14] |
| 52,000 | 300 | 7.2 | - | 0.41 | 500 | 14.7 | Fe-Fe | - | 3 | 3 | 283.85 | 76.9 | 0.2709 | [33] |
| 52,000 | 300 | 7.2 | - | 0.41 | 500 | 17.9 | Fe-Fe | - | 3 | 3 | 283.85 | 84.5 | 0.2977 | [33] |
| 3360 | 500 | 7.5 | - | - | 500 | 35.9 | Fe-Fe | 12.8 | 3 | 2 | 1969.37 | 50 | 0.0254 | [2] |
| 3360 | 500 | 7.5 | - | - | 500 | 71.8 | Fe-Fe | 12.8 | 3 | 2 | 3938.74 | 88 | 0.0223 | [2] |
| 2500 | 500 | 6 | 30 ± 2 (constant) | - | - | 30 | Fe-Fe | 45 | 1 | 4 | 15552.00 | 62 | 0.0040 | [9] |
| 2000 | 2000 | 2 | Room | - | - | 3 | Fe-Fe | 100 | 2 | 4 | 1080.00 | 20.5 | 0.0190 | [10] |
| 2000 | 2000 | 6 | Room | - | - | 3 | Fe-Fe | 100 | 2 | 4 | 1080.00 | 81.3 | 0.0753 | [10] |
| 2000 | 2000 | 10 | Room | - | - | 3 | Fe-Fe | 100 | 2 | 4 | 1080.00 | 75.5 | 0.0699 | [10] |
| 2000 | 2000 | 6 | Room | - | - | 1 | Fe-Fe | 100 | 2 | 4 | 360.00 | 25.8 | 0.0715 | [10] |
| 2000 | 2000 | 6 | Room | - | - | 5 | Fe-Fe | 100 | 2 | 4 | 1800.00 | 92.5 | 0.0514 | [10] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syaichurrozi, I.; Sarto, S.; Sediawan, W.B.; Hidayat, M. Effect of Current and Initial pH on Electrocoagulation in Treating the Distillery Spent Wash with Very High Pollutant Content. Water 2021, 13, 11. https://doi.org/10.3390/w13010011
Syaichurrozi I, Sarto S, Sediawan WB, Hidayat M. Effect of Current and Initial pH on Electrocoagulation in Treating the Distillery Spent Wash with Very High Pollutant Content. Water. 2021; 13(1):11. https://doi.org/10.3390/w13010011
Chicago/Turabian StyleSyaichurrozi, Iqbal, Sarto Sarto, Wahyudi Budi Sediawan, and Muslikhin Hidayat. 2021. "Effect of Current and Initial pH on Electrocoagulation in Treating the Distillery Spent Wash with Very High Pollutant Content" Water 13, no. 1: 11. https://doi.org/10.3390/w13010011
APA StyleSyaichurrozi, I., Sarto, S., Sediawan, W. B., & Hidayat, M. (2021). Effect of Current and Initial pH on Electrocoagulation in Treating the Distillery Spent Wash with Very High Pollutant Content. Water, 13(1), 11. https://doi.org/10.3390/w13010011





